Abstract
目的
探究LncRNA SNHG8调控miR-494-3p表达减轻脑缺血再灌注损伤的发生机制。
方法
构建小鼠脑缺血再灌注损伤模型,通过TTC染色检测梗死面积,ELISA检测脑组织中炎症因子IL-1β、IL-6和TNF-α含量,RT-qPCR检测脑组织中LncRNA SNHG8和miR-494-3p的表达。构建过表达LncRNA SNHG8的OGD/R模型小胶质细胞,细胞分为OGD/R+oe-NC组或OGD/R+oe-SNHG8组,并通过ELISA和流式细胞术检测细胞的炎症反应和凋亡情况。双荧光素酶实验验证LncRNA SNHG8和miR-494-3p的靶向关系。在oe-SNHG8的OGD/R模型小胶质细胞中进一步过表达miR-494-3p,细胞分为OGD/R+oe-SNHG8+mimic NC组或OGD/R+oe-SNHG8+miR-494-3p mimic组,检测细胞炎症反应和凋亡的变化。
结果
小鼠脑缺血再灌注损伤模型中,脑组织出现明显的梗死区,炎症因子IL-1β、IL-6和TNF-α的含量明显增加(P < 0.001),lncRNA SNHG8低表达(P < 0.01),而miR-494-3p高表达(P < 0.01)。过表达LncRNA SNHG8明显抑制OGD/R模型小胶质细胞的炎症反应和凋亡(P < 0.01)。过表达LncRNA SNHG8能抑制miR-494-3p的表达(P < 0.01)。在oe-SNHG8的OGD/R模型小胶质细胞中进一步过表达miR-494-3p,部分促进细胞的炎症反应和凋亡(P < 0.05)。
结论
LncRNA SNHG8通过抑制miR-494-3p表达,抑制炎症反应和细胞凋亡,从而改善脑缺血再灌注损伤。
Keywords: 脑缺血再灌注损伤, LncRNA SNHG8, miR-494-3p, 炎症反应, 细胞凋亡
Abstract
Objective
To explore the mechanism by which LncRNA SNHG8 regulates miR-494-3p expression to alleviate cerebral ischemia-reperfusion injury.
Methods
A mouse model of cerebral ischemia-reperfusion injury was established, and TTC staining was used to determine the infarct area; ELISA was used to detect the contents of the inflammatory factors IL-1β, IL-6 and TNF-α in the brain tissue, and RT-qPCR was performed to detect the expression levels of LncRNA MALAT1 and miR-155-5p. A microglial cell model overexpressing LncRNA SNHG8 was exposed to oxygen-glucose deprivation/reoxygenation (OGD/R), and inflammatory reaction and apoptosis of the cells were detected using ELISA and flow cytometry. A luciferase reporter assay was used to detect the targeting relationship between LncRNA SNHG8 and miR-494-3p. We further constructed a microglial cell model overexpressing both LncRNA SNHG8 the miR-494-3p, and examined inflammatory reactions and apoptosis of the cells following OGD/R exposure.
Results
In the mouse model of cerebral ischemia-reperfusion injury, the contents of inflammatory factors IL-1β, IL-6 and TNF-α increased significantly in the brain tissue (P < 0.001), where LncRNA SNHG8 expression was lowered (P < 0.01) and miR-494-3p expression increased significantly (P < 0.01). In the microglial cells, overexpression of LncRNA SNHG8 significantly inhibited the inflammatory reaction and apoptosis following OGD/R exposure (P < 0.01), and overexpression of LncRNA SNHG8 strongly inhibited the expression of miR-494-3p (P < 0.01). Overexpression of miR-494-3p in microglia overexpressing SNHG8 partially promoted inflammatory reaction and cell apoptosis in response to OGD/R (P < 0.05).
Conclusion
LncRNA SNHG8 can improve cerebral ischemia-reperfusion injury in mice by inhibiting the expression of miR-494-3p and suppressing inflammatory reactions and apoptosis of the microglia.
Keywords: cerebral ischemia-reperfusion injury, LncRNA SNHG8, miR-494-3p, inflammatory reaction, apoptosis
脑卒中是全球范围内导致长期重度残疾和死亡的最常见原因之一[1]。80%~85%的脑卒中病例是由脑缺血引起的,脑缺血通常由大脑大动脉栓塞或血栓栓塞性闭塞所致[2]。介入治疗需要恢复血流,从而导致再灌注损伤。脑缺血再灌注损伤(CIRI)是指随着血流灌注短期恢复,缺血缺氧引起的神经损伤进一步加重的病理过程[3]。越来越多的证据表明,缺血常涉及一系列的神经事件,如缺氧、氧化应激和炎性反应等[4],最终导致缺血脑的急性坏死、凋亡和自噬[5]。目前,组织型纤溶酶原激活剂是治疗CIRI的唯一有效方法[6]。因此,有必要且迫切地为脑卒中患者寻找新的有效治疗靶点,探讨脑缺血的潜在分子机制。
长链非编码RNA(lncRNA)是一种含有200多个碱基的非编码RNA,可调节基因的转录和翻译、表观遗传机制、细胞分化和其他生理/病理活动[7]。许多研究表明,lncRNA还影响神经系统的许多生理和病理过程,它们已被确定为脑卒中的潜在生物标志物[8]。例如,有证据表明,LncRNA SNHG8表达降低与脑缺血的进展密切相关[9]。LncRNA SNHG8不仅通过调节miR-425-5p保护小胶质细胞免受缺血诱导的炎症反应,而且还缓解了体外和体内脑微血管内皮细胞的损伤[10]。有趣的是,lncRNA的功能通常是通过调控microRNA介导的,microRNA通过在转录后与3'UTR结合来调节mRNA表达[11]。例如,LncRNA CASC15通过直接靶向抑制miR-338-3p的表达,然后促进CIRI的细胞凋亡和炎症反应[12]。因此,我们试图进一步了解LncRNA SNHG8在CIRI中的发生机制。
MicroRNA(miRNA)是一类被广泛研究的内源性RNA,它们不被翻译成蛋白质,但在许多细胞过程中起着至关重要的作用,包括正常的生理和异常病理过程[13]。许多miRNA与脑缺血再灌注损伤有关。例如,已知miR-532-3p下调通过靶向NOX2而加重缺血/再灌注损伤[14]。Zuo等[15]发现miR-652可以通过抑制NOX2而保护缺血/再灌注损伤。此外,据报道,miR-494-3p通过抑制BHLHE40的表达加剧CIRI后的脑损伤和神经元损伤[16]。然而,LncRNA SNHG8是否能够介导miR-494-3p表达而缓解脑缺血再灌注损伤,目前尚未见到相关报道。
1. 材料和方法
1.1. 细胞株
人胚肾细胞HEK-293T、小鼠小胶质细胞BV2(武汉普诺赛生命科技有限公司)。
1.2. 实验动物
从湖南斯莱克景达实验动物有限公司[SCXK(湘)2019-0004]购买SPF级的6周龄雄性C57BL/6J小鼠(20~25 g)。将所有小鼠饲养在环境控制的房间中,在12小时的光照/黑暗循环下随意获取食物和水。实验方案经长沙市第一医院医学伦理委员会审核批准后实施(2021伦审【临研】第(61)号)。
1.3. 主要试剂
胎牛血清、MEM培养基(武汉普诺赛生命科技有限公司);Lipofectamine 3000TM转染试剂盒、TRIzol试剂、细胞凋亡检测试剂盒(Thermo Fisher Scientific);异氟醚(上海麦克林生化科技有限公司);6-0尼龙缝合线(Doccol);戊巴比妥钠、TTC溶液(Sigma-Aldrich);4%多聚甲醛、RIPA裂解液(上海碧云天生物技术有限公司);PrimeScriptTMRT试剂盒、SYBR® Premix Ex TapTM Ⅱ试剂盒(TakaRa Bio);ELISA检测试剂盒IL-1β、IL-6和TNF-α(R&D);双荧光素酶报告基因检测试剂盒(Abcam)。
1.4. 主要仪器
二氧化碳培养箱(深圳市瑞沃德生命科技有限公司);ABI7500实时荧光定量PCR仪(Thermo Fisher Scientific);酶标仪(Biotek);流式细胞检测仪(深圳迈瑞生物医疗电子股份有限公司);高速冷冻型微量台式离心机[大龙兴创实验仪器(北京)股份公司)]。
1.5. 细胞培养与处理
HEK-293T细胞和BV2细胞在含有10%胎牛血清和1%青霉素-链霉素溶液的MEM培养基中培养。对于缺氧缺糖/再灌注(OGD/R)细胞模型,将全培养基换成无葡萄糖DMEM,将细胞置于37 ℃厌氧室(95% N2和5% CO2)中2 h,然后重新加入全培养基,保持正常培养状态(37 ℃,5% CO2,饱和湿度)继续培养。48 h后收集细胞用于后续试验。在对照组中,细胞没有被剥夺氧和葡萄糖,正常培养。
1.6. 细胞转染
oe-SNHG8、miR-494-3p mimic及相应对照均购自GenePharma(中国上海)。转染oe-NC或oe-SNHG8后再进行剥夺氧和葡萄糖处理,将细胞分为OGD/R+oe-NC组或OGD/R + oe-SNHG8组;将oe-SNHG8与mimic NC或miR-494-3p mimic共同转染后再进行剥夺氧和葡萄糖处理,将细胞分为OGD/R+oe-SNHG8+mimic NC组和OGD/R+oe-SNHG8+miR-494-3p mimic组。对于转染,根据制造商的说明,将所需质粒与LipofectamineTM 3000混合,并转染到指定细胞中。
1.7. 小鼠脑缺血再灌注损伤模型建立
通过大脑中动脉闭塞手术建立脑缺血再灌注损伤模型[17]。用异氟醚(4%诱导浓度和2%维持浓度)麻醉小鼠,沿中线切开颈部皮肤,小心地分离左侧颈总动脉、颈内动脉和颈外动脉。接下来,将一根带有圆形尖端的6-0尼龙丝从左侧颈总动脉插入颈内动脉的末端,以阻塞右大脑中动脉的起源。闭塞2 h后,去除尼龙线,让血液通过左侧颈内动脉回流(再灌注),缝合皮肤切口。再灌注24 h后,腹腔注射1% 戊巴比妥钠(150 mg/kg)对小鼠实施安乐死,取小鼠大脑组织进行后续实验。术前禁食12 h,自由饮水。术中保留大鼠自主呼吸,维持直肠温36.5~37.5 ℃,术中和术后维持室温25 ℃。Sham组只暴露左侧颈总动脉,不结扎处理,其余均行相同手术操作。
1.8. 小鼠神经损伤评估
小鼠神经损伤程度采用神经功能缺失评分和大脑水肿程度确定。小鼠神经功能缺失评分:血液再灌注后24 h,大鼠神经功能缺失通过Longa评分标准确定[18];大脑水肿程度通过标准的大脑湿重-大脑干质量来确定[19]。大脑提取后迅速称取质量,随后在100 ℃下干燥24 h,称质量。大脑的水肿程度通过公式:(湿质量-干质量)/湿质量×100%。
1.9. TTC染色
将小鼠大脑组织样品在冰箱中于-20 ℃冷冻30 min以进行切片。简而言之,将大脑组织切成2.0 mm厚的冠状切片。并使用2% TTC溶液在37 ℃下对这些切片染色20 min。然后,将切片浸入4%多聚甲醛中固定过夜。然后照相,与正常区域的红色相比,脑损伤区域呈现白色。染色切片用AutoCAD分析软件(San Rafael,CA)成像和分析。
1.10. RT-qPCR检测LncRNA SNHG8和miR-494-3p的表达
按照制造商的说明,使用Trizol试剂从细胞和大脑组织的大脑皮层梗死区域中提取总RNA。使用逆转录试剂盒将总RNA反向转录为cDNA。对浓度和纯度符合要求的RNA样品,稀释到合适的浓度,利用PrimeScriptTM RT试剂盒进行逆转录。采用ABI7500实时荧光定量PCR仪,使用SYBR®Premix Ex TapTMⅡ试剂盒,运用Applied Biosystems 7500HT系统进行PCR,并通过2-ΔΔCt方法分析数据。每个样品重复3次,所有反应引物如下表 1所示。LncRNA SNHG8用GAPDH为内参,miR-494-3p的相对表达用U6为内参。数据分析采用2-ΔΔCt法,公式如下:ΔΔCt=[Ct(目的基因)-Ct(内参基因)]实验组-[Ct(目的基因)-Ct(内参基因)]对照组。
表 1.
引物序列
Primer
| Gene name | Primer sequenc (5'-3') | |
| LncRNA SNHG8 | Forward | CCCGAGAACCGTCAGTTTGA |
| Reverse | CCGGCACCCTCTAGGTTTTT | |
| miR-494-3p | Forward | ACATACACGGGAAACCTC |
| Reverse | GAACATGTCTGCGTATCTC | |
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG |
| Reverse | ACCACCCTGTTGCTGTAGCCAA | |
| U6 | Forward | CTCGCTTCGGCAGCACAT |
| Reverse | TTTGCGTGTCATCCTTGCG |
1.11. 流式细胞仪检测细胞凋亡
使用Annexin V-FITC/PI细胞凋亡检测试剂盒对细胞进行凋亡分析。将各组细胞悬液调整密度为105/mL,每个样品收集1 mL细胞悬液于10 mL离心管中,500 r/min离心5 min,弃去培养液。PBS洗涤,500 r/min离心5 min,弃上清。用100 μL的标记溶液重悬细胞,室温下避光孵育10 min。加入5 μL的Annexin V-荧光素异硫氰酸酯(Annexin V-FITC)和5 μL的碘化丙啶(PI),轻轻混匀,室温避光反应10 min。流式细胞仪检测FITC和PI荧光,分析细胞凋亡率。
1.12. ELISA检测细胞中IL-1β、IL-6和TNF-α含量
收集细胞上清和小鼠大脑组织的大脑皮层梗死区域匀浆上清液对炎症因子IL-1β、IL-6和TNF-α进行ELISA分析。部分脑组织在冰上超声匀浆30 min,将脑组织匀浆和各组细胞4 ℃、12 000 r/min离心10 min,收集其上清液。通过ELISA试剂盒检测IL-1β、IL-6和TNF-α含量。所有操作严格按照试剂盒说明书进行。
1.13. 荧光素酶报告实验检测LncRNA SNHG8和miR-494-3p的靶向调控关系
根据生物信息学软件Starbase预测结果,设计LncRNA SNHG8和miR-494-3p结合位点的野生序列和突变序列。通过生工生物工程有限公司合成LncRNA SNHG8野生型Wt-SNHG8和3'UTR结合序列突变型Mut-SNHG8,将野生序列和突变序列片段克隆并分别与pmirGLO载体结合,将Wt-SNHG8或Mut-SNHG8和mimic NC或miR-494-3p mimic使用LipofectamineTM 3000共转染到HEK-293T细胞中。转染48 h后,使用双荧光素酶双报告基因试剂盒进行荧光素酶活性检测。
1.14. 统计学分析
使用SPSS 23.0进行数据统计学分析,计量资料以均数±标准差表示。所有实验独立重复3次。数据若服从正态分布及各组间满足方差齐性时,采用t检验或单因素方差分析;若数据不服从正态分布,则采用秩和检验进行分析验。P < 0.05为差异有统计学意义。
2. 结果
2.1. LncRNA SNHG8在脑缺血再灌注损伤小鼠中低表达,而miR-494-3p高表达
Sham组未观察到梗死,I/R组出现明显的梗死区(图 1)。神经功能缺失评分结果显示I/R组小鼠神经严重损伤,而且脑水肿程度严重(P < 0.001,图 2、3)。ELISA检测结果显示,与Sham组相比,I/R组小鼠脑组织中炎症因子IL-1β、IL-6和TNF-α的表达水平明显增加(P < 0.001,图 4)。RT-qPCR结果显示,I/R组中LncRNA SNHG8低表达(P < 0.01),而miR-494-3p高表达(P < 0.01,图 5)。
图 1.

Sham组和I/R组小鼠脑组织TTC染色
TTC staining of the brain tissue in Sham group and I/R group.
图 2.

Sham组和I/R组小鼠神经功能缺失评分
Score of neurological deficit in Sham group and I/R group. ***P < 0.001.
图 3.

Sham组和I/R组小鼠脑水肿程度评估
Evaluation of brain edema degree in Sham group and I/R group mice. ***P < 0.001.
图 4.

Sham组和I/R组小鼠脑组织中炎症因子IL-1β、IL-6和TNF-α表达水平
Expression levels of inflammatory factors IL-1β, IL-6 and TNF-α in the brain tissue of Sham group and I/R group. ***P < 0.001.
图 5.

Sham组和I/R组小鼠脑组织中LncRNASNHG8和miR-494-3p表达
Expression of LncRNA SNHG8 and miR-494-3p in brain tissue of Sham group and I/R group mice. **P < 0.01.
2.2. 过表达LncRNA SNHG8抑制OGD/R模型小胶质细胞的炎症反应
RT-qPCR结果显示(图 6),LncRNA SNHG8在OGD/R组中的表达明显降低(P < 0.01),过表达后LncRNA SNHG8的表达明显升高(P < 0.01)。ELISA结果显示,与Control组相比,OGD/R组细胞内炎症因子IL-1β、IL-6和TNF-α的表达水平明显增加(P < 0.01);与OGD/R+oe-NC组相比,OGD/R+oe-SNHG8组IL-1β、IL-6和TNF-α的含量明显减少(P < 0.01),OGD/R组与OGD/R+oe-NC之间差异性不显著(图 7)。
图 6.

LncRNASNHG8在各组细胞中的表达水平
Expression level of LncRNA SNHG8 in microglial cells of each group. **P < 0.01.
图 7.

各组细胞中炎症因子IL-1β、IL-6和TNF-α表达水平
Expression levels of inflammatory factors IL-1β, IL-6 and TNF-α in cells of each group. **P < 0.01.
2.3. 过表达LncRNA SNHG8抑制OGD/R模型小胶质细胞的凋亡
流式细胞术检测结果显示(图 8),与Control组相比,OGD/R组细胞凋亡率明显增加(P < 0.01);与OGD/R+oe-NC组相比,OGD/R+oe-SNHG8组细胞凋亡率明显降低(P < 0.01)。OGD/R组与OGD/R+oe-NC之间差异没有统计学意义(P>0.05)。
图 8.

各组细胞凋亡率比较
Comparison of apoptosis rates among the groups. **P < 0.01.
2.4. LncRNA SNHG8靶向负调节miR-494-3p
通过生物信息学网站Starbase预测了LncRNA SNHG8和miR-494-3p结合的靶位点(图 9),并且设计了野生型LncRNA SNHG8(Wt-SNHG8)和突变型LncRNA SNHG8(Mut-SNHG8)。双荧光素酶报告实验结果(图 10)显示,与mimic NC相比,miR-494-3p mimic组明显减弱了Wt-SNHG8的荧光素酶信号(P < 0.01),而对Mut-SNHG8的荧光素酶信号没有显著性差异(P>0.05)。与OGD/R+oe-NC组比较,转染OGD/R+oe-SNHG8的小胶质细胞中miR-494-3p的表达水平显著降低(P < 0.01,图 11)。
图 9.

生物信息学预测LncRNA SNHG8和miR-494-3p的靶向调控位点
Bioinformatic prediction of the targeted regulatory sites of LncRNASNHG8 and miR-494-3p.
图 10.

双荧光素酶报告实验
Luciferase reporter assay. **P < 0.01.
图 11.

miR-494-3p在两组细胞中的表达水平
Expression level of miR-494-3p in two groups of cells. **P < 0.01.
2.5. 过表达miR-494-3p逆转了oe-SNHG8对OGD/R模型小胶质细胞炎症反应和凋亡的抑制作用
RT-qPCR检测显示,进一步过表达miR-494-3p的OGD/R模型小胶质细胞中miR-494-3p的水平降低(P < 0.01,图 12)。与OGD/R+oe-SNHG8+mimic NC相比较,OGD/R+oe-SNHG8+miR-494-3p mimic炎症因子IL-1β、IL-6和TNF-α的含量增加(P < 0.05,图 13),细胞凋亡率增加(P < 0.01,图 14)。
图 12.
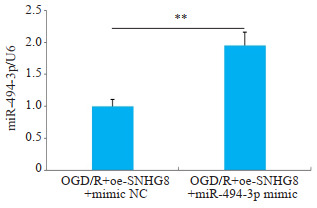
miR-494-3p在两组细胞中的表达水平
Expression level of miR-494-3p in two groups of cells. **P < 0.01.
图 13.

两组细胞IL-1β、IL-6和TNF-α含量比较
Comparison of the contents of IL-1β, IL-6 and TNF-α between the two groups. *P < 0.05.
图 14.

两组细胞凋亡率比较
Comparison of apoptosis rate between the two groups. **P < 0.01.
3. 讨论
缺血性中风是一种常见的神经系统疾病,是全球死亡和长期残疾的主要原因[20, 21]。由颅内动脉闭塞或颅外颈动脉闭塞引发的急性缺血性脑卒中约占总脑卒中的85%[22]。在缺血性中风后的几个小时内,神经元会永久受损并经历细胞死亡[23]。因此,拯救受损神经元的治疗选择很重要;然而,迄今为止,只有少数治疗药物被报道可以缓解中风后的神经功能障碍。临床治疗,例如组织纤溶酶原激活剂介导的溶栓,通常受到治疗时间窗口狭窄和长期效果不足的限制[24, 25]。在缺血性中风情况下,小胶质细胞发挥着双刃剑的作用,吞噬组织碎片并分泌促炎和抗炎介质,这可能会加剧缺血性损伤或诱导修复[26]。小胶质细胞活化和增殖的损伤已被证明可增加缺血性损伤引起的梗死面积和细胞凋亡[27]。研究发现,MSC-Exos(间充质干细胞来源的外泌体)可以降低OGD/R诱导的BV-2细胞凋亡[28];在OGD/R模型中,BV2细胞凋亡率升高,儿茶素(100 µmol/L)也可以有效地抑制细胞凋亡[29]。在本研究中,我们发现CIRI小鼠动物模型中,LncRNA SNHG8低表达,而miR-494-3p高表达。过表达LncRNA SNHG8可抑制OGD/R模型小胶质细胞的炎症反应和细胞凋亡。LncRNA SNHG8靶向负调控miR-494-3p的表达。过表达miR-494-3p逆转了oe-SNHG8对OGD/R模型小胶质细胞凋亡和炎症反应的抑制作用。从机制上讲,我们的结果表明:LncRNA SNHG8通过抑制miR-494-3p表达,抑制炎症反应和细胞凋亡,从而改善脑缺血再灌注损伤。
近年来,一些lncRNAs已被证明在CIRI中失调[30, 31]。LncRNA是脑卒中等缺血性损伤后脑血管内皮中新发现的一类调节因子[32]。lncRNA已成为治疗缺血性脑卒中的新靶点[33, 34]。例如,抑制LncCHRF通过调节miR-126/SOX6轴来减少缺血性损伤[35]。敲低LncRNA AK038897可通过调节DAPK1的miR-26a-5靶向来预防脑缺血再灌注损伤[36]。因此,lncRNA作为脑缺血再灌注损伤进展中的新治疗靶点具有巨大的潜力。例如,沉默MALAT1可以抑制OGD-R诱导的细胞凋亡[37]。此外,Zhang等[9]报道lncRNASNHG8通过miR-449c-5p/SIRT1/FoxO1途径抑制小胶质细胞活化和血脑屏障通透性,从而引起对缺血性脑损伤的保护作用。在本研究中,我们通过调节LncRNA SNHG8表达证明了过表达LncRNA SNHG8对脑缺血再灌注损伤具有保护作用。
为了识别LncRNA SNHG8的下游靶标,我们通过StarBase生物信息学网站分析发现LncRNA SNHG8与miR-494-3p存在结合位点。MicroRNA(miRNA)是长度约为18~21个核苷酸的短非编码RNA,可以通过靶向其3'非翻译区(3'-UTR)来调节mRNA翻译[38]。超过20%的miRNA在缺血性脑中异常表达,提示miRNA参与缺血性脑卒中的发病机制和发展[39]。miR-424的过表达可以通过抑制小胶质细胞活化来减少缺血性脑损伤[40]。增加miR-224-3p表达可通过降低FAK家族相互作用蛋白(FIP200)的表达来减轻脑缺血/再灌注损伤[41]。miR-494-3p加剧CIRI后的脑损伤和神经元损伤[16],这与我们的研究结果相似。在本研究中,荧光素酶报告基因检测显示miR-494-3p是CIRI中LncRNA SNHG8的直接靶基因,过表达LncRNA SNHG8抑制miR-494-3p的表达,证明LncRNA SNHG8可以负向调控miR-494-3p的表达。此外,本实验还发现,进一步过表达miR-494-3p,部分逆转了oe-SNHG8对OGD/R模型小胶质炎症反应和细胞凋亡的抑制作用。
总之,LncRNA SNHG8通过抑制miR-494-3p表达,抑制炎症反应和细胞凋亡,从而改善脑缺血再灌注损伤。这些结果表明LncRNA SNHG8参与脑缺血再灌注损伤的进展,为脑缺血再灌注损伤的治疗提供了一个新的潜在靶点。
Biography
曹天然,副主任医师,E-mail: caotianran1027@sina.com
Funding Statement
湖南省自然科学基金-科卫联合项目(2022JJ70129)
Contributor Information
曹 天然 (Tianran CAO), Email: caotianran1027@sina.com.
张 雪红 (Xuehong ZHANG), Email: zhangxuehong1027@163.com.
References
- 1.Joseph B, Miller, Md M, et al. The advanced reperfusion era: implications for emergency systems of ischemic stroke care. Ann Emerg Med. 2017;69(2):192–201. doi: 10.1016/j.annemergmed.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 2.Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn) 2017;23(1, Cerebrovascular Disease):15–39. doi: 10.1212/CON.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 3.Yin P, Wei YF, Wang X, et al. Roles of specialized pro-resolving lipid mediators in cerebral ischemia reperfusion injury. Front Neurol. 2018;9:617. doi: 10.3389/fneur.2018.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Liu YW, Yin J, et al. Oxygen-glucose-deprivation/reoxygenation-induced autophagic cell death depends on JNK-mediated phosphorylation of bcl-2. Cell Physiol Biochem. 2016;38(3):1063–74. doi: 10.1159/000443057. [DOI] [PubMed] [Google Scholar]
- 6.Cornu C, Amsallem E, Assia SJ A. Thrombolytic therapy for acute ischemic stroke. Am J Cordiovosc Drugs. 2001;1(4):281–92. doi: 10.2165/00129784-200101040-00006. [DOI] [PubMed] [Google Scholar]
- 7.Morlando M, Ballarino M, Fatica A. Long non-coding RNAs: new players in hematopoiesis and leukemia. Front Med (Lausanne) 2015;2:23. doi: 10.3389/fmed.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Lu MY, Zhang XF, et al. Isosteviol sodium protects against ischemic stroke by modulating microglia/macrophage polarization via disruption of GAS5/miR-146a-5p sponge. Sci Rep. 2019;9:12221. doi: 10.1038/s41598-019-48759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang DB, Pan N, Jiang C, et al. LncRNA SNHG8 sponges miR-449c-5p and regulates the SIRT1/FoxO1 pathway to affect microglia activation and blood-brain barrier permeability in ischemic stroke. J Leukoc Biol. 2022;111(5):953–66. doi: 10.1002/JLB.1A0421-217RR. [DOI] [PubMed] [Google Scholar]
- 10.Tian JN, Liu YH, Wang ZQ, et al. LncRNA Snhg8 attenuates microglial inflammation response and blood-brain barrier damage in ischemic stroke through regulating miR-425-5p mediated SIRT1/NF-κB signaling. J Biochem Mol Toxicol. 2021;35(5):e22724. doi: 10.1002/jbt.22724. [DOI] [PubMed] [Google Scholar]
- 11.Perry N, Volin M, Toledano H. microRNAs in Drosophila regulate cell fate by repressing single mRNA targets. Int J Dev Biol. 2017;61(3/4/5):165–70. doi: 10.1387/ijdb.160271ht. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Wang LJ, Wang L, et al. LncRNA CASC15 promotes cerebral ischemia/reperfusion injury via miR-338-3p/ETS1 axis in acute ischemic stroke. Int J Gen Med. 2021;14:6305–13. doi: 10.2147/IJGM.S323237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L, Zuo ML, Wang AP, et al. Low expression of miR-532-3p contributes to cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Mol Med Rep. 2020;22(3):2415–23. doi: 10.3892/mmr.2020.11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo ML, Wang AP, Song GL, et al. miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed Pharmacother. 2020;124:109860. doi: 10.1016/j.biopha.2020.109860. [DOI] [PubMed] [Google Scholar]
- 16.Sun LJ, Ji DD, Zhi F, et al. miR-494-3p upregulation exacerbates cerebral ischemia injury by targeting Bhlhe40. Yonsei Med J. 2022;63(4):389. doi: 10.3349/ymj.2022.63.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng YY, Pan CF, Chen ML, et al. miR-29a ameliorates ischemic injury of astrocytes in vitro by targeting the water channel protein aquaporin 4. Oncol Rep. 2019;41(3):1707–17. doi: 10.3892/or.2019.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chen WA, Huang SS, et al. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol. 2016;771:145–51. doi: 10.1016/j.ejphar.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang MQ, Tang MM, Wu Q, et al. LncRNA DANCR attenuates brain microvascular endothelial cell damage induced by oxygen-glucose deprivation through regulating of miR-33a-5p/XBP1s. Aging. 2020;12(2):1778–91. doi: 10.18632/aging.102712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He LZ, Huang GN, Liu HX, et al. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci Adv. 2020;6(12):eaay9751. doi: 10.1126/sciadv.aay9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YC, Li X, Shen YT, et al. PERK (protein kinase RNA-like ER kinase) branch of the unfolded protein response confers neuroprotection in ischemic stroke by suppressing protein synthesis. Stroke. 2020;51(5):1570–7. doi: 10.1161/STROKEAHA.120.029071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fifield KE, Vanderluit JL. Rapid degeneration of neurons in the penumbra region following a small, focal ischemic stroke. Eur J Neurosci. 2020;52(4):3196–214. doi: 10.1111/ejn.14678. [DOI] [PubMed] [Google Scholar]
- 24.Wu XH, Gong YS, Ding XB, et al. Retrovirus-mediated transfection of the tissue-type plasminogen activator gene results in increased thrombolysis of blood clots. Biochem Genet. 2019;57(2):234–47. doi: 10.1007/s10528-018-9887-0. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, Min SK, Usoltseva R, et al. Thrombolytic fucoidans inhibit the tPA-PAI1 complex, indicating activation of plasma tissue-type plasminogen activator is a mechanism of fucoidan-mediated thrombolysis in a mouse thrombosis model. Thromb Res. 2018;161:22–5. doi: 10.1016/j.thromres.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Patel AR, Ritzel R, McCullough LD, et al. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol. 2013;5(2):73–90. [PMC free article] [PubMed] [Google Scholar]
- 27.Lalancette-Hebert M, Swarup V, Beaulieu JM, et al. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci. 2012;32(30):10383–95. doi: 10.1523/JNEUROSCI.1498-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu ZZ, Yuan Y, Zhang XL, et al. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxidative Med Cell Longev. 2021;2021:1–14. doi: 10.1155/2021/6219715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CM, Wu CT, Yang TH, et al. Green tea catechin prevents hypoxia/reperfusion-evoked oxidative stress-regulated autophagy-activated apoptosis and cell death in microglial cells. J Agric Food Chem. 2016;64(20):4078–85. doi: 10.1021/acs.jafc.6b01513. [DOI] [PubMed] [Google Scholar]
- 30.Chen FH, Zhang LX, Wang EW, et al. LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem Biophys Res Commun. 2018;496(1):184–90. doi: 10.1016/j.bbrc.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Guo D, Ma J, Yan L, et al. Down-regulation of lncrna MALAT1 attenuates neuronal cell death through suppressing Beclin1-dependent autophagy by regulating mir-30a in cerebral ischemic stroke. Cell Physiol Biochem. 2017;43(1):182–94. doi: 10.1159/000480337. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Matsuura K, Kleiner DE, et al. Analysis of long noncoding RNA expression in hepatocellular carcinoma of different viral etiology. J Transl Med. 2016;14(1):1–11. doi: 10.1186/s12967-015-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao MH, Szeto V, Yang BB, et al. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018;9:281. doi: 10.1038/s41419-018-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandran R, Mehta SL, Vemuganti R. Non-coding RNAs and neuroprotection after acute CNS injuries. Neurochem Int. 2017;111:12–22. doi: 10.1016/j.neuint.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gai HY, Wu C, Zhang Y, et al. Long non-coding RNA CHRF modulates the progression of cerebral ischemia/reperfusion injury via miR-126/SOX6 signaling pathway. Biochem Biophys Res Commun. 2019;514(2):550–7. doi: 10.1016/j.bbrc.2019.04.161. [DOI] [PubMed] [Google Scholar]
- 36.Wei R, Zhang L, Hu W, et al. Long non-coding RNA AK038897 aggravates cerebral ischemia/reperfusion injury via acting as a ceRNA for miR-26a-5p to target DAPK1. Exp Neurol. 2019;314:100–10. doi: 10.1016/j.expneurol.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Xin JW, Jiang YG. Long noncoding RNA MALAT1 inhibits apoptosis induced by oxygen-glucose deprivation and reoxygenation in human brain microvascular endothelial cells. Exp Ther Med. 2017;13(4):1225–34. doi: 10.3892/etm.2017.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 39.Tan JR, Tan KS, Koo YX, et al. Blood microRNAs in low or no risk ischemic stroke patients. Int J Mol Sci. 2013;14(1):2072–84. doi: 10.3390/ijms14012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao HP, Wang J, Gao L, et al. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. 2013;44:1706–13. doi: 10.1161/STROKEAHA.111.000504. [DOI] [PubMed] [Google Scholar]
- 41.Deng YM, Ma G, Dong QH, et al. Overexpression of miR-224-3p alleviates apoptosis from cerebral ischemia reperfusion injury by targeting FIP200. J Cell Biochem. 2019;120:17151–8. doi: 10.1002/jcb.28975. [DOI] [PubMed] [Google Scholar]


