Abstract
Background
Since the publication of the 2010 Canadian antiplatelet guidelines, several large randomized controlled trials (RCTs) have evaluated the role of aspirin (ASA) use in primary prevention. We evaluated the effect of ASA use, compared with no ASA, on ischemic and bleeding events in patients without known atherosclerotic cardiovascular diseases.
Methods
We updated a published systematic review and meta-analysis by searching MEDLINE, Embase, and CENTRAL for the period up to March 2023. We included RCTs that enrolled patients for primary prevention of atherosclerotic cardiovascular diseases, and compared use of ASA to no ASA. We assessed risk of bias (RoB) using the Cochrane RoB tool, and certainty of evidence using the grading recommendations, assessment, development, and evaluation (GRADE) criteria. The primary efficacy outcome was major adverse cardiovascular events (MACE) (death, myocardial infarction, or stroke). The primary safety outcomes were intracranial hemorrhage and extracranial major bleeding events. We used a random-effects model to generate pooled risk ratios (RRs) and 95% confidence intervals (CIs).
Results
We included 14 RCTs (n = 167,587) at overall low RoB, with a median follow-up of 5 years. Compared to no ASA, ASA use reduced the incidence of MACE (RR 0.90, 95% CI 0.86-0.94), with a higher risk of intracranial hemorrhage (RR 1.33, 95% CI 1.13-1.56) and extracranial major bleeding (RR 1.67, 95% CI 1.36-2.06). In prespecified subgroups of age, sex, and diabetes, effect estimates were consistent.
Conclusions
ASA use in primary prevention is associated with a consistent reduction in MACE, but at the expense of major bleeding events. Patient values and preferences should be taken into account when considering ASA use for primary prevention.
Résumé
Contexte
Depuis la publication, en 2010, des lignes directrices canadiennes sur les agents antiplaquettaires, plusieurs importants essais contrôlés randomisés (ECR) ont été menés pour évaluer le rôle de l’aspirine (AAS) en prévention primaire. Nous avons comparé l’effet de l’AAS à la non-utilisation de l’AAS sur les événements ischémiques et hémorragiques chez des patients présentant une maladie cardiovasculaire athéroscléreuse (MCVAS) connue.
Méthodologie
Nous avons mis à jour une revue systématique et une méta-analyse déjà publiées en effectuant une recherche dans les bases de données MEDLINE, Embase et CENTRAL jusqu’en mars 2023. Nous avons inclus les ECR ayant porté sur la prévention primaire de la MCVAS chez les patients et comparé l’AAS et la non-utilisation de l’AAS. Nous avons évalué le risque de biais à l’aide de l’outil RoB de Cochrane, et le degré de certitude des données probantes au moyen des critères GRADE. Le principal critère d’évaluation de l’efficacité était les événements cardiovasculaires indésirables majeurs (ECIM; décès, infarctus du myocarde ou accident vasculaire cérébral). Les principaux critères d’évaluation de l’innocuité étaient les hémorragies intracrâniennes (HIC) et les saignements extracrâniens majeurs (SECM). Nous avons utilisé un modèle à effets aléatoires afin de générer les rapports de risque (RR) et les intervalles de confiance (IC) à 95 % regroupés.
Résultats
Nous avons inclus 14 ECR (n = 167 587) associés à un faible risque de biais et dont le suivi médian était de 5 ans. Comparativement à la non-utilisation d’AAS, l’AAS a réduit les ECIM (RR : 0,90; IC à 95 % : 0,86-0,94), mais était associé à un risque plus élevé d’HIC (RR : 1,33; IC à 95 % : 1,13-1,56) et de SECM (RR : 1,67; IC à 95 % : 1,36-2,06). Les estimations de l’effet étaient constantes dans les sous-groupes définis au préalable selon l’âge, le sexe et le diabète.
Conclusion
En prévention primaire, l’AAS est associé à une réduction systématique des ECIM, mais au détriment de manifestations hémorragiques majeures. Les valeurs et les préférences du patient doivent être prises en compte lorsqu’on envisage l’AAS en prévention primaire.
After the publication of the 2010 Canadian Cardiovascular Society antiplatelet guidelines recommending against the routine use of aspirin (acetylsalicylic acid [ASA]) in primary prevention, neither the 2012 nor the 2018 guideline update revised this position.1, 2, 3 In 2016, the European Society of Cardiology (ESC) guidelines on cardiovascular disease (CVD) prevention recommended against the use of antiplatelet therapy in individuals without established CVD, based on an increased risk of major bleeding.4 But the 2019 American College of Cardiology/American Heart Association primary prevention guidelines recommended low-dose ASA for selected adults aged 40 to 70 years who are deemed to be at higher risk for atherosclerotic cardiovascular disease (ASCVD) but do not have clinical features suggesting an increased bleeding risk.5
Since the publication of the 2010 Canadian Cardiovascular Society Antiplatelet Guidelines, a large body of additional evidence has been reported, comprising more than 50,000 patients in total.6, 7, 8, 9 We therefore sought to provide an updated synthesis of the available data regarding the use of ASA for the primary prevention of CVD events in terms of both safety and efficacy among a broad population, as well as in prespecified subgroups of interest according to age, sex, and diabetes, with the goal of providing clinicians with helpful guidance when considering use of ASA for primary prevention of ASCVD.
Methods
We updated a published systematic review and meta-analysis in accordance with the methodology outlined in the Cochrane Handbook for Systematic Review and Interventions and reported according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10,11
Search strategy, study selection, and data extraction
We updated the search used in the previous systematic review in MEDLINE, Embase, and CENTRAL up to March 2023.12 In duplicate, reviewers screened references and then potentially eligible full-text articles.
We included randomized controlled trials (RCTs) that selected patients for primary prevention of ischemic vascular events and compared ASA administration to no ASA. To be included, articles had to report original comparative outcomes in terms of ischemic CVD events or major bleeding among patients exposed vs not exposed to daily ASA as a primary prevention strategy.
One reviewer (E.B.-C.) performed all database searches and imported the records into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Subsequently, 2 reviewers (E.B.-C. and C.L.) independently screened article titles and abstracts and reviewed full-text articles for inclusion. The same reviewers independently extracted the following data from each newly included study using a standardized data collection form: study acronym, lead author, publication year, sample size, baseline characteristics, follow-up duration, and data on all prespecified outcomes.
Assessment of risk of bias and certainty of evidence
Two reviewers (E.B.-C. and A.F.) independently evaluated trial-level risk of bias (RoB) using the Cochrane RoB tool.13 We then rated the outcome-level certainty of the evidence using the grading recommendations, assessment, development, and evaluation (GRADE) framework, which incorporates risk of bias, imprecision, inconsistency, indirectness, and publication bias.14
Outcomes
The primary efficacy outcome of interest was major adverse cardiovascular events (MACE) (composite of death from any cause, myocardial infarction [MI], and stroke). The primary safety outcomes were intracranial hemorrhage (ICH) and extracranial major bleeding (ECMB). Secondary outcomes were all-cause mortality and major gastrointestinal bleeding (GIB).
Statistical analysis
We pooled dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) using using the Mantel-Haenszel method for all outcomes. We evaluated statistical heterogeneity with visual inspection of the forest plot and quantified the percentage of the variability that is due to heterogeneity between trials using the I2 statistic. We assessed whether age, sex, and diabetes were effect modifiers in prespecified subgroups analyses. We conducted all analyses using Review Manager version 5.4 (Cochrane, Copenhagen, Denmark).
Results
Search and selection of studies
From 4450 unique citations identified in the updated search algorithm, we screened 9 in full-text and found 1 additional eligible trial,6 for a total of 14 RCTs with a combined population 167,587 patients (Fig. 1).6,7,9,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 The included studies with patient characteristics are summarized in Table 1. Across included RCTs, the median age was 63 years, 51% were female, and 19% had diabetes. The ASA dose ranged from 75 to 500 mg, although the majority of studies (12 of 14 studies, representing 84% of the total studied population) used low-dose ASA, and the median follow-up was 5 years. Overall, RoB was deemed to be low in 8 of 14 trials and high in the other 6 (Supplemental Table S1). Visual inspection of the funnel plots did not suggest publication bias. We rated the certainty of evidence as high for MACE, all-cause mortality, ICH, and ECMB.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of study selection for the meta-analysis.
Table 1.
Summary of retained RCTs of ASA in primary prevention
| Study author (y) | Design | Sample size, n | Average follow-up, y | Mean age, y | Proportion of women, % | Proportion with diabetes, % | ASA dose studied, mg | Primary outcome | Secondary outcomes | Primary safety outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Fowkes et al.15 (2010) | Double-blind RCT | 3350 | 8.2 | 62 | 72 | 3 | 100 | Initial fatal or nonfatal coronary event, stroke, or revascularization | 1. All initial vascular events defined as a composite of the primary endpoint event or angina, intermittent claudication, or TIA 2. All-cause mortality |
None specified |
| Gaziano et al.9 (2018) | Double-blind RCT | 12,546 | 5 | 64 | 30 | 0 | 100 | CV death, MI, unstable angina, stroke, or TIA | MI and stroke | GI bleeding |
| Bowman et al.7 (2018) | Double-blind RCT | 15,480 | 7.4 | 63 | 37 | 100 | 100 | Vascular death, MI, or stroke/TIA | Nonfatal MI, intracranial hemorrhage, GI hemorrhage, GI cancer | Any major bleed, defined as any confirmed intracranial hemorrhage, sight-threatening eye bleeding, or any other serious bleeding episode (ie, requiring hospitalization or transfusion, or fatal or disabling) |
| McNeil et al.16 (2018) | Double-blind RCT | 19,114 | 4.7 | 74 | 56 | 11 | 100 | Disability-free survival composite (all-cause death, dementia, or physical disability) |
Major hemorrhage, any intracranial bleeding, upper GI bleeding, CV disease (fatal CV disease, MI, stroke, or hospitalization for heart failure), all-cause mortality, cancer mortality | None specified |
| Peto et al. 17 (1988) | Open-label RCT |
5139 | 6 | 60 | 0 | 2 | 500 | Nonfatal MI, nonfatal stroke, or TIA | Noncerebral bleed, hypertension, arrhythmia, acute thrombotic event (pulmonary, venous, or other), peptic ulcer, nonfatal malignant neoplasms, respiratory: acute infections, chronic bronchitis, emphysema, asthma, cataract, migraine, musculoskeletal disorders for which medical advice sought | None specified |
| Hansson et al.18 (1998) | Double-blind RCT | 18,790 | 3.8 | 61.5 | 47 | 8 | 75 | Nonfatal MI, nonfatal stroke, and CV death | Fatal bleeds (GI, cerebral, other), nonfatal major bleeds (GI, cerebral, nasal, other), minor bleeds (GI, nasal, purpura, other) | |
| Ogawa et al.19 (2008) | Open-label RCT | 2539 | 4.37 | 65.0 | 45 | 100 | 81 or 100 | Fatal or nonfatal IHD, fatal or nonfatal stroke, and PAD | Included each primary endpoint and combinations of primary endpoints as well as death from any cause | GI bleeding events and any hemorrhagic events other than hemorrhagic stroke |
| Ikeda et al.20 (2014) | Open-label RCT | 14,658 | 5.02 | 70.5 | 57.7 | 33.9 | 100 | Death from CV causes, nonfatal stroke, nonfatal MI | Composite including primary outcomes, plus TIA, angina pectoris, and arteriosclerotic disease requiring surgery or intervention; death from CV disease, death from non-CV causes, nonfatal stroke (ischemic or hemorrhagic), nonfatal MI, TIA, angina pectoris, arteriosclerotic disease requiring surgery or intervention, and serious extracranial hemorrhage requiring transfusion or hospitalization | Serious extracranial hemorrhage requiring transfusion or hospitalization; GI hemorrhage; gastroduodenal ulcer, reflux oesophagitis; erosive gastritis; stomach or abdominal discomfort, pain or pressure; heartburn; nausea |
| Physicians’ Health Study21 (1989) | Double-blind RCT | 22,071 | 5 | ∗ | 0 | 2.4 | 325 | Death with confirmed cause, nonfatal MI, or nonfatal stroke | Ischemic strokes, hemorrhagic strokes | GI discomfort, upper GI ulcers, other noninfectious disorders of the digestive tract, miscellaneous symptoms of the digestive tract, Bleeding (easy bruising, hematemesis, melena, nonspecific GI bleeding, epistaxis, other), bleeding requiring transfusions, death from GI bleed |
| Belch et al.22 (2008) | Double-blind RCT | 1276 | 6.7 | 60.2 | 55.8 | 100 | 100 | Death from coronary heart disease or stroke, non-fatal MI or stroke, or amputation above the ankle for critical limb ischemia† | All-cause mortality, nonfatal MI, other vascular events including stroke, TIA, coronary or peripheral arterial bypass surgery, coronary or peripheral arterial angioplasty, angina, claudication or critical limb ischemia | Adverse events: Malignancy, GI bleeding, GI symptoms including dyspepsia, arrhythmia, allergy including skin rash |
| Roncaglioni25 PPP (2001) | Open-label RCT | 4495 | 3.6 | 64.4 | 57.5 | 17 | 100 | CV death, nonfatal MI, and nonfatal stroke | CV deaths, total deaths, total CV events (CV death, nonfatal MI, nonfatal stroke, angina pectoris, TIA, PAD, and revascularization procedures | Cancer, bleeding (GI, intracranial not parenchymal, ocular, epistaxis, other), GI disease (except bleeding), other events |
| Thrombosis prevention trial23 (1998) | Double-blind RCT | 2540 | 6.8 | 57 | 0 | ∗ | 75 | Coronary death and fatal and nonfatal MI | Stroke | Bleeding episodes: Major episodes; confirmed cerebral haemorrhages and fatal or life-threatening haemorrhages at other sites that required transfusion and/or surgery Intermediate episodes; include macroscopic hematuria, larger bruises, and prolonged nose bleeds Minor episodes; ie, bruising, nose bleeds, rectal bleeding, and pink or red urine |
| Ridker et al.24 (2005) | Double-blind RCT | 39,876 | 10.1 | 54.6 | 100 | 2.6 | 100 every other day | Combination of major CV events, including nonfatal MI, nonfatal stroke, and death from CV causes | Fatal or nonfatal MI, fatal or nonfatal stroke, ischemic stroke, hemorrhagic stroke, and death from CV causes Additional analyses included the incidence of death from any cause, TIA, and the need for coronary revascularization |
Fatal GI hemorrhages, GI bleeding requiring transfusion; self-reported hematuria, easy bruising and epistaxis, symptoms suggestive of gastric upset †The presence of gastrointestinal bleeding or peptic ulcer was confirmed by a specific follow-up questionnaire. |
| Yusuf et al.6 (2021) | Double-blind RCT | 5713 | 4.6 | 63.9 | 52.9 | 36.7 | 75 | Death from CV causes, MI, stroke (ASA vs placebo comparison only) |
Major CV events and the composite of the primary outcome plus angina with evidence of ischemia / death from any cause, first and recurrent CV events, cancer | Major bleeding, minor bleeding, GI bleeding Major bleeding based on ISTH criteria‡ |
Follow-up is mean or median, as reported in each study.
ASA, acetylsalicylic acid (aspirin); CV, cardiovascular, GI, gastrointestinal; IHD, ischemic heart disease; ISTH, International Society on Thrombosis and Hemostasis; MI, myocardial infarction; PAD, peripheral artery disease; PPP, Primary Prevention Project; RCT, randomized controlled trials; TIA, transient ischemic attack.
Not reported.
This study included 2 hierarchical composite primary outcomes. The most comprehensive composite is described.
The ISTH criteria are defined as follows: (i) fatal bleeding; (ii) bleeding in a critical site or area (retroperitoneal, cardiac tamponade, hemoptysis, intraocular, intracranial, definite hemorrhagic stroke or subarachnoid hemorrhage); or (iii) bleeding causing a fall in hemoglobin level of 20 g/L or more or leading to transfusion of 2 or more units of blood.34
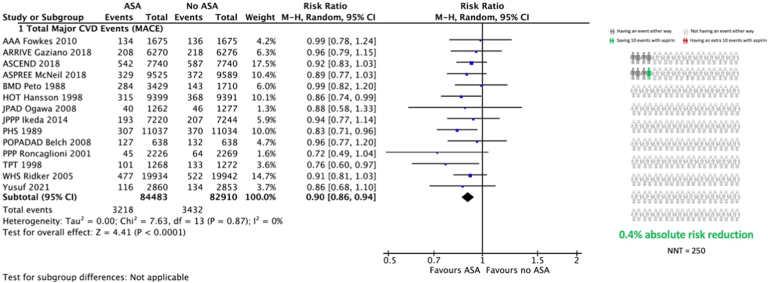
Major adverse cardiovascular events
All 14 studies reported MACE. A 10% relative risk reduction occurred with ASA, compared to no ASA, in favor of primary prevention (relative risk [RR] 0.90, 95% CI 0.86-0.94, I2 = 0%; Fig. 2; Table 2), translating to 4 fewer events per 1000 patients treated with ASA for 5 years (95% CI, 2 to 6 fewer per 1000). The treatment effect was similar in direction and magnitude irrespective of age (P-interaction = 0.51; Supplemental Fig. S1), sex (P-interaction = 0.28; Supplemental Fig. S2), or diabetes (P-interaction = 0.80; Supplemental Fig. S3).
Figure 2.
Meta-analysis of the effect of acetylsalicylic acid (ASA; aspirin) primary prevention on major adverse cardiovascular events (MACE). Risk ratio refers to the relative risk of the event compared to no ASA. Squares represent individual study risk ratios. The diamond represents the pooled risk ratio after meta-analysis. Lines represent the 95% confidence intervals (CIs) of the individual studies. The right panel represents the absolute risk increase or reduction with ASA use, compared to no ASA. AAA, Aspirin for Asymptomatic Atherosclerosis Trial; ARRIVE, Aspirin to Reduce Risk of Aspirin to Reduce Risk of Initial Vascular Events in Patients at Moderate Risk of Cardiovascular Disease; ASCEND, A Study of Cardiovascular Events in Diabetes; ASPREE, Aspirin in Reducing Events in the Elderly trial; BMD, British Male Doctors Study; CVD, cardiovascular; HOT, Hypertension Optimal Treatment randomised trial; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirine for Diabetes Trial; JPPP, Japanese Primary Prevention Project; M-H, Mantel-Haenszel; NTT, number needed to treat; PHS, Physicians’ Health Study; POPADAD, Prevention of Progression of Arterial Disease and Diabetes; PPP, Primary Prevention Project; TPT, Thrombosis Prevention Trial; WHS, Women’s Health Study.
Table 2.
Summary of meta-analysis findings in the overall primary prevention population
| Outcome | Certainty of evidence | Effect estimate |
|
|---|---|---|---|
| RR (95% CI) | Absolute change, per 1000 | ||
| MACE | High | 0.90 (0.86–0.94) | 4 fewer (from 6 to 2 fewer) |
| ICH | High | 1.33 (1.13–1.56) | 1 more (from 0 to 2 more) |
| ECMB | High | 1.67 (1.36–2.06) | 5 more (from 3 to 8 more) |
CI, confidence interval; ECMB, extracranial major bleeding; ICH, intracranial hemorrhage; MACE, major adverse cardiovascular events; RR, risk ratio (relative risk).
Intracranial hemorrhage
Thirteen studies compared the risk of intracranial bleeding with ASA use in primary prevention.7,9,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Although 3 small studies (representing only 8.8% of all studied patients) demonstrated a reduced risk of ICH among patients receiving ASA,9,18,22 meta-analysis of all 13 studies showed an increased risk of ICH in the ASA group (RR 1.33, 95% CI 1.13-1.56, I2 = 0%; Fig. 3; Table 2), translating to 1 more ICH event per 1000 patients treated with ASA for primary prevention over 5 years (95% CI 0 to 2 more per 1000). The effect of ASA on ICH risk resulted in similar effect sizes in the prespecified subgroups, without significant heterogeneity (Supplemental Figs. S4-S6).
Figure 3.
Meta-analysis of the effect of acetylsalicylic (ASA) primary prevention on intracranial hemorrhage (ICH). Risk ratio refers to the relative risk of the event compared to no ASA. Squares represent individual study risk ratios. The diamond represents the pooled risk ratio after meta-analysis. Lines represent the 95% confidence intervals (CIs) of the individual studies. The right panel represents the absolute risk increase or reduction with ASA use, compared to no ASA. AAA, Aspirin for Asymptomatic Atherosclerosis Trial; ARRIVE, Aspirin to Reduce Risk of Aspirin to Reduce Risk of Initial Vascular Events in Patients at Moderate Risk of Cardiovascular Disease; ASCEND, A Study of Cardiovascular Events in Diabetes; ASPREE, Aspirin in Reducing Events in the Elderly trial; BMD, British Male Doctors Study; CVD, cardiovascular; HOT, Hypertension Optimal Treatment randomised trial; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirine for Diabetes Trial; JPPP, Japanese Primary Prevention Project; M-H, Mantel-Haenszel; NTT, number needed to treat; PHS, Physicians’ Health Study; POPADAD, Prevention of Progression of Arterial Disease and Diabetes; PPP, Primary Prevention Project; TPT, Thrombosis Prevention Trial; WHS, Women’s Health Study.
Extracranial major bleeding
Thirteen studies evaluated the risk of ECMB with and without ASA for primary prevention.6,7,9,15, 16, 17, 18, 19,21,23, 24, 25 The risk of ECMB events among patients taking ASA was increased, compared to the risk in those without ASA (RR 1.67, 95% CI 1.36-2.06, I2 = 63%; Fig. 4; Table 2), translating to 5 more ECMB events over 5 years per 1000 patients treated with ASA for primary prevention (95% CI 3 to 8 more per 1000). Subgroup ECMB rates were inconsistently reported, but ASA consistently increased the risk of total major bleeding (consisting of any type of extracranial or intracranial major bleeding episode) in all prespecified subgroups (Supplemental Figs. S7-S9).
Figure 4.
Meta-analysis of the effect of acetylsalicylic acid (ASA) primary prevention on extracranial major bleeding. Risk ratio refers to the relative risk of the event compared to no ASA. Squares represent individual study risk ratios. The diamond represents the pooled risk ratio after meta-analysis. Lines represent the 95% confidence intervals (CIs) of the individual studies. The right panel represents the absolute risk increase or reduction with ASA use, compared to no ASA. AAA, Aspirin for Asymptomatic Atherosclerosis Trial; ARRIVE, Aspirin to Reduce Risk of Aspirin to Reduce Risk of Initial Vascular Events in Patients at Moderate Risk of Cardiovascular Disease; ASCEND, A Study of Cardiovascular Events in Diabetes; ASPREE, Aspirin in Reducing Events in the Elderly trial; BMD, British Male Doctors Study; HOT, Hypertension Optimal Treatment randomised trial; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirine for Diabetes Trial; JPPP, Japanese Primary Prevention Project; M-H, Mantel-Haenszel; NTT, number needed to treat; PHS, Physicians’ Health Study; PPP, Primary Prevention Project; TPT, Thrombosis Prevention Trial; WHS, Women’s Health Study.
Other clinical events
All 14 studies also reported all-cause mortality.6,7,9,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Two showed an increased mortality risk among patients receiving ASA,16,23 but our overall meta-analysis showed no significant difference in all-cause mortality with the use of ASA for primary prevention (RR 0.97, 95% CI 0.93-1.01, I2 = 0%; Supplemental Fig. S10). Neither age, sex, nor diabetes was an effect modifier (Supplemental Figs. S11-S13).
Twelve studies evaluated the risk of major (GIB) among those receiving ASA for primary prevention.6,7,9,15, 16, 17, 18, 19,21,23, 24, 25 The risk of GIB events among patients taking ASA was increased, compared to the risk among those not taking ASA (RR 1.59, 95% CI 1.32-1.91, I2 = 0.27; Supplemental Fig. S14).
Discussion
This comprehensive updated meta-analysis demonstrates, with a high level of certainty, a reduction in MACE with the use of ASA, compared to no ASA, in a primary prevention population that is consistent across all prespecified subgroups but is associated with a similarly consistent increased risk of major bleeding events. These competing risks must be carefully considered prior to prescribing ASA for primary prevention.
The 10% relative risk reduction in MACE with ASA for primary prevention deserves particular attention. A 2019 meta-analysis of ASA use for primary prevention found a similar reduction in MACE (cardiovascular death, nonfatal MI, and nonfatal stroke) with the number needed to treat (NNT) being 241 patients, but with an increased risk of major bleeding with the number needed to harm (NNH) being 210 patients over the same timeframe.12 (These findings are very consistent with the findings of the present analysis: number needed to treat, 250; number needed to harm, 200 over 5 years.) The US Preventive Services Task Force (USPSTF) provided an updated evidence report and systematic review that found ASA was associated with a similar significant decrease in MACE (10%), with similar reductions in the individual components of the composite outcome, but with significant increases in major bleeding events. However, the investigators found no difference in cardiovascular mortality and all-cause mortality (similar to our results) with ASA.26 Given these findings, the US Preventive Services Task Force suggested a role for ASA in primary prevention in those aged 40-59 years with an estimated 10-year risk of 10% or greater for CVD, but at low risk of bleeding.11 However, an important point to note is that a strategy of selective prescription of ASA for primary prevention of ASCVD based on estimated risk of ischemic or bleeding events has not yet been evaluated prospectively, and estimating risk based on pooled cohort equations comes with inherent limitations, particularly in older, multimorbid, or obese patients,27,28 such that individualized approaches are needed. The benefits of adding ASA in the context of contemporary primary prevention arguably are also smaller, with more-intensive approaches to lipid and blood pressure lowering29; conversely, the benefits might be greater among patients with more-extensive atherosclerotic burden on noninvasive imaging. Other factors that have yet to find their way into clinical practice may also influence the decision for or against ASA use in primary prevention. For example, analyses from the Aspirin in Reducing Events in the Elderly (ASPREE) trial and the Women’s Health Study suggest that individuals with liproprotein(a) genetic variants may derive a higher benefit from ASA use in primary prevention.30,31
Whereas the ischemic advantages of ASA use are apparent, the risk of bleeding is certainly concerning. Also, segregating ischemic risk from bleeding risk is difficult, especially as age is a strong predictor of both outcomes.29 Although the risk of ICH with ASA use (1 additional event for every 1000 patients treated over 5 years) was low, this risk may not be acceptable to individuals contemplating ASA use for primary prevention. Moreover, a dynamic approach to balancing risk during a patient’s lifetime needs to be considered, as both the baseline risk of ICH and the increased risk with ASA use evolve with comorbidities and age. We also demonstrated an increased risk of ECMB (5 additional events for every 1000 patients treated over 5 years) and GIB (3 additional events for every 1000 patients treated over 5 years) with ASA use.
ASA inhibits the cyclo-oxegenase-1 (COX-1) pathway, which alters the biosynthesis of prostanoids such as prostaglandins, which are known to protect against gastric and duodenal mucosal damage. Although enteric-coated ASA formulations in theory reduce GIB risk, this reduction has not been supported by recent findings, and the observed increase in major bleeding events that we report stems from studies that nearly all used enteric-coated ASA.32 Another formulation, phospholipid-aspirin complex, is associated with reduced gastrointestinal injury and predictable absorption that may mitigate the GIB risk.33,34 Whether this formulation provides a more favourable safety profile in a primary prevention population remains to be demonstrated. Also unclear is whether coadministration of ASA and a gastroprotective agent, such as a proton pump inhibitor, sufficiently mitigates the GIB risk,35 such that ASA would be more broadly acceptable in a primary prevention context, particularly given the uncertainty regarding the cost-effectiveness of this approach.
Whether to prescribe ASA for the primary prevention of ischemic vascular events, and to whom, continues to generate debate among experts. Professional society recommendations therefore vary. The American Diabetes Association recommends using 75-162 mg of ASA daily for patients with diabetes mellitus who are at increased cardiovascular risk.36 The American Heart Association, in 2011, before the publication of much of the current body of evidence, established targeted recommendations for ASA use in women, patients with diabetes, and those aged over 65 years if blood pressure is well controlled and the ischemic benefits are deemed to outweigh the bleeding risk.37 The more recent American College of Cardiology/American Heart Association guidelines for primary prevention subsequently limited low-dose ASA use to adults aged 40-70 years.5 The current body of evidence, however, as summarized in this systematic review, does not support using ASA in primary prevention at any dose on the basis of sex, age, or diabetes status alone. Indeed, given the wide Cis in many of the subanalyses, any nonsignificant subgroup effect likely is due to insufficient statistical power rather than a true lack of effect.
Given that the benefit of ASA on ischemic events is counterbalanced by an increased risk of bleeding (and in the absence of a proven mortality benefit), a shared decision-making model appears appropriate when considering the possibility of ASA use in primary prevention among selected patients at increased risk of ASCVD with an acceptable bleeding profile. Patient values and preferences are integral in judging the balance of benefit and harm. Most studies on patient valuation of risk and benefit have been conducted in secondary prevention populations, and they may not represent the preferences of a primary prevention population. However, given the lack of a proven mortality benefit of ASA in primary prevention, avoiding ICH may drive decision-making. Mühlbacher and Bethge found that German patients with acute coronary syndrome valued a reduction in mortality twice as much as a reduction in bleeding in a discrete-choice experiment.38,39 Yuan et al. found that American participants considered disabling stroke to be an outcome of the same order of desirability as death, and a reduction in both of these outcomes was preferred strongly over a reduction in MI.40 Whether the respondents in this study would have considered intracranial hemorrhage equivalent in desirability to disabling stroke is unclear, but Pinto et al. found that United Kingdom patients with MI considered intracranial hemorrhage a fate worse than death, based on a discrete-choice experiment.41 Patient outcome valuations did not differ according to sex in the discrete-choice experiments, and the effect of sex was not reported in the study by Yuan et al.40 An interesting finding by Pinto et al. is that patients at higher bleeding risk (with at least one clinical risk factor) valued a reduction in bleeding events more than did those at lower risk (with no risk factors).41 Similarly, patients at higher ischemic risk (thrombosis in MI [TIMI] risk score ≥ 3) valued a reduction in ischemic events more than did those at lower risk.41 The fact that patients appear to be able to consider events tacitly for which they are most at risk further supports empowerment of the patient as a decision maker via arming them with the best available evidence.41
Future research should seek to evaluate the role of ASA in enriched (higher-risk) primary prevention populations, such as those with higher pooled ischemic risk estimates, lipoprotein (Lp)(a) variants or otherwise elevated Lp(a) levels, higher atherosclerotic burden on noninvasive or invasive imagin, and those failing to obtain target blood pressure, hemoglobin A1c, low-density lipoprotein cholesterol, apolipoprotein B, or Lp(a) levels despite receiving optimal guideline-directed therapy.
Our analysis comes with limitations. Because this analysis is based on study-level data, we could not stratify results by baseline ASCVD risk. Also, the influence of other primary prevention therapies (ie, 3-hydroxy-3-methylglutaryl coenzyme A [HMG-CoA] reductase inhibitors) could not be ascertained. Given these limitations, risk differences are not adjusted for population differences across studies. Finally, the interaction between evolving comorbid risk and frailty over time could not be addressed.
Conclusion
Our comprehensive meta-analysis of ASA use in primary prevention suggests that it reduces ischemic events at the expense of major bleeding, without a demonstrated mortality benefit. An individualized, informed, patient-centric approach may identify patients with a high ischemic, but low bleeding risk who could benefit from ASA use via reduction of vascular events. However, routine broad prescription of ASA for primary prevention of ASCVD is not supported by the present analysis.
Acknowledgments
Ethics Statement
This meta-analysis was conducted on studies that either explicitly stated having or were understood to have complied with all appropriate ethical standards. Our analysis was rigorously conducted based on the available data.
Patient Consent
This meta-analysis was conducted on de-identified aggregate patient data as presented in published reports. Therefore, individual patient consent was not required.
Funding Sources
This analysis was funded in part by the Canadian Cardiovascular Society (CCS) to support the development of society guideline recommendations. The funder had no input regarding the contents of the present manuscript. E.B.-C. is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. G.M.-G. and B.J.P. are each supported by a Fonds de recherche du Québec en Santé (FRQS) Junior 1 Clinician-Scientist Award. M.L. is a Canada Research Chair in Platelets As Biomarkers and Vectors. The other authors have no funding sources to declare.
Disclosures
Unrelated to this work, E.B.-C. has received research grants from Bayer, BMS-Pfizer, and Roche Diagnostics, and consulting fees from Trimedic Therapeutics Inc. G.M.-G. has received research grants from Bayer Canada and speaker honoraria from Bayer Canada and Jamp Pharma. M.L. has received speaker honoraria from Bayer Canada and research grants to the institution from Idorsia; has served on a national advisory board for Servier Canada; and has received in-kind and financial support for investigator-initiated grants from Fujimori Kogyo. B.J.P. has received research funding from Bayer Canada, Novartis Canada, and Boehringer-Ingelheim Canada and has received honoraria from Novartis Canada. The other authors have no conflicts of interest to disclose.
Footnotes
See page 889 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.08.011.
Supplementary Material
References
- 1.Bell A.D., Roussin A., Cartier R., et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2011;27(suppl A):S1–S59. doi: 10.1016/j.cjca.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Tanguay J.F., Bell A.D., Ackman M.L., et al. Focused 2012 update of the Canadian Cardiovascular Society guidelines for the use of antiplatelet therapy. Can J Cardiol. 2013;29:1334–1345. doi: 10.1016/j.cjca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Mehta S.R., Bainey K.R., Cantor W.J., et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi: 10.1016/j.cjca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 5.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf S., Joseph P., Dans A., et al. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med. 2021;384:216–228. doi: 10.1056/NEJMoa2028220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman L., Mafham M., Stevens W., et al. ASCEND: A Study of Cardiovascular Events iN Diabetes: characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J. 2018;198:135–144. doi: 10.1016/j.ahj.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahady S.E., Margolis K.L., Chan A., et al. Major GI bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial. Gut. 2021;70:717–724. doi: 10.1136/gutjnl-2020-321585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaziano J.M., Brotons C., Coppolecchia R., et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036–1046. doi: 10.1016/S0140-6736(18)31924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) 2021;74:790–799. doi: 10.1016/j.rec.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 11.US Preventive Services Task Force. Davidson K.W., Barry M.J., et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:1577–1584. doi: 10.1001/jama.2022.4983. [DOI] [PubMed] [Google Scholar]
- 12.Zheng S.L., Roddick A.J. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321:277–287. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A.C., Savovic J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Balshem H., Helfand M., Schunemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Fowkes F.G., Price J.F., Stewart M.C., et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 16.McNeil J.J., Wolfe R., Woods R.L., et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peto R., Gray R., Collins R., et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988;296:313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson L., Zanchetti A., Carruthers S.G., et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa H., Nakayama M., Morimoto T., et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda Y., Shimada K., Teramoto T., et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510–2520. doi: 10.1001/jama.2014.15690. [DOI] [PubMed] [Google Scholar]
- 21.Physicians' health study aspirin and primary prevention of coronary heart disease. N Engl J Med. 1989;321:1825–1828. doi: 10.1056/NEJM198912283212610. [DOI] [PubMed] [Google Scholar]
- 22.Belch J., MacCuish A., Campbell I., et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council's General Practice Research Framework. Lancet. 1998;351:233–241. [PubMed] [Google Scholar]
- 24.Ridker P.M., Cook N.R., Lee I.M., et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 25.Roncaglioni M.C. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 26.Guirguis-Blake J.M., Evans C.V., Perdue L.A., et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:1585–1597. doi: 10.1001/jama.2022.3337. [DOI] [PubMed] [Google Scholar]
- 27.Khera R., Pandey A., Ayers C.R., et al. Performance of the pooled cohort equations to estimate atherosclerotic cardiovascular disease risk by body mass index. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanna M.G., Peterson E.D., Wojdyla D., et al. The accuracy of cardiovascular pooled cohort risk estimates in U.S. older adults. J Gen Intern Med. 2020;35:1701–1708. doi: 10.1007/s11606-019-05361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S.U., Lone A.N., Kleiman N.S., et al. Aspirin with or without statin in individuals without atherosclerotic cardiovascular disease across risk categories. JACC Adv. 2023;2 doi: 10.1016/j.jacadv.2022.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacaze P., Bakshi A., Riaz M., et al. Aspirin for primary prevention of cardiovascular events in relation to lipoprotein(a) genotypes. J Am Coll Cardiol. 2022;80:1287–1298. doi: 10.1016/j.jacc.2022.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chasman D.I., Shiffman D., Zee R.Y., et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Bianco-Rondeau M., Robert-Halabi M., Bloom S., et al. Aspirin for primary cardiovascular prevention in patients with diabetes: uncertainties and opportunities. Thromb Haemost. 2022;122:1443–1453. doi: 10.1055/s-0042-1743469. [DOI] [PubMed] [Google Scholar]
- 33.Angiolillo D.J., Prats J., Deliargyris E.N., et al. Pharmacokinetic and pharmacodynamic profile of a novel phospholipid aspirin formulation. Clin Pharmacokinet. 2022;61:465–479. doi: 10.1007/s40262-021-01090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franchi F., Schneider D.J., Prats J., et al. Pharmacokinetic and pharmacodynamic profiles of a novel phospholipid-aspirin complex liquid formulation and low dose enteric-coated aspirin: results from a prospective, randomized, crossover study. J Thromb Thrombolysis. 2022;54:373–381. doi: 10.1007/s11239-022-02687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostom A., Moayyedi P., Hunt R., et al. Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 2009;29:481–496. doi: 10.1111/j.1365-2036.2008.03905.x. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S103–S123. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 37.Mosca L., Benjamin E.J., Berra K., et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mühlbacher A.C., Bethge S., Kaczynski A. Treatment after acute coronary syndrome: analysis of patient's priorities with analytic hierarchy process. Int J Technol Assess Health Care. 2016;32:284–291. doi: 10.1017/S0266462316000428. [DOI] [PubMed] [Google Scholar]
- 39.Mühlbacher A.C., Bethge S. Reduce mortality risk above all else: a discrete-choice experiment in acute coronary syndrome patients. Pharmacoeconomics. 2015;33:71–81. doi: 10.1007/s40273-014-0223-1. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Z., Levitan B., Burton P., et al. Relative importance of benefits and risks associated with antithrombotic therapies for acute coronary syndrome: patient and physician perspectives. Curr Med Res Opin. 2014;30:1733–1741. doi: 10.1185/03007995.2014.921611. [DOI] [PubMed] [Google Scholar]
- 41.Pinto C.A., Chua G.N., Bridges J.F.P., et al. Comparing patient preferences for antithrombotic treatment during the acute and chronic phases of myocardial infarction: a discrete-choice experiment. Patient. 2022;15:255–266. doi: 10.1007/s40271-021-00548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.