Abstract
Background
Ejection fraction (EF) is often used as a prognostic indicator and for classifying heart failure (HF) patients. This study evaluates the association of echocardiographic parameters with HF with improved EF (HFimpEF).
Methods
This single-centre study retrospectively included patients with HF with reduced EF (HFrEF) from a cohort of admitted patients over 2018-2020, who were then followed up prospectively until 2023. The control group was categorized as patients with non-recovered HFrEF, and the population group was categorized as patients with HFimpEF.
Results
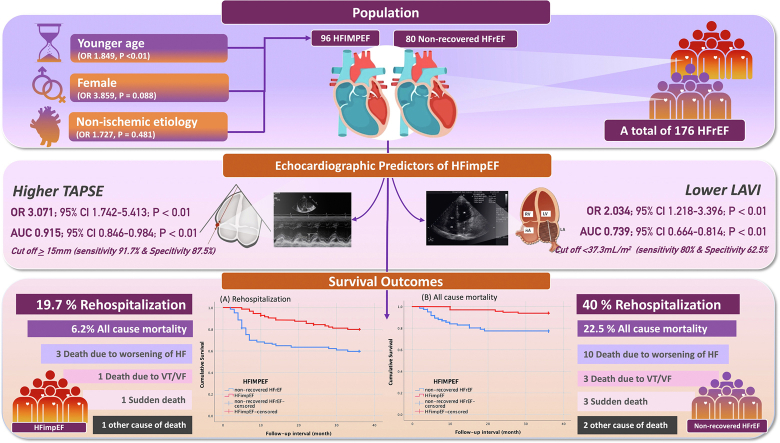
A total of 176 patients with HFrEF were included in the study. Non-ischemic etiology was found to be the most prevalent cause of HFimpEF. The baseline echocardiography examination revealed that the HFimpEF group exhibited significantly higher values for tricuspid annular plane systolic excursion (TAPSE; P < 0.001) and inferior vena cava diameter (P < 0.001). The non-recovered HFrEF group demonstrated higher baseline left atrial volume index (LAVi) values (P < 0.001). In multivariate analysis, a higher value of TAPSE (odds ratio 3.071; P = 0.008) and a lower value of LAVi (odds ratio 2.034; P = 0.008) were independent echocardiography variables associated with HFimpEF. After a mean follow-up duration of 32.5 ± 9.1 months, the HFimpEF group had higher survival from rehospitalization due to worsening HF and lower all-cause mortality (log rank P < 0.001 and P = 0.005, respectively).
Conclusions
Higher TAPSE and lower LAVi in baseline were associated with the transition from HFrEF to HFimpEF. The HFimpEF group had better survival compared to those with non-recovered HFrEF.
Graphical abstract
Résumé
Contexte
La fraction d’éjection est souvent utilisée comme indicateur pronostique et comme élément de classification des patients atteints d’insuffisance cardiaque. La présente étude visait à évaluer l’association entre les paramètres échocardiographiques et l’insuffisance cardiaque avec fraction d’éjection améliorée (ICFEA).
Méthodologie
Cette étude monocentrique a été menée de façon rétrospective auprès d’une cohorte de patients atteints d’insuffisance cardiaque avec fraction d’éjection réduite (ICFER) traités entre 2018 et 2020, et cette cohorte a été suivie de façon prospective jusqu’en 2023. Les patients du groupe témoin ont été classés comme ayant une ICFER ne s’étant pas résorbée, et les patients de la population étudiée ont été classés comme ayant une ICFEA.
Résultats
Au total, 176 patients présentant une ICFER ont été inclus dans l’étude. La cause la plus fréquente d’ICFER était une étiologie non ischémique. Lors de l’évaluation échocardiographique initiale, les patients du groupe ayant progressé vers l’ICFEA présentaient des valeurs significativement plus élevées en ce qui concerne l’excursion systolique du plan de l’anneau tricuspide (TAPSE pour tricuspid annular plane systolic excursion) (p < 0,001) et le diamètre de la veine cave inférieure (VCI) (p < 0,001). D’autre part, les patients du groupe dont l’ICFER ne s’est pas résorbée présentaient des valeurs initiales plus élevées à l’indice de volume auriculaire gauche (IVAG) (p < 0,001). Lors d’une analyse multivariée, des valeurs de TAPSE plus élevées (rapport de cotes [RC] de 3,071; p = 0,008) et des valeurs plus faibles d’IVAG (RC de 2,034; p = 0,008) étaient deux variables échocardiographiques indépendantes associées avec la progression vers l’ICFEA. Après un suivi d’une durée moyenne de 32,5 ± 9,1 mois, le groupe présentant une ICFEA présentait un taux plus élevé de survie sans réhospitalisation due à une aggravation de l’IC et un taux plus faible de mortalités toutes causes confondues que le groupe dont l’ICFER ne s’était pas résorbée (p selon le test logarithmique par rangs < 0,001 et p = 0,005, respectivement).
Conclusions
Une valeur de TAPSE élevée et un IVAG faible à l'évaluation initiale étaient associés à un passage de l’ICFER à l’ICFEA. La survie de patients présentant une ICFEA était supérieure à celle des patients présentant une ICFER non résorbée.
Heart failure (HF) is a clinical syndrome characterized by cardinal symptoms and signs resulting from structural or functional abnormalities of the heart. The left ventricular ejection fraction (LVEF) derived from echocardiographic parameters is often used as a prognostic indicator and for the classification of HF patients.1 Treatment of HF is indicated in all patients with left ventricular (LV) dysfunction, regardless of symptoms. The treatment objectives encompass improving the survival rate, alleviating symptoms, reducing morbidity, and minimizing rehospitalization.1,2 Generally, morbidity and mortality reduction can be attained through restoration of LV function in patients with HF with reduced ejection fraction (HFrEF).1, 2, 3 According to numerous studies, LVEF can improve with or without treatment.2 Based on these findings, the Heart Failure Society of America, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society defined HF with improved ejection fraction (HFimpEF) as HF with a baseline LVEF of ≤ 40%, with a minimum increase of 10 points from the baseline LVEF measurement, and subsequently reaching an LVEF > 40% following a second LVEF assessment.4, 5, 6
Although the improvement in ejection fraction is associated with enhanced quality of life and event-free survival, this specific population continues to be susceptible to future adverse outcomes.7 Various demographic and clinical parameters also have been identified as predictors of improved ejection fraction in HF patients.8 Although the echocardiographic examination is crucial in assessing the success of therapy and patient prognosis, the baseline echocardiographic parameters used to predict the improvement from HFrEF to HFimpEF are still imprecise. Moreover, no studies have investigated rehospitalization survival and the all-cause mortality outcome of HFimpEF, compared with non-recovered HFrEF.
This study aimed to assess the clinical outcome and baseline echocardiographic parameters of HFrEF patients that are associated with the development of HFimpEF. To our knowledge, no study has used baseline echocardiography as a predictive model for HFimpEF based on the Universal Definition and Classification of Heart Failure (Heart Failure Society of America, the Heart Failure Association of the European Society of Cardiology and the Japanese Heart Failure Society).
Methods
In this cohort study, patient medical records were identified and analyzed retrospectively using the International Classification of Diseases, 10th revision (ICD-10) code. Patients diagnosed with HFrEF who were referred to Prof. Dr. I.G.N.G Ngoerah General Hospital during 2019 and 2020 were enrolled in the study. All patients underwent a full echocardiogram evaluation once they were stable. The second echocardiography examination was performed within 1 year following the initial evaluation. The subsequent echocardiography was performed to stratify patients into distinct cohorts of those with HFimpEF and those with HFrEF. These categorizations enabled the subsequent tracking and evaluation of patients over a span of 3 years, until January 2023, for survival analysis, yielding crucial insights into their prognoses and clinical outcomes. A cardiology resident carried out the examination, and it was validated by an echocardiography consultant cardiologist. Echocardiographic measurements were performed on a Philips-EPIQ 7 ultrasound system (Andover, MA). Standard techniques were adopted to obtain M-mode, 2-dimensional, and Doppler measurements, following the American Society of Echocardiography's guidelines.9 LVEF was measured using the Simpson biplane method, unless use of this method was not possible. Patients were categorized according to the improvement in ejection fraction using the Classification of Heart Failure for HFimpEF definition based on the Heart Failure Association of the European Society of Cardiology, which required an improvement of 10 points from baseline LVEF and an LVEF of > 40% upon second measurement.6
The control group consisted of patients who did not meet these criteria and were categorized as those with non-recovered HFrEF. Patients who received cardiac resynchronization therapy, those who did not undergo a second echocardiogram, and those who underwent a second echocardiogram more than 1 year following the initial echocardiography were excluded from the study to ensure the integrity of the data analysis. All patients received optimal medical therapy according to our hospital standards, per discharge records from the first admission. Rehospitalization due to worsening HF was defined as the hospitalization of patients diagnosed with acute decompensated HF, based on ICD-10 codes. Prospective collection of all-cause mortality data was conducted through medical records, commencing from the second echocardiogram and continuing until January 2023 or death of the patient.
All categorical data were presented in frequencies and percentages. The normality of the metric data was assessed using the Kolmogorov Smirnov test. The sociodemographic and clinical characteristics and outcomes were compared for the cases vs control groups using Pearson's χ2 test for categorical variables, and the t-test or Mann-Whitney U test for metric variables. Moreover, variables demonstrating a P value < 0.25 were subsequently incorporated into the logistic regression model using multivariate analysis. Independent variables on echocardiographic parameters for HFimpEF were re-evaluated using the receiver operating characteristic (ROC) to find cutoff values, along with their sensitivity and specificity in predicting improvement from HFrEF to HFimpEF. Survival from HF rehospitalization and all-cause mortality were evaluated using the Kaplan-Meier method, and the log-rank test was used to make comparisons. All data were analyzed using the SPSS 22 (IBM, Armonk NY) statistical package, and a P value of < 0.05 was considered statistically significant. The study was conducted in accordance with the International Conference on Harmonization—Good Clinical Practice (ICH-GCP). Ethical clearance (1469/UN14.2.2.VII.14/LT/2023) was received, with protocol number 2023.02.1.0713, from the Udayana Research Ethics Commission Unit.
Results
A total of 176 HFrEF patients were included in this study. In the follow-up, 96 patients were identified as having HFimpEF and 80 patients were classified as having non-recovered HFrEF (control group).
The majority of the sample in our study was male (65.4%), with more male patients in the HFimpEF group, although the difference was not significant (70.8% vs 60%; Table 1). In terms of age, the groups had no significant difference (HFimpEF, mean age of 51.15 years vs HFrEF non-recovered, mean age of 54.62 years, P = 0.218). However, non-ischemic etiology was observed to be significantly more prevalent in those with HFimpEF compared to those with non-recovered HFrEF (58.3% vs 35.0%; P = 0.029). No significant differences were present in the other parameters, including comorbidities, medications, and vital signs, at the time of the initial echocardiographic measurements, with all P values > 0.05 (Table 1).
Table 1.
Baseline characteristics of heart failure with improved ejection fraction (HFimpEF) and non-recovered heart failure with reduced ejection fraction (non-recovered HFrEF)
| Characteristic | HFrEF non-recovered (n = 80) | HFimpEF (n = 96) | P |
|---|---|---|---|
| Age, y | 54.62 ± 15.09 | 51.15 ± 14.23 | 0.218 |
| Sex | 0.286 | ||
| Male | 48 (60) | 68 (70.8) | |
| Female | 32 (40) | 28 (29.2) | |
| BMI | 0.544 | ||
| Underweight | 6 (3.4) | 2 (1.1) | |
| Normal | 28 (15.9) | 28 (15.9) | |
| Pre-obese | 14 (8.0) | 18 (10.2) | |
| Obese | 48 (27.3) | 32 (18.2) | |
| Obese 2 | 2 (5) | 1 (2.1) | |
| Smoking | 24 (13.6) | 12 (10.2) | 0.218 |
| Etiology of HFrEF | 0.029∗ | ||
| Ischemic etiology | 52 (65.0) | 40 (41.7) | |
| Non-ischemic etiology | 28 (35.0) | 56 (58.3) | |
| Diabetes mellitus | 26 (32.5) | 24 (25.0) | 0.437 |
| Hypertension | 42 (52.5) | 36 (37.5) | 0.158 |
| Atrial fibrillation | 28 (35.0) | 32 (33.3) | 0.870 |
| Rheumatic heart disease | 6 (7.5) | 6 (6.2) | 0.815 |
| Dyslipidemia | 26 (32.5) | 21 (21.8) | 0.338 |
| Cerebrovascular event | 3 (3.7) | 2 (2.1) | 0.875 |
| Glomerular filtration rate, mL/min | 0.817 | ||
| > 60 | 50 (62.5) | 70 (72.9) | |
| 30–60 | 16 (20) | 16 (16.6) | |
| < 30 | 14 (17.5) | 10 (10.4) | |
| Heart rate, bpm | 81.50 ± 34.25 | 75.25 ± 12.22 | 0.156 |
| Respiration rate, breaths/min | 15.21 ± 3.04 | 15.50 ± 2.22 | 0.845 |
| SBP, mm Hg | 128.15 ± 36.47 | 125.81 ± 25.58 | 0.863 |
| DBP, mm Hg | 91.88 ± 12.22 | 88.02 ± 10.53 | 0.772 |
| Temperature, oC | 36.58 ± 0.62 | 36.79 ± 0.88 | 0.891 |
| SpO2, % | 97.50 ± 1.15 | 98.05 ± 1.00 | 0.805 |
| Beta-blocker | 73 (91.25) | 90 (93.75) | 0.925 |
| ACEi | 68 (85.0) | 76 (79.2) | 0.480 |
| MRA | 50 (62.5) | 70 (72.9) | 0.296 |
| Diuretic | 70 (87.5) | 82 (85.4) | 0.777 |
| Digoxin | 26 (32.5) | 28 (29.1) | 0.885 |
| P2Y12 inhibitor | 52 (65.0) | 40 (41.7) | 0.029∗ |
| Nitrate | 18 (22.5) | 14 (14.5) | 0.232 |
| Anticoagulant | 28 (35.0) | 32 (33.3) | 0.870 |
| Others | 60 (75.0) | 68 (70.8) | 0.662 |
| Revascularization during period of inclusion to 2nd echocardiography | 10 (12.5) | 7 (7.2) | 0.155 |
Values are mean (± standard deviation) or n (%), unless otherwise indicated.
ACEi, angiotensin-converting enzyme inhibitor; BMI, body mass index; bpm, beats per minure; DBP, diastolic blood pressure; MRA, mineralocorticoid receptor antagonist; P2Y12, purinergic receptor P2Y G protein-coupled 12; RAAS, renin angiotensin aldosterone system; SBP, systolic blood pressure; SpO2, oxygen saturation.
Significance.
The median follow-up time between the initial echocardiographic measurement and the second measurement was 5 months (range: 4-8 months) in the HFimpEF group, and 5 months (range: 5-7 months) in the control group. In the HFimpEF group, the mean increase in LVEF was 15.04%, which differed significantly (P < 0.05) between the LVEF as measured at baseline vs post-follow-up. In contrast, the non-recovered HFrEF group displayed a relatively modest average increase of 1.17% in LVEF, which was not statistically significantly different relative to the baseline value (P = 0.310; Table 2). For baseline echocardiographic parameters, no significant difference was present in the mean LVEF, the LV internal diameter end systole (LVIDs), or the LV internal diameter end diastole (LVIDd). Patients with HFimpEF had a significantly lower baseline left atrial volume index (LAVi; 39.08 ± 18.32 mL/m2 vs 49.54 ± 15.96 mL/m2; P < 0.001), compared to that for patients with non-recovered HFrEF (Table 3). Relative wall thickness (RWT) did not differ significantly between the 2 groups at baseline, but both groups showed a significant increase after follow-up, with the mean improvement in the HFimpEF group being higher (pre, 0.31 vs post, 0.38; P < 0.001) than that in the nonrecovered HFrEF group (pre, 0.31 vs post, 0.33; P = 0.019; Tables 2 and 3).
Table 2.
Paired sample statistical analysis of heart failure with improved ejection fraction (HFimpEF) and non-recovered heart failure with reduced ejection fraction (non-recovered HFrEF) at initial and post–follow-up measurement
| Parameter | Non-recovered HFrEF (n = 80) |
P | HFimpEF (n = 96) |
P | ||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||
| LVEF | 32.88 ± 5.03 | 34.05 ± 9.5 | 0.310 | 33.57 ± 4.50 | 48.61 ± 4.93 | < 0.001∗ |
| Ao Diam | 2.01±0.18 | 1.98 ± 0.21 | 0.206 | 2.01 ± 0.35 | 1.99 ± 0.36 | 0.862 |
| LA Diam | 3.77 ± 0.55 | 3.74 ± 0.54 | 0.110 | 4.02 ± 0.91 | 3.99 ± 0.92 | 0.227 |
| La/Ao | 1.86 ± 0.38 | 1.90 ± 0.33 | 0.739 | 2.09 ± 0.72 | 2.10 ± 0.82 | 0.507 |
| FS | 23.01 ± 8.93 | 22.04 ± 7.98 | 0.064 | 25.02 ± 6.99 | 26.31 ± 6.46 | < 0.001∗ |
| IVSd | 1.80 ± 3.14 | 1.82 ± 3.12 | 0.214 | 1.04 ± 0.44 | 1.07 ± 0.55 | 0.186 |
| IVSs | 1.58 ± 0.58 | 1.56 ± 0.38 | 0.343 | 1.67 ± 0.46 | 1.94 ± 0.48 | < 0.001∗ |
| LVIDd | 6.04 ± 0.68 | 5.38 ± 0.75 | 0.293 | 5.32 ± 0.66 | 4.74 ± 0.62 | < 0.001∗ |
| LVIDs | 3.41 ± 0.87 | 3.49 ± 0.93 | 0.047∗ | 3.22 ± 0.69 | 2.81 ± 0.56 | < 0.001∗ |
| LVPWd | 1.22 ± 1.24 | 1.27 ± 1.26 | 0.225 | 1.02 ± 0.32 | 1.07 ± 0.36 | 0.080 |
| LVPWs | 1.56 ± 0.32 | 1.58 ± 0.34 | 0.465 | 1.68 ± 0.44 | 1.74 ± 0.48 | 0.001∗ |
| RWT | 0.31 ± 0.02 | 0.33 ± 0.58 | 0.019∗ | 0.31 ± 0.33 | 0.38 ± 0.27 | < 0.001∗ |
| TAPSE | 13.5 ± 4.80 | 13.9 ± 5.00 | 0.056 | 19.2 ± 4.30 | 20.8 ± 4.20 | < 0.001∗ |
| RV S' | 10.20 ± 2.17 | 10.31 ± 2.36 | 0.423 | 10.72 ± 3.36 | 11.52 ± 3.21 | < 0.001∗ |
| LAVi | 49.54 ± 15.96 | 45.19 ± 15.88 | < 0.001∗ | 39.08 ± 18.32 | 29.45 ± 11.26 | < 0.001∗ |
| Mitral E/A | 1.59 ± 0.80 | 1.56 ± 0.72 | 0.639 | 1.62 ± 0.78 | 1.34 ± 0.68 | < 0.001∗ |
| E/e’ (septal) | 12.78 ± 6.71 | 13.51 ± 7.52 | 0.025∗ | 13.92 ± 7.38 | 12.22 ± 5.19 | 0.001∗ |
| IVC diam | 12.0 ± 2.90 | 12.6 ± 29.2 | < 0.001∗ | 18.7 ± 0.30 | 16.9 ± 0.28 | < 0.001∗ |
Ao, aortic root; d, end diastole; diam, diameter; FS, fractional shortening; IVC, inferior vena cava; IVS, interventricular septal; LA, left atrial; LAVi, left atrial volume index; LV, left ventricular; LVEF, LV ejection fraction; LVID, LV internal diameter; LVPW, LV posterior wall; RV S′, systolic excursion velocity; RWT, relative wall thickness; s, end systole; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Significance.
Table 3.
Baseline echocardiographic parameters of heart failure with improved ejection fraction (HFimpEF) and non-recovered heart failure with reduced ejection fraction (non-recovered HFrEF)
| Parameter | Non-recovered HFrEF (n = 80) |
HFimpEF (n = 96) |
P | ||
|---|---|---|---|---|---|
| Mean ± SD | Min, max | Mean ± SD | Min, max | ||
| LVEF | 32.88 ± 5.03 | (22.00, 39.20) | 33.57 ± 4.50 | (22.00, 39.20) | 0.540 |
| Ao diam | 2.01 ± 0.18 | (1.70, 2.70) | 2.01 ± 0.35 | (1.70, 2.70) | 0.892 |
| LA diam | 3.77 ± 0.55 | (2.90, 4.80) | 4.02 ± 0.91 | (2.10, 6.10) | 0.154 |
| La/Ao | 1.86 ± 0.38 | (0.60, 2.47) | 2.09 ± 0.72 | (1.10, 3.83) | 0.296 |
| FS | 23.01 ± 8.93 | (12.00, 49.00) | 25.02 ± 6.99 | (12.50, 48.95) | 0.059 |
| IVSd | 1.80 ± 3.14 | (0.60, 2.01) | 1.04 ± 0.44 | (0.30, 2.01) | 0.223 |
| IVSs | 1.58 ± 0.58 | (0.30, 2.40) | 1.67 ± 0.46 | (0.50,2.40) | 0.705 |
| LVIDd | 5.32 ± 0.66 | (3.54,6.90) | 5.04 ± 0.68 | (3.47, 6.20) | 0.085 |
| LVIDs | 3.41 ± 0.87 | (2.01, 5.70) | 3.22 ± 0.69 | (2.01, 4.43) | 0.378 |
| LVPWd | 1.22 ± 1.24 | (0.43, 2.01) | 1.02 ± 0.32 | (0.63, 2.01) | 0.332 |
| LVPWs | 1.56 ± 0.32 | (1.00, 2.10) | 1.68 ± 0.44 | (1.00, 2.62) | 0.269 |
| RWT | 0.31 ± 0.02 | (0.28, 0.40) | 0.31 ± 0.33 | (0.26, 0.48) | 0.990 |
| TAPSE | 13.5 ± 4.80 | (10.0, 28.0) | 19.2 ± 4.30 | (10.3, 28.0) | < 0.001∗ |
| RV S′ | 10.20 ± 2.17 | (5.51, 15.00) | 10.72 ± 3.36 | (6.09, 22.00) | 0.706 |
| LAVi | 49.54 ± 15.96 | (25.60,102.0) | 39.08 ± 18.32 | (21.60, 140.7) | < 0.001∗ |
| Mitral E/A | 1.59 ± 0.80 | (0.30, 3.10) | 1.62 ± 0.78 | (0.30, 4.19) | 0.993 |
| E/e′ (septal) | 12.78 ± 6.71 | (2.16, 33.00) | 13.92 ± 7.38 | (5.00, 33.00) | 0.721 |
| IVC diam | 12.0 ± 29.0 | (8.23, 21.72) | 18.71 ± 3.02 | (8.22, 24.77) | < 0.001∗ |
Ao, aortic root; d, end diastole; diam, diameter; FS, fractional shortening; IVC, inferior vena cava;; IVS, interventricular septal; LA, left atrial; LAVi, left atrial volume index; LV, left ventricular; LVEF, LV ejection fraction; LVID, LV internal diameter; LVPW, LV posterior wall; max, maximum; min, minimum; RV S′, systolic excursion velocity; RWT, relative wall thickness; s, end systole; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Significance.
Baseline LV diastolic parameters, such as the ratios E/A and E/e', did not differ significantly between the 2 groups. Despite the inferior vena cava (IVC) diameter being within the normal range, a significantly higher value was observed at baseline in the HFimpEF group, compared to that in the non-recovered HFrEF group (18.7 mm vs 12.0 mm). Right ventricle (RV) systolic function was assessed by tricuspid annular plane systolic excursion (TAPSE) and was found to be significantly higher in the HFimpEF group (19.2 mm vs 13.5 mm; P < 0.001; Table 3). Patients with HFimpEF demonstrated a significant improvement in LV contractile function, as evidenced by a substantial increase of 15.04% in LVEF (pre, 33.57% vs post, 48.61%; P < 0.001). Additionally, a notable enhancement occurred in RV function, reflected by a significant increase in TAPSE of 1.6 mm (pre, 19.2 mm vs post, 20.8 mm; P < 0.001), compared to the baseline measurement (Table 2). Improved cardiac chamber geometry in patients with HFimpEF was evidenced by a reduction of the left atrial volume index (LAVI) value of 9.63 mL/m2 (pre, 39.08 mL/m2 vs post, 29.45 mL/m2; P < 0.001) on measurements at follow-up, compared to baseline values (Table 2). LV diastolic parameters such as the E/A and E/e' ratios also showed significant improvement at follow-up measurements in the population with HFimpEF.
Patients with HFimpEF had a significant reduction of the IVC value of 1.8 mm (pre, 18.7 mm vs post, 16.9 mm; P = 0.001; Table 4). No significant LVEF increase occurred in the non-recovered HFrEF group, but a reduction in mean LAVI occurred, of 4.35 mL/m2 (pre, 49.54 mL/m2 vs post, 45.19 mL/m2; P < 0.001). Other measures, such as LVIDs, E/e' septal, and IVC diameter, were increased in HFrEF patients after follow-up (Table 2).
Table 4.
Regression analysis of potential prognostic factors of heart failure with improved ejection fraction
| Factor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Younger age | < 0.001 | 1.042 (1.018–1.066) | < 0.001 | 1.849 (1.786–1.914) |
| Female sex | 0.012 | 2.219 (1.386–3.552) | 0.088 | 3.859 (0.818–18.205) |
| Nonischemic etiology | 0.045 | 2.974 (1.022–8.653) | 0.481 | 1.727 (0.378–7.891) |
| Higher TAPSE | 0.008 | 2.816 (1.298–6.109) | < 0.001 | 3.071 (1.742–5.413) |
| Lower LAVi | < 0.001 | 2.212 (1.409–3.472) | 0.006 | 2.034 (1.218–3.396) |
| Higher IVC | 0.231 | 3.113 (0.487–19.898) | NI | |
| Smoking | 0.581 | 1.259 (0.555–2.856) | NI | |
| Hypertension | 0.106 | 2.567 (0.817–8.065) | NI | |
| Heart rate | 0.756 | 1.235 (0.326–4.678) | NI | |
| P2Y12 inhibitor | 0.217 | 3.494 (0.478–25.539) | NI | |
| Nitrate | 0.268 | 0.123 (0.003–5.043) | NI | |
| Revascularization | 0.093 | 0.525 (0.247–1.115) | NI | |
| LA diameter | 0.121 | 2.816 (0.761–10.421) | NI | |
| FS | 0.495 | 1.252 (0.656–2.389) | NI | |
| IVSd | 0.208 | 1.568 (0.778–3.161) | NI | |
| LVIDd | 0.071 | 2.521 (0.881–7.213) | NI | |
CI, confidence interval; d, end diastole; FS, fractional shortening; IVC, inferior vena cava; IVS, interventricular septal; LA, left atrial; LVID,left ventricular internal diameter; LAVi, left atrial volume index; NI, not included; OR, odds ratio; P2Y12, purinergic receptor P2Y G protein-coupled 12; TAPSE, tricuspid annular plane systolic excursion.
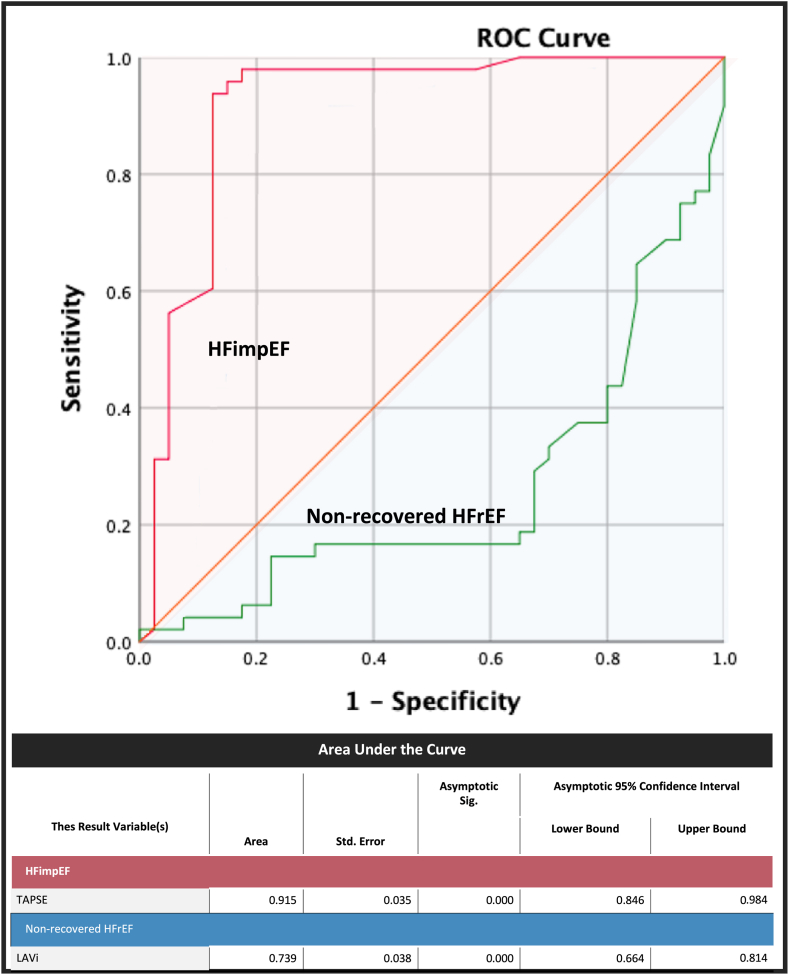
The baseline characteristics with a P value < 0.25 on univariate analysis were then tested with a multivariate analysis on Cox regression (Table 4). Female sex and non-ischemic etiology of HFrEF were significant in the univariate analysis for HFimpEF (P = 0.012 and P = 0.045, respectively), but in multivariate analysis, younger age was associated with HFimpEF (odds ratio [OR] 1.849; 95% confidence interval [CI] 1.786-1.914; P < 0.001). Echocardiographic parameters of TAPSE (OR 3.071; 95% CI 1.742-6.752; P < 0.001) and LAVi (OR 2.034; 95% CI 1.218-3.217; P = 0.006) were independently associated with the development of HFimpEF on multivariate analysis and were then tested further through ROC analysis to obtain the optimal cutoff value (Fig. 1). Baseline LAVi demonstrated a negative predictive value for the development of HFimpEF and was found to be a predictor of non-recovered HFrEF (P = 0.001). The area under the curve was calculated to be 0.739, with an optimal cutoff value of > 37.3 mL/m2 (80% sensitivity and 62.5% specificity). Moreover, baseline TAPSE emerged as a significant predictor for the development of HFimpEF (P = 0.001), with an area under the curve of 0.915 and an optimal cutoff value of ≥ 15 mm (91.7% sensitivity and 87.5% specificity).
Figure 1.
Receiver operating characteristics (ROC) analysis for predicting heart failure with improved ejection fraction (HFimpEF) or non-recovered heart failure with reduced ejection fraction (non-recovered HFrEF). LAVi, left atrial volume index; Sig, significance; Std., standard; TAPSE, tricuspid annular plane systolic excursion.
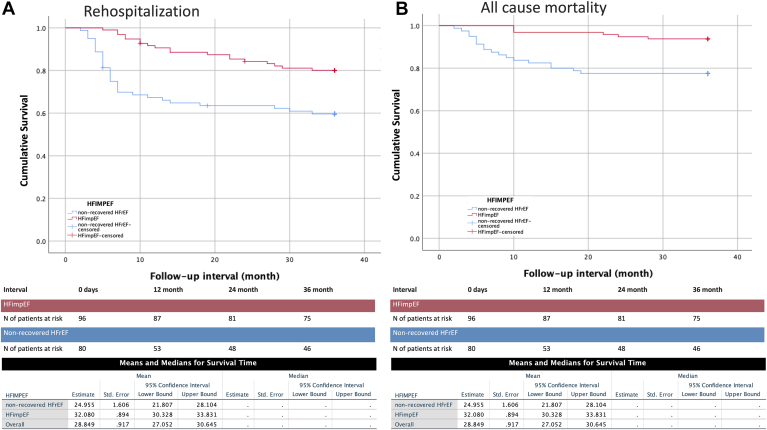
After a mean of 32.5 ± 9.1 months, 32.18% of patients experienced at least 1 rehospitalization due to worsening HF, and a total of 13.6% of patients died (Table 5). No patients were lost to follow-up. In the Kaplan-Meier analysis, the HFimpEF group had better survival from all-cause mortality and rehospitalization due to worsening HF, compared to those with nonrecovered HFrEF (log-rank P < 0.001 and P = 0.005, respectively; Fig. 2).
Table 5.
Rehospitalization and all-cause mortality of heart failure with improved ejection fraction (HFimpEF) and non-recovered heart failure with reduced ejection fraction (non-recovered HFrEF) after follow-up
| Rehospitalization and all-cause mortality outcomes | Non-recoverd HFrEF (n = 80) | HFimpEF (n = 96) | P |
|---|---|---|---|
| Rehospitalization for HF | 34 (42.5) | 21 (21.86) | 0.004∗ |
| Aborted cardiac arrest | 4 (5) | 3 (3.13) | 0.529 |
| Revascularizations of CAD | 7 (8.75) | 10 (10.41) | 0.709 |
| CRT implant | 4 (5) | 2 (2.08) | 0.303 |
| ICD implant | 3 (3.75) | 2 (2.08) | 0.513 |
| Death | 24 (13.6) | ||
| Progressive HF | 10 (12.5) | 3 (3.125) | 0.028∗ |
| VAs | 3 (3.75) | 1 (1.04) | 0.261 |
| Sudden death at home | 3 (3.75) | 1 (1.04) | 0.261 |
| COVID-19 infection | 2 (2.5) | 0 | 0.243 |
| Ischemic stroke | 0 | 1 (1.04) | 0.571 |
Values are n (%), unless otherwise indicated.
CAD, coronary artery disease; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; HF, heart failure; VA, ventricular arrhythmia.
Significance.
Figure 2.
Kaplan-Meier curves for survival of (A) those with rehospitalization due to worsening heart failure (HF) and (B) all-cause mortality compared between those with HF with improved ejection fraction (HFimpEF) and those with non-recovered HF with reduced ejection fraction (non-recovered HFrEF). N, number; Std., standard.
Discussion
In this cohort study of 176 patients with HFrEF, the overall mean LVEF was increased by 8.1% from baseline during the second echocardiogram assessment at follow-up. Our study revealed numerous important findings, including the following: (i) young age was an independent demographic variable associated with HFimpEF; (ii) echocardiographic parameters including TAPSE and LAVi were predictors of LVEF recovery, with cutoffs of ≥ 15 mm and < 37.3 mL/m2, respectively; and (iii) the HFimpEF group had reduced HF rehospitalization and all-cause mortality, compared to the non-recovered HFrEF group over a 3-year follow-up period.
In our study, approximately 55% of the population had an LVEF increase of more than 10 points and more than 40% on the second echocardiogram. Compared to large cohort studies that used the same HFimpEF criteria we did, this proportion was comparatively higher. Previous cohort studies already were utilizing the most current and recommended medications, such as angiotensin receptor-neprilysin inhibitors and sodium-glucose cotransporter-2 inhibitors. However, an important point to mention is that these specific medications were not observed within our research cohort. This discrepancy could be due to the rigorous inclusion criteria we implemented during the participant selection process, which were designed to mitigate bias. Consequently, this approach also influenced the composition of the HFrEF control groups; so it would seem that the proportions between the study and control populations relatively similar. Additionally, baseline echocardiography differences between the HFimpEF and the non-recovered HFrEF groups were not reported in this prospective cohort study. A point to note is that the outcome of HFimpEF in these 3 previous cohort studies was a secondary outcome of the effectiveness of angiotensin receptor-neprilysin inhibitors and sodium-glucose cotransporter-2 inhibitors; therefore, the reason for the different proportion, compared with that in the present study, is difficult to determine.10, 11, 12 Compared with a retrospective study that correlated echocardiographic characteristics among patients who experienced LVEF improvement, the proportion of patients experiencing an increase in LVEF 10 points was relatively similar to that in our study. However, these studies did not use the latest HFimpEF criteria based on classification of HF.13,14
Baseline clinical characteristics remain controversial and unclear as reliable predictors of LVEF recovery. The Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) study,13 which included 3994 HF patients, concluded the opposite result of our study findings. They found that age was not associated with an increase in ejection fraction, but the duration of their follow-up in determining LVEF increase was 24 months. In a distinct prospective study conducted by Park et al.,14 noteworthy associations were also identified between various factors and the recovery of ejection fraction. Specifically, younger age and female gender, coupled with de novo heart failure, hypertension, and atrial fibrillation, exhibited a positive predictive influence on the restoration of ejection fraction. Conversely, the presence of ischemic heart disease and diabetes mellitus emerged as predictors of adverse outcomes, highlighting their negative impact on the likelihood of ejection fraction recovery.14
The study by Park et al.14 bears resemblance to our investigation in regard to the study population, as both encompass a significant proportion of Asian individuals and have comparatively shorter durations of echocardiographic follow-up. However, disparities between their findings and ours may be attributed to several factors. First and foremost, our study targeted exclusively those individuals who exhibited initial episodes of HFrEF. By doing so, we aimed to eliminate potential confounding factors that could impact ejection fraction, such as the use of medications that have the potential to improve cardiac function, prior to the onset of HF. The observed significant difference in beta-blocker (BB) utilization as a predictor of HFimpEF can be attributed to the comparable proportions of BB usage between the 2 groups in our study, which were not statistically significantly different. Furthermore, the divergent significance of comorbidities can be attributed to our study's classification, which dichotomized patients into those with ischemic vs nonischemic etiologies. A point worth noting is that certain studies also have explored the role of genetic factors, which were not explored specifically in our study.15,16 Additionally, other investigations have highlighted the association between medication use and adherence with improvements in ejection fraction; however, our study did not observe significant differences in these aspects between the 2 groups.14,17
Echocardiography, a crucial diagnostic tool, assesses ventricular dysfunction and prognosis in HF.18 LVEF is the primary predictor for HF severity, prognosis, and classification.19, 20, 21 Given the prognostic implications of LVEF in HF patients, our study aimed to compare echocardiographic parameters between the group with non-recovered HFrEF vs the group with HFimpEF. Our study revealed that no significant differences were present in the initial LVEF between the non-recovered HFrEF and the HFimpEF groups (33.57% vs 32.88%, respectively), and the mean LVEF recovery value was greater in the HFimpEF group, with an increase of 15.04% vs 1.17% in the non-recovered HFrEF group, which conflicts with the findings of the IMPROVE HF study.14 However, a point to note is that the HFrEF classification used in that study did not adhere to the < 40% threshold. Additionally, the criteria for HFimpEF used in our investigation are relatively novel, and no previous study has classified patients with HFimpEF according to our new classification. The findings of echocardiographic parameters being predictors of HFimpEF in our study were similar to the results of a study conducted by Shah et al., who reported that LAVi and TAPSE are independent echocardiographic variables in LVEF recovery, together with LVIDd.22 The nonsignificance of LVIDd in our study was not surprising because the 2D linear measurement was not an accurate representation of the actual size of the left ventricle, owing to several assumptions, which were confirmed by the retrospective study by Moon et al. and the subanalysis of the IMPROVE HF study by Wilcox et al.13,23 Nonetheless, the HFimpEF group in our study showed significant improvement in both the LVIDd and LVIDs parameters at the second measurement. These enhancements were substantiated by favourable alterations in RWT. LV RWT is a marker of remodelling that various studies have investigated,24,25 demonstrating that reverse remodelling occurs in those with HFimpEF and improves the LV architecture.
Another parameter—LAVi—also was reported to have a role in predicting LV function improvement with high specificity and as a prognostic factor in HF patients.13,26,27 During the multivariate analysis and the ROC analysis, we discovered a robust link between higher left atrial volume and the likelihood of not recovering in the HFrEF population. The ideal threshold of ≥ 37.3 mL/m2 was associated with 80% sensitivity and 62.5% specificity. However, the group with non-recovered HFrEF also showed significant improvement from LAVi, although it was still at an abnormal value (baseline, 49.54 mL/m2 vs post, 45.19 mL/m2; P < 0.001). A point worth mentioning is that the categorization of LAVi as mild (29-33 mL/m2), moderate (34-39 mL/m2), or severe (≥ 40 mL/m2) provided a standardized approach for assessing the severity of left atrial enlargement.28 This improved value of LAVi may be due to our HF population undergoing optimal HF therapy with better volume status at follow-up.
Another echocardiographic parameter that can predict long-term outcomes in HF patients is RV systolic dysfunction.29 Gando et al.25 suggested that biventricular involvement is more common in non-ischemic cardiomyopathy than in ischemic etiology, resulting in a higher degree of RV dilatation and dysfunction. Consistent with this theory, our research revealed that patients diagnosed with HFimpEF exhibited a slightly elevated IVC diameter, although it was still within the normal range. This observation may serve as a potential indicator of improved LV function. However, the difference in IVC diameter, which describes the right atrial pressure, was not significant in subsquent multivariate analysis. This lack of difference can be attributed to the dynamic nature of the IVC, which limits its ability to accurately describe the anatomic and functional characteristics of the right ventricle. TAPSE, a marker of RV function, also is known to be used for risk stratification in HFrEF.26 Multivariate analysis identified that a higher TAPSE value was linked with better RV systolic function and was independently associated with LV function recovery.
Our study revealed compelling evidence indicating that patients with HFimpEF exhibit a notably improved prognosis in terms of survival and rehospitalization due to worsening HF, compared to individuals with nonrecovered HFrEF. Despite the continued presence of clinical manifestations associated with HF, such as signs and symptoms, patients with HFimpEF experienced more favourable outcomes. Numerous theories have been proposed to explain this favourable prognosis for patients with HFimpEF. One such hypothesis is that reverse remodelling occurs, accompanied by a more favourable neurohormonal profile.26 Anatomic and neurohormonal reverse remodelling are widely recognized as being able to enhance the physiological function of the heart, optimizing blood flow throughout the body. Therefore, these mechanisms may contribute to the improved outcomes observed in patients with HFimpEF. Additionally, our cohort of patients with HFimpEF exhibited a lower prevalence of coronary artery disease, aligning with previous investigations conducted in this field.7,13 This observation suggests that the etiology of HFimpEF may differ from that of nonrecovered HFrEF, in which coronary artery disease often plays a more prominent role. The difference in the prevalence of coronary artery disease between these 2 groups may contribute to the contrasting clinical characteristics and outcomes observed in our study.
Coronary artery disease as a comorbidity can potentially influence rehospitalization rates and mortality in patients with nonrecovered HFrEF, introducing a potential bias in assessing outcomes. The findings of the IMPROVE HF study aligned with those in our own research. Specifically, they found that the absence of previous myocardial infarction and a nonischemic etiology of HF were associated with a greater increase, of 10%, in LVEF.13 The higher response to guideline-directed medical therapy observed in the HFimpEF population was likely caused by better structural conditions early in the course of the disease and a greater number of viable myocardial cells. Additionally, genetic variability may have played a role in the differential treatment response between patients with HFimpEF and those with non-recovered HFrEF.30 Such a role has already been proven in HF patients who receive BB,31,32 renin-angiotensin-aldosterone axis inhibitor,33,34 and cardiac resynchronization therapy.35,36
Despite the comprehensive nature of our study, acknowledgement of certain limitations is important. One of the limitations of our study is the inability to explain the onset of chronic HF and remodelling, as the patients included in the study were admitted at their first occurrence of HFrEF. Consequently, a potential bias related to the duration of HF cannot be completely ruled out. Additionally, brain natriuretic peptide/pro b-type natriuretic peptide (BNP/pro-BNP) levels, known to be important markers related to the severity and prognosis of HF patients, were not recorded, because not all patients had their levels checked routinely. Also, our study lacks longitudinal data on HF pharmacologic therapies and rather has only available baseline data, which affects the echocardiography parameters. Lastly, new echocardiographic techniques such as tissue strain analysis and 3D-echocardiography may also be useful in predicting the likelihood of recovery in patients with HFrEF, but these parameters are not measured routinely in our centre.
Conclusions
Baseline echocardiographic parameters, specifically TAPSE and LAVi, have demonstrated significant associations with the transition from HFrEF to HFimpEF. Additionally, the HFimpEF group exhibited superior outcomes in terms of rehospitalization rates and all-cause mortality, compared to the non-recovered HFrEF group. These findings underscore the value of these parameters as possible prognostic indicators and predictors of disease progression in HF patients.
Acknowledgments
Ethics Statement
Ethical clearance (1469/UN14.2.2.VII.14/LT/2023) was received, with protocol number 2023.02.1.0713, from the Udayana Research Ethics Commission Unit.
Patient Consent
This is a retrospective study using de-identified data; therefore, the IRB did not require consent from the patient.
Funding Sources
All authors have no funding sources to declare.
Disclosures
All authors have no conflict of interest to disclose.
Footnotes
See page 868 for disclosure information.
References
- 1.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W., Januzzi J.L., Jr., Allen L.A., et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–230. doi: 10.1016/j.jacc.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt B., Coats A.J.S., Tsutsui H., et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 4.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui H., Isobe M., Ito H., et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure—digest version. Circ J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 7.Basuray A., French B., Ky B., et al. Heart failure with recovered ejection fraction clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. doi: 10.1161/CIRCULATIONAHA.113.006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati G., Udelson J.E. Heart failure with improved ejection fraction: Is it possible to escape one’s past? JACC Heart Fail. 2018;6:725–733. doi: 10.1016/j.jchf.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Lang R.M., Badano L.P., Victor M.A., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Vardeny O., Fang J.C., Desai A.S., et al. Dapagliflozin in heart failure with improved ejection fraction: a prespecified analysis of the DELIVER trial. Nat Med. 2022;28:2504–2511. doi: 10.1038/s41591-022-02102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong M.G., Suh J., Moon I.K., et al. Heart failure with improved ejection fraction (HFimpEF) in patients treated with sacubitril/valsartan (SV) Eur Heart J. 2022;43(suppl 2) [Google Scholar]
- 12.Alsindi F., Manla Y., Bader F. Patient characteristics of heart failure with improved ejection fraction (HFimpEF) following treatment with sacubitril/valsartan: a real-world experience from The Middle East. J Card Fail. 2022;28:S85. [Google Scholar]
- 13.Wilcox J.E., Fonarow G.C., Yancy C.W., et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J. 2012;163:49–56.e2. doi: 10.1016/j.ahj.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Park C.S., Park J.J., Mebazaa A., et al. Characteristics, outcomes, and treatment of heart failure with improved ejection fraction. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware J.S., Li J., Mazaika E., et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374:233–241. doi: 10.1056/NEJMoa1505517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicoira M., Rigolli M., Bergamini C., et al. Progression of left ventricular dysfunction and remodelling under optimal medical therapy in CHF patients: role of individual genetic background. Cardiol Res Pract. 2011;2011 doi: 10.4061/2011/798658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florea V.G., Rector T.S., Anand I.S., Cohn J.N. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the Valsartan Heart Failure Trial. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003123. [DOI] [PubMed] [Google Scholar]
- 18.Marwick T.H. The role of echocardiography in heart failure. J Nucl Med. 2015:31S–38S. doi: 10.2967/jnumed.114.150433. [DOI] [PubMed] [Google Scholar]
- 19.Damy T., Viallet C., Lairez O., et al. Comparison of four right ventricular systolic echocardiographic parameters to predict adverse outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:818–824. doi: 10.1093/eurjhf/hfp111. [DOI] [PubMed] [Google Scholar]
- 20.Nauta J.F., Hummel Y.M., van der Meer P., et al. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20:1303–1311. doi: 10.1002/ejhf.1220. [DOI] [PubMed] [Google Scholar]
- 21.Ciampi Q., Villari B. Role of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunction. Cardiovasc Ultrasound. 2007;5:34. doi: 10.1186/1476-7120-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah M.A., Soofi M.A., Jafary Z., et al. Echocardiographic parameters associated with recovery in heart failure with reduced ejection fraction. Echocardiography. 2020;37:1574–1582. doi: 10.1111/echo.14859. [DOI] [PubMed] [Google Scholar]
- 23.Moon J., Shim C.Y., Kim Y.J., et al. Left atrial volume as a predictor of left ventricular functional recovery in patients with dilated cardiomyopathy and absence of delayed enhancement in cardiac magnetic resonance. J Card Fail. 2016;22:265–271. doi: 10.1016/j.cardfail.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Wong M., Staszewsky L., Latini R., et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT Echocardiographic Study. J Am Coll Cardiol. 2002;40:970–975. doi: 10.1016/s0735-1097(02)02063-6. [DOI] [PubMed] [Google Scholar]
- 25.Gando Y., Kawano H., Yamamoto K., et al. Age and cardiorespiratory fitness are associated with arterial stiffening and left ventricular remodelling. J Hum Hypertens. 2010;24:197–206. doi: 10.1038/jhh.2009.57. [DOI] [PubMed] [Google Scholar]
- 26.Katsiki N., Mikhailidis D., Papanas N. Left atrial volume: an independent predictor of cardiovascular outcomes. Int J Cardiol. 2018;265:234–235. doi: 10.1016/j.ijcard.2018.04.121. [DOI] [PubMed] [Google Scholar]
- 27.Norton G.R., Peterson V.R., Robinson C., et al. Independent of left ventricular mass, circulating inflammatory markers rather than pressure load are associated with concentric left ventricular remodelling. Int J Cardiol. 2019;274:342–347. doi: 10.1016/j.ijcard.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 28.Lang R.M., Bierig M., Devereux R.B., et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Carluccio E., Biagioli P., Alunni G., et al. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.006894. [DOI] [PubMed] [Google Scholar]
- 30.Kalogeropoulos A.P., Fonarow G.C., Georgiopoulou V., et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. doi: 10.1001/jamacardio.2016.1325. [DOI] [PubMed] [Google Scholar]
- 31.Lobmeyer M.T., Gong Y., Terra S.G., et al. Synergistic polymorphisms of beta1 and alpha2C-adrenergic receptors and the influence on left ventricular ejection fraction response to beta-blocker therapy in heart failure. Pharmacogenet Genomics. 2007;17:277–282. doi: 10.1097/FPC.0b013e3280105245. [DOI] [PubMed] [Google Scholar]
- 32.Ramahi T.M., Longo M.D., Cadariu A.R., et al. Left ventricular inotropic reserve and right ventricular function predict increase of left ventricular ejection fraction after beta-blocker therapy in nonischemic cardiomyopathy. 2001;37:818–824. doi: 10.1016/s0735-1097(00)01162-1. [DOI] [PubMed] [Google Scholar]
- 33.Levine T.B., Levine A.B., Bolenbaugh J., Stomel R.J. Impact of left ventricular size on pharmacologic reverse remodeling in heart failure. Clin Cardiol. 2000;23:355–358. doi: 10.1002/clc.4960230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cicoira M., Zanolla L., Rossi A., et al. Failure of aldosterone suppression despite angiotensin-converting enzyme (ace) inhibitor administration in chronic heart failure is associated with ACE DD genotype. J Am Coll Cardiol. 2001;37:1808–1812. doi: 10.1016/s0735-1097(01)01237-2. [DOI] [PubMed] [Google Scholar]
- 35.Kutyifa V., Kloppe A., Zareba W., et al. The influence of left ventricular ejection fraction on the effectiveness of cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2013;61:936–944. doi: 10.1016/j.jacc.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 36.Pezzali N., Curnis A., Specchia C., et al. Adrenergic receptor gene polymorphism and left ventricular reverse remodelling after cardiac resynchronization therapy: preliminary results. Europace. 2013;15:1475–1481. doi: 10.1093/europace/eut136. [DOI] [PubMed] [Google Scholar]