Abstract
Fluorescence imaging is an invaluable tool to study biological processes and further progress depends on the development of advanced fluorogenic probes that reach intracellular targets and label them with high specificity. Excellent fluorogenic rhodamine dyes have been reported, but they often require long and low-yielding syntheses, and are spectrally limited to the visible range. Here we present a general strategy to transform polymethine compounds into fluorogenic dyes using an intramolecular ring-closure approach. We illustrate the generality of this method by creating both spontaneously blinking and no-wash, turn-on polymethine dyes with emissions across the visible and near-infrared spectrum. These probes are compatible with self-labelling proteins and small-molecule targeting ligands, and can be combined with rhodamine-based dyes for multicolour and fluorescence lifetime multiplexing imaging. This strategy provides access to bright, fluorogenic dyes that emit at wavelengths that are more red-shifted compared with those of existing rhodamine-based dyes.
Subject terms: Chemical tools, Small molecules
Polymethine dyes are bright and red-shifted fluorophores that lack an intrinsic turn-on mechanism, which leads to non-specific staining when applied to biological samples. Now the fluorescence of polymethine dyes was masked through an intracellular cyclization strategy that gets reversed upon binding an intended macromolecular target, providing specificity for live-cell imaging.
Main
Fluorescence microscopy is crucial to study the structure and function of cells. Fluorescent protein tags allow for the dynamic observation of proteins in living cells, but their brightness and photostability are often inferior to those of small-molecule fluorophores1. Fluorogenic dyes conjugated to self-labelling protein tags such as HaloTag2 or SNAP-tag3 combine the excellent photophysical properties of small-molecule dyes with the precise labelling of genetically encoded tags and have been widely used for fluorescence microscopy and nanoscopy.
The development of fluorogenic probes has predominantly focused on rhodamine-based scaffolds4,5. Rhodamine dyes exist in an equilibrium between a fluorescent zwitterionic (open) and a non-fluorescent spirocyclic (closed) form. This equilibrium is sensitive to the microenvironment of the probe. In media of low polarity and high pH, rhodamines are primarily present in the closed form, whereas polar media and low pH favour the open form6–8. Rhodamine dyes have been systematically optimized for fluorogenicity by varying the electron density of their conjugated core and by modulating the electronic character of the intramolecular nucleophile that closes the spirocycle9,10. These dyes, which span the visible range, display substantial fluorescence increase upon target binding and have been used for no-wash, multicolour, live-cell fluorescence imaging experiments. In contrast, the development of novel rhodamines with excitation and emission wavelengths in the near-infrared region (>700 nm) has been hampered by their complicated, long and low-yielding syntheses, as well as by their tendency to remain in the non-fluorescent form even upon binding to their target11.
Polymethine dyes are some of the most used fluorophores in cell, tissue and whole-organism imaging due to their simple and highly modular synthesis, high extinction coefficients, biocompatibility, and tunable emission wavelengths spanning from the green to the shortwave infrared wavelengths. Members of this class of fluorophores include indoleninium-based dyes such as carbocyanines (Cy)12–15 and squaraines16–18, flavylium-based dyes19,20, and coumarin–hemicyanine hybrid scaffolds21–24, among others. The variety of applications of these dyes is illustrated by two classic examples: Indocyanine Green25, a clinically approved, near-infrared dye for optical imaging of the vasculature, and Cy5 derivatives (for example, AlexaFluor 647), which are the most widely used fluorophores in single-molecule localization microscopy (SMLM)26. Many elegant turn-on mechanisms for polymethine dyes have been reported.27–30 However, so far there is no reported general strategy to impart binding-induced fluorogenicity to polymethine dyes and thus their applicability in live-cell imaging of specific protein targets remains very limited.
Unlike rhodamine-based dyes, polymethine fluorophores do not possess a built-in intramolecular cyclization equilibrium that could be leveraged to induce fluorogenicity; however, the addition of nucleophilic side chains has been used as a strategy to create fluorogenic polymethine dyes. For instance, Cy5 and Cy7 dyes have been decorated with alcohols, amines and thiols as nucleophiles, thereby forming oxazines, diazines and thiazines, respectively31–34. Coumarin–hemicyanine hybrid fluorophores have been generated with a p-nitrophenol group that exists mainly in the spirocyclic form but can undergo a photoinduced and reversible interconversion into its open fluorescent form24. Furthermore, coumarin–hemicyanine hybrid fluorophores have been developed with a hydroxyethyl ring-closing moiety to generate esterase-activatable fluorescent probes21. Despite these efforts, cyclization reactions in polymethine dyes have not been as efficient as in rhodamines, and the creation of robustly fluorogenic polymethine dyes has so far remained elusive. In this work we applied classic organic chemistry heuristics to design favourable cyclization reactions that led to a general strategy for the creation of fluorogenic polymethine dyes for live-cell imaging.
Results and discussion
Probe design and validation
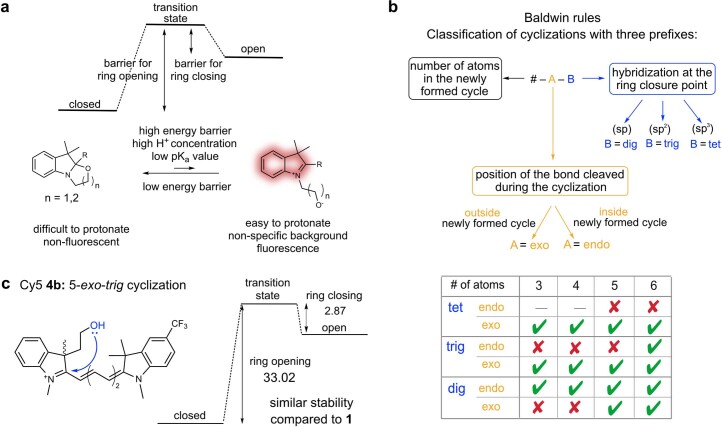
An efficient fluorogenic dye must exist exclusively in the non-fluorescent form unless it is bound to its intended target. In the case of cyclization-based fluorogenicity, it means that the barrier of ring opening and closure must be high and low, respectively (Extended Data Fig. 1). The favourability of polar cyclization reactions can be estimated using heuristic guidelines known as the Baldwin rules (Extended Data Fig. 1)35. We noticed that in past attempts to create fluorogenic polymethine dyes, the intramolecular cyclization was of the 5-endo-trig type, which is unfavourable according to the Baldwin rules (Extended Data Fig. 1). This cyclization (Fig. 1a) is classified as 5-endo-trig because it involves the formation of a five-membered ring that contains (endo) the single bond formed from the double bond of the trigonal (trig) carbon attacked by the nucleophile (Fig. 1a). Although 6-endo-trig cyclizations should be more favourable than 5-endo-trig, past attempts at using 6-endo-trig cyclizations to impart fluorogenicity did not lead to more stable cyclic isomers31–34. Based on these observations, we hypothesized that a 5-exo-trig cyclization—in which the single bond that forms upon attack of the double bond is not contained (exo) within the newly formed ring (Fig. 1b)—would be a more efficient alternative.
Extended Data Fig. 1. Probe design and Baldwin rules.
a) Cyclization energy landscape that favours fluorogenicity: A large barrier of ring opening and a small barrier of ring closure. b) Explanation of the Baldwin rules. c) Structure and calculated energy of ring opening and closing of Cy5 derivative 4b. Calculations were carried out at the B3LYP/DGTZVP level of theory and energy values are reported in kcal mol–1.
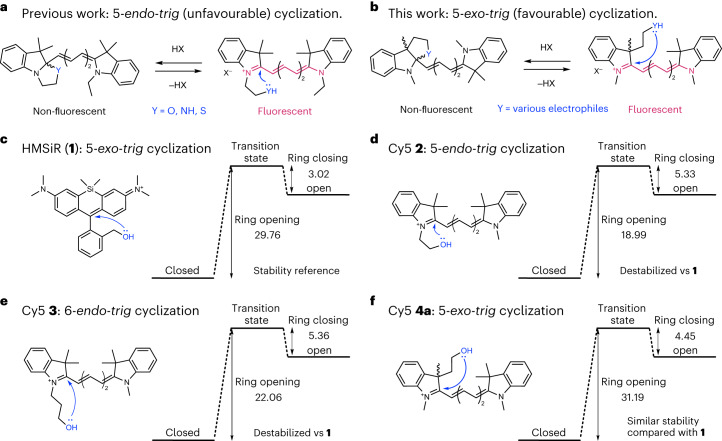
Fig. 1. Intramolecular cyclization reactions of carbocyanines and HMSiR (1).
a, Example of previous work using a 5-endo-trig cyclization to create fluorogenic carbocyanine dyes31–34. b, 5-exo-trig cyclization as a robust and general strategy towards fluorogenicity in polymethine dyes. c–f, Structures and calculated energies of ring opening and closing of rhodamine HMSiR (1) (c), and Cy5 derivatives 2 (d), 3 (e) and 4a (f). Energies (kcal mol–1) were calculated at the B3LYP/DGTZVP level of theory. A summary of calculated energies using the B3LYP or M06-2X functional can be found in Supplementary Table 1.
To test our hypothesis, we used density functional theory (DFT) to calculate the energies of ring opening and closure for HMSiR (1)—a silicon rhodamine derivative with an efficient 5-exo-trig intramolecular cyclization reaction (Fig. 1c)36. We then compared these values with those calculated for Cy5 derivatives 2, 3 and 4a, which undergo 5-endo-trig, 6-endo-trig and 5-exo-trig cyclizations, respectively (Fig. 1d–f). HMSiR (1) has larger ring-opening and smaller ring-closing energies than Cy5 derivatives 2 and 3, which undergo 5- and 6-endo-trig cyclizations, respectively (Fig. 1c,d). In contrast, the energies of ring opening and closure in Cy5 derivative 4a—which undergoes a 5-exo-trig cyclization—compare favourably with the computed values for HMSiR (1) (Fig. 1c,f). We also observed that judicious addition of electron-withdrawing groups to the Cy5 dye further increased the stability of the closed isomer (for example, derivative 4b; Extended Data Fig. 1), allowing for additional modulation of the energies of ring opening and closure. These predictions suggested that more robust fluorogenic polymethine dyes could be obtained simply by employing a 5-exo-trig cyclization instead of a 5-endo-trig one.
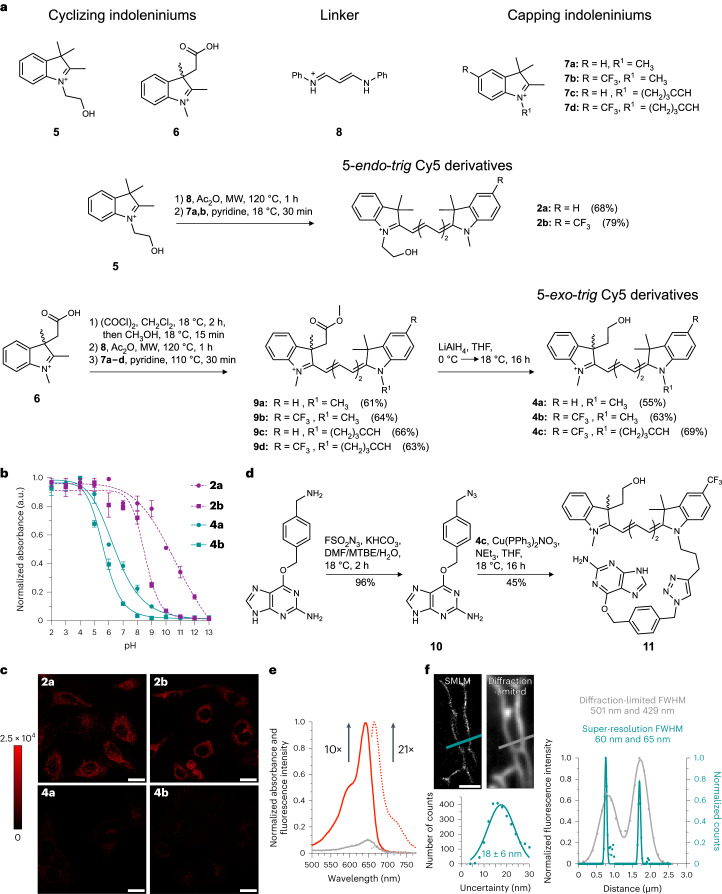
To test these computational predictions, we prepared the indoleninium building blocks 5, 6, 7a–d and linker 8 (Fig. 2a and Supplementary Information) according to published procedures37–40. We next synthesized the 5-endo-trig Cy5 probes 2a and 2b in a two-step, microwave-assisted procedure (Supplementary Information). The 5-exo-trig Cy5 probes 4a and 4b were prepared in two steps via methyl ester intermediates 9a–d, followed by reduction with LiAlH4 (Fig. 2a). We performed pH titrations of these Cy5 derivatives to evaluate the stability of the closed isomer (Fig. 2b and Supplementary Fig. 1). The pH titrations of 5-endo-trig probes revealed ring-opening pKa values above physiological pH (11.1 for 2a and 8.5 for 2b), indicating that these probes would be highly fluorescent as free small molecules in cells. By contrast, the ring-opening pKa values of 5-exo-trig probes were below 7 (6.4 for 4a and 5.7 for 4b), suggesting that they might only show some fluorescence in acidic compartments. Furthermore, 5-exo-trig compounds 4a and 4b have a much higher propensity to cyclize in medium of low dielectric constant than their 5-endo-trig counterparts 2a and 2b (Supplementary Fig. 2).
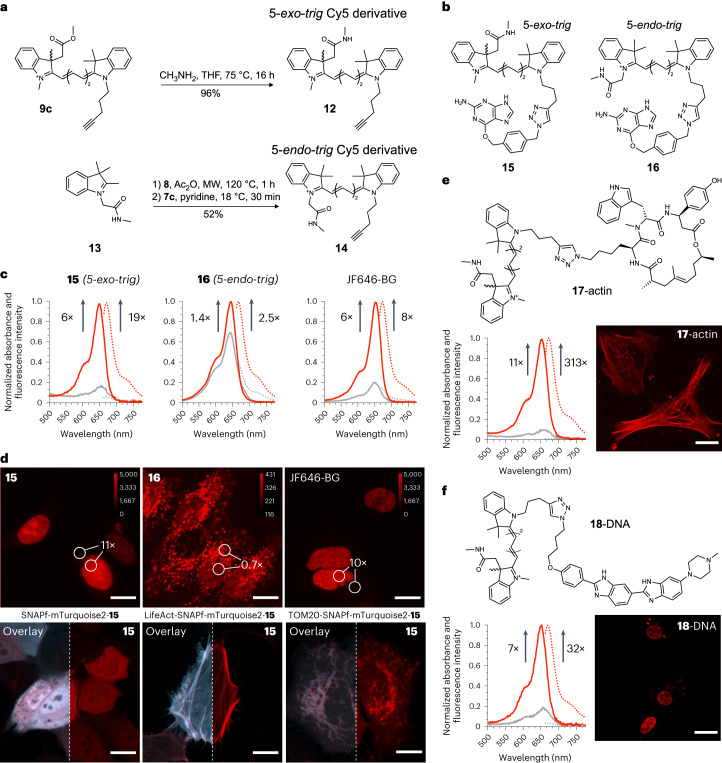
Fig. 2. Synthesis and imaging properties of 5-endo-trig and 5-exo-trig Cy5 derivatives.
a, Synthesis of 5-endo-trig and 5-exo-trig Cy5 dyes with a hydroxy group as a ring-closing moiety. b, The pH profiles of dyes 2a, 2b, 4a and 4b. Symbols indicate the mean of three independent experiments and error bars represent the s.d. c, Intracellular fluorescence of untargeted 5-endo-trig (2a,b) and 5-exo-trig Cy5 derivatives (4a,b) in HeLa cells. The calibration bar indicates raw pixel values. Micrographs are representative of three independent biological samples measured over three separate imaging sessions. d, SNAP-tag functionalization of 4c to yield SNAP-tag-reactive probe 11. e, Protein-binding turn-on of 11 (2.5 µM) incubated with purified SNAP-tag protein (5 µM) in phosphate-buffered saline (PBS) for 1.5 h. The lines indicate absorbance (solid) or fluorescence (dotted) of the free dye in solution (grey) or the dye–protein conjugate (red). Spectra are the mean from three independent experiments. f, Single-molecule localization microscopy imaging of 11 (100 nM) in live HeLa cells transfected with β-tubulin-SNAP-tag. A comparison between a super-resolved and a diffraction-limited image (upper left), and localization precision of single-molecules of probe 11 in live cells is also shown (bottom left). Scale bar, 20 µm (c) and 2 µm (f). Data are representative of three independent experiments performed in three different imaging sessions. FWHM, full-width at half-maximum.
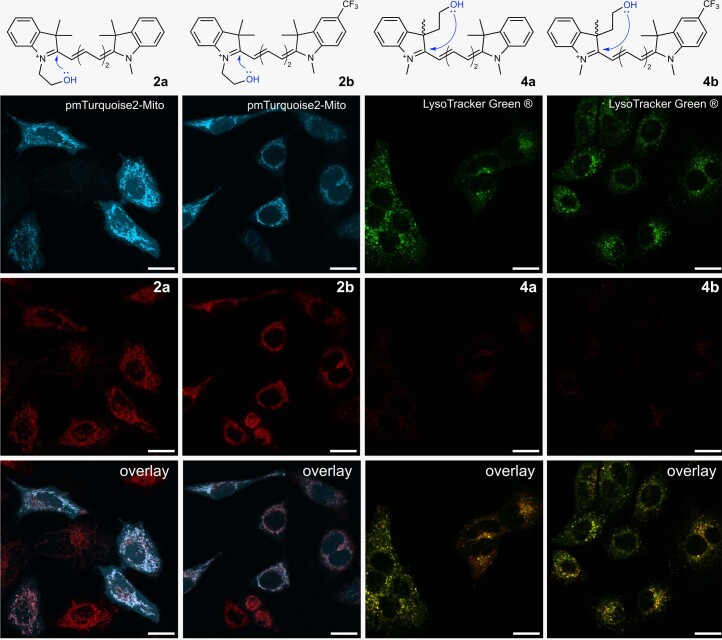
In live-cell imaging experiments (Fig. 2c), the 5-endo-trig probes 2a and 2b exhibited bright fluorescence in mitochondria (Fig. 2c and Extended Data Fig. 2). This subcellular localization is probably a consequence of the delocalized positive charge of the open Cy5 scaffold. By contrast, 5-exo-trig probes 4a and 4b exhibited only very faint fluorescence, which was mostly localized in the lysosomes (Fig. 2c and Extended Data Fig. 2). These results confirm that the closed form of the probes that undergo 5-exo-trig cyclizations (4a and 4b) is very stable under physiological conditions and greatly minimizes non-specific background in live-cell imaging.
Extended Data Fig. 2. Subcellular localization of untargeted dyes 2a, 2b, 4a, and 4b.
For 5-endo-trig probes 2a and 2b, HeLa cells were transfected with a plasmid encoding for COX8A-mTurquoise2 and incubated with 250 nM 2a or 2b for 2 h. For 5-exo-trig probes 4a and 4b, HeLa cells were incubated with 250 nM 4a or 4b for 2 h. LysoTracker Green® (100 nM) was added 15 min prior to imaging. Scale bars: 15 µm. Micrographs are representative of three independent biological samples measured over three separate imaging sessions.
We next investigated whether we could induce fluorescence turn-on upon conjugation of dye 4b to a self-labelling protein tag. Given that HaloTag has been thoroughly optimized for rhodamine-based dyes2,41, we chose to work with SNAP-tag42, thereby providing an orthogonal system that could be used in multiplexing studies with rhodamines and HaloTag. We synthesized the azide-modified SNAP-tag ligand 10 using the diazotizing reagent FSO2N3 (ref. 43) and combined it with alkyne-modified Cy5 dye 4c in a click reaction to generate probe 11 (Fig. 2d and Methods). This dye displayed a 10-fold turn-on in absorbance and a 21-fold turn-on in fluorescence when incubated with purified SNAP-tag protein (Fig. 2e and Methods), demonstrating its fluorogenicity upon binding to a self-labelling tag. Live-cell experiments using SNAP-tag fused to a fragment of histone H2B demonstrated that although the cellular uptake of compound 11 is not enhanced compared to its 5-endo-trig derivative, it labels SNAP-tag more efficiently and display much less non-specific signal (Supplementary Fig. 3).
Importantly, probe 11 was designed to mimic the cyclization equilibrium in HMSiR (1), which is a spontaneously blinking fluorophore useful for SMLM. Thus, to assess the spontaneous blinking properties of probe 11, we expressed SNAP-tag fused to β-tubulin in HeLa cells and treated these cells with compound 11 (Methods and Supplementary Tables 2 and 3). We performed live-cell SMLM imaging in growth medium without any blinking or anti-fading agents. These experiments revealed that probe 11 indeed displays spontaneous blinking, allowing for the acquisition of super-resolved fluorescence images (Fig. 2f). Single molecules of probe 11 could be localized with a precision of 18 ± 6 (mean ± s.d.) nm, and widths of about 60 nm were measured for microtubules in living cells (Fig. 2f). These results are comparable to those obtained with HMSiR (1)36.
A fluorogenic Cy5 derivative
We next applied the 5-exo-trig ring-closing strategy to develop a no-wash, fluorogenic Cy5 derivative. After synthesizing a few derivatives and testing the effect of pH and polarity on their cyclization equilibrium (Supplementary Discussion 1), we transformed the methyl ester group of 9c into N-methyl amide 12 and prepared the corresponding 5-endo-trig indoleninium 13 and Cy5 probe 14 (Fig. 3a). These alkyne-bearing derivatives were conjugated to benzyl guanine 10 to generate probes 15 and 16 (Fig. 3b). Incubation of these dyes with purified SNAP-tag protein led to a 6-fold increase in absorbance and 19-fold increase in fluorescence for compound 15. The larger turn-on in fluorescence indicates that binding to SNAP-tag not only induces ring opening but also increases the quantum yield of emission of compound 15 compared with the free dye in solution (Supplementary Table 4). In comparison, compound 16 underwent only a 1.4-fold and 2.5-fold increase in absorbance and fluorescence, respectively, upon binding to SNAP-tag (Fig. 3c). The in vitro fluorogenicity of probe 15 is comparable with that of the popular JF646 dye functionalized with a benzyl guanine (JF646-BG) for SNAP-tag labelling (Fig. 3c). This observation confirms that the 5-exo-trig cyclization of polymethine dyes can be reversed upon binding to protein targets, analogously to the turn-on mechanism of rhodamines.
Fig. 3. Synthesis, in vitro evaluation and live-cell validation of fluorogenic Cy5 derivatives.
a, Synthesis of 5-exo-trig and 5-endo-trig Cy5 derivatives with an N-methyl amide as a ring-closing moiety. b, Structures of compounds 15 and 16 functionalized with benzyl guanine for SNAP-tag labelling. c, Protein-binding turn-on of 15, 16 or JF646-BG with SNAP-tag protein. For protein-binding studies, probes (2.5 µM) were incubated with 5 µM purified SNAP-tag protein in PBS for 1.5 h at pH 7.4. d, No-wash, live-cell imaging of HeLa cells that were transfected with H2B-SNAPf-mTurquoise2 (upper images) or with the indicated plasmid (lower images) and incubated with 15 (50 nM), 16 (50 nM) or JF646-BG (50 nM) as indicated. The calibration bars indicate raw pixel values. e, Structure, protein-binding turn-on and no-wash live-cell imaging in HeLa cells of 17-actin. The concentration of 17-actin was 2.5 µM for protein-binding studies and 250 nM for cell imaging. f, Structure, DNA-binding turn-on and live-cell imaging in HeLa cells of 18-DNA. The concentration of 18-DNA was 1 µM for DNA-binding studies and 500 nM for cell imaging. Scale bars, 15 µm. Spectra are the mean from three independent experiments and the lines indicate absorbance (solid) or fluorescence (dotted) of the free dye in solution (grey) or the dye–macromolecule conjugate. Micrographs are representative of three independent biological samples measured over three separate imaging sessions.
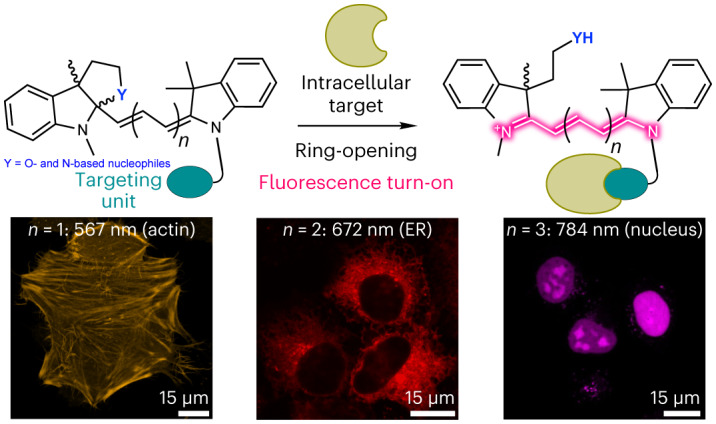
We then performed no-wash, live-cell imaging to compare the performance of probes 15, 16 and JF646. We transfected HeLa cells with a plasmid that encodes the fusion protein H2B-SNAPf-mTurquoise2 and incubated them with dyes 15, 16 or JF646-BG (Fig. 3d). Probe 15 exhibited bright fluorescence signal in the nucleus and only very faint unspecific background signals. This performance was comparable with that of JF646-BG (Fig. 3d). Probe 16, on the other hand, displayed a >10-fold-weaker fluorescence signal, indicating that it might be less membrane-permeant (Fig. 3d). Furthermore, probe 16 exhibited most of its fluorescent signal in vesicles, confirming that 5-endo-trig Cy5 derivatives are not suitable as no-wash turn-on dyes. Finally, we tested the generality of labelling with probe 15 by transfecting HeLa cells with plasmids encoding for an untargeted SNAPf-mTurquoise2 fusion protein (whole cell), LifeAct-SNAPf-mTurquoise2 (actin) or TOM20-SNAPf-mTurquoise2 (mitochondria). In all cases, labelling was specific, as judged by the excellent overlay between the signal of the reference fluorescent protein mTurquoise2 and that of 15 linked to SNAP-tag (Fig. 3d).
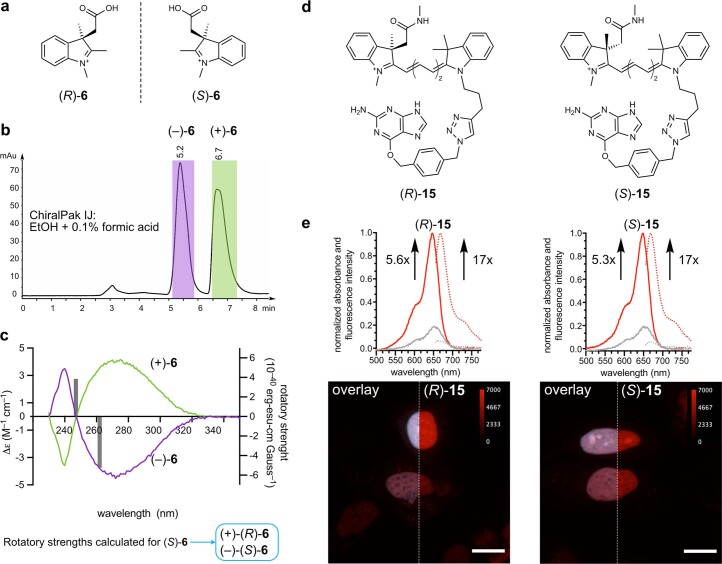
The 5-exo-trig ring-closure design leads to an indolenine fragment that contains a chiral carbon (Extended Data Fig. 3). To investigate the effect of stereochemistry on the ring opening of the dye upon binding to SNAP-tag, we separated the enantiomers of 6, (+)-6 and (–)-6 using a chiral stationary phase. We employed electronic circular dichroism and time-dependent density functional theory (TD-DFT)44 to assign the absolute configuration of the two enantiomers (Extended Data Fig. 3 and Methods), and found that (–)-6 has S absolute configuration ((+)-(R)-6 and (–)-(S)-6). We used each enantiomer separately to prepare enantiomerically pure probes (R)-15 and (S)-15. We did not observe a substantial difference in fluorogenicity upon binding of each enantiomer to either purified SNAP-tag or in live-cell imaging (Extended Data Fig. 3). We hypothesize that the relatively long distance between the chiral centre of the dye and the protein surface alleviates any potential enantioselective binding interactions; however, we argue that such interactions could be leveraged to further increase the fluorogenicity of 5-exo-trig polymethine probes upon binding to chiral targets.
Extended Data Fig. 3. Separation and evaluation of enantiomers (S)-15 and (R)-15.
a) Structure of the enantiomers (S)-6 and (R)-6. b) Separation of enantiomers (S)-6 and (R)-6 by HPLC under the conditions indicated. c) Determination of the absolute configuration of the enantiomers of 6 by electronic circular dichroism and comparison with the calculated rotatory strengths (grey bars) of (S)-6 using TD-DFT (CAM-B3LYP/DGTZVP). Spectra were measured once. d) Structures of the enantiomeric dyes (S)-15 and (R)-15. e) Protein-binding turn-on of probes (S)-15 and (R)-15 (2.5 µM) incubated with 5 µM purified SNAP-tag protein in PBS, pH 7.4, for 1.5 h. No-wash confocal microscopy of live HeLa cells transfected with H2B-SNAPf-mTurq2 and incubated with (S)-15 (50 nM) and (R)-15 (50 nM). Scale bars: 15 µm. Spectra are the mean of three independent experiments and micrographs are representative of three independent biological samples measured over three separate imaging sessions. Grey lines indicate absorbance (solid line) or fluorescence (dotted line) of the free dye in solution whereas coloured lines indicate absorbance (solid line) or fluorescence (dotted line) of the dye-protein conjugate.
We next tested whether binding to other macromolecular targets also induced fluorescence turn-on in probe 15. For this purpose, we prepared compounds 17-actin and 18-DNA. These probes were composed of a 5-exo-trig Cy5 derivative linked through a triazole-containing alkane to either the actin-binding cyclo-depsipeptide jasplakinolide (Fig. 3e)45,46 or to Hoechst 33342 (Fig. 3f)—a dye that binds to the minor groove of double-stranded DNA (dsDNA) and has been used to target fluorogenic rhodamine dyes to the cell nucleus47. Probe 17-actin showed fluorogenic behaviour with an 11-fold turn-on in absorbance and 313-fold turn-on in fluorescence upon binding to actin filaments in vitro (Fig. 3e). The much larger fluorescence turn-on compared to absorbance increase indicates that binding to actin considerably enhances the quantum yield of carbocyanine 17. In live-cell imaging experiments, 17-actin labelled actin fibres selectively in live, unmodified HeLa cells. Similar results were obtained for 18-DNA, which displayed a 7-fold increase in absorbance and 32-fold increase in fluorescence upon binding to double-stranded DNA (Fig. 3f). Live-cell imaging also confirmed specific staining of the cell nucleus (Fig. 3f). These results demonstrate that the fluorogenicity of 5-exo-trig Cy5 derivatives is not limited to SNAP-tag, and other macromolecules can also induce fluorescence turn-on.
Fluorogenic Cy3 and Cy7 derivatives
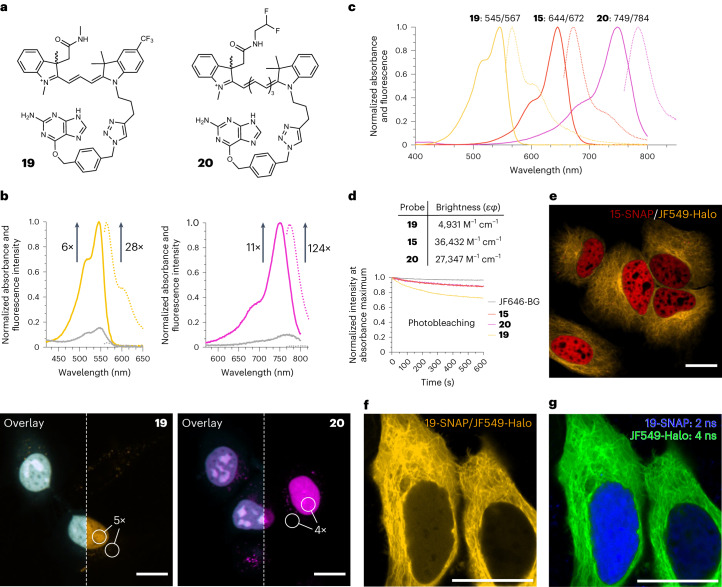
Carbocyanine dyes can cover a large spectral range by varying the number of conjugated carbon atoms in between the two indoleninium moieties. We therefore explored whether the 5-exo-trig fluorogenic strategy could be extended to Cy3 and Cy7 dyes, providing fluorophores for two extra imaging channels. We suspected that the Cy3 derivative would have a higher LUMO energy than Cy5, whereas the opposite would be true for the Cy7 derivative; thus, we expected the Cy3 dyes to be more likely to adopt the open form than Cy5 dyes, whereas Cy7 dyes would tend to adopt the closed form. To balance these trends, we added a CF3 moiety on the capping indoleninium to favour the closed form of Cy3. Similarly, we replaced the nucleophilic N-methyl amide with an electron-deficient amide to facilitate ring-opening in our Cy7 design. From a synthetic point of view, compared with the preparation of Cy5 derivatives, we only changed the commercially available linker and adjusted the temperatures during the microwave-assisted protocol (Supplementary Information).
We prepared Cy3 derivative 19 and Cy7 derivative 20 (Fig. 4a) for SNAP-tag labelling. Both probes displayed fluorogenic behaviour upon binding to purified SNAP-tag protein, with increases in absorbance of 6-fold for 19 and 11-fold for 20, and increases in fluorescence of 28-fold for 19 and 124-fold for 20 (Fig. 4b). Similar to compound 15, the large turn-on in fluorescence indicates that binding to SNAP-tag considerably increases the quantum yield of emission of these probes compared with the free dyes in solution (Supplementary Table 4). Probes 19 and 20 were then used in no-wash live-cell imaging experiments with HeLa cells transiently transfected with the H2B-SNAPf-mTurquoise2 plasmid described before. We observed excellent co-localization of the mTurquoise2 reference signal with the yellow and near-infrared fluorescence signals of 19 and 20, respectively, confirming the specificity of the probes (Fig. 4b). Labelling of other cellular structures was performed with similar results (Extended Data Fig. 4). Probes 15, 19 and 20 cover a substantial portion of the visible spectrum and reach into the near-infrared region. Probes 15 and 20 display excellent brightness and good photostability when bound to SNAP-tag (Fig. 4c,d, Supplementary Table 4, and Supplementary Fig. 4 and 5). Cy3 derivative 19 is not as bright or photostable, but these properties could be further tuned by introducing substituents or by making the polymethine chain more rigid48.
Fig. 4. Synthesis, in vitro evaluation and live-cell validation of fluorogenic Cy3 and Cy7 derivatives.
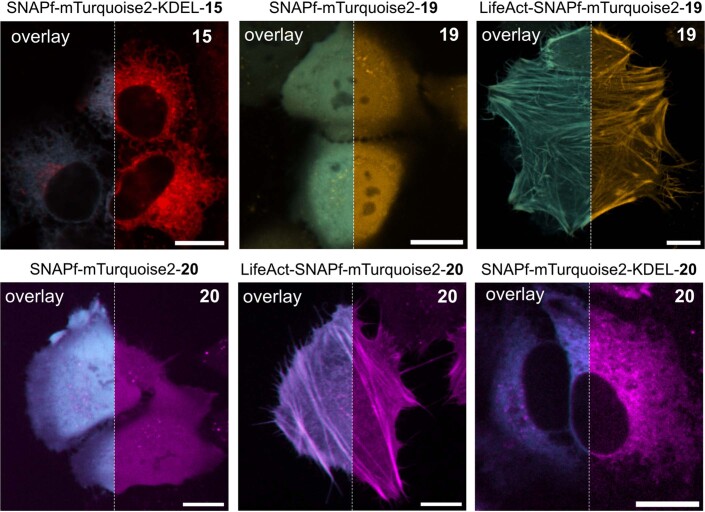
a, Structures of Cy3 and Cy7 derivatives 19 and 20. b, SNAP-tag-binding turn-on and no-wash live-cell imaging of HeLa cells transfected with H2B-SNAPf-mTurquoise2 and treated with 19 (50 nM) or 20 (250 nM). The lines indicate absorbance (solid) or fluorescence (dotted) of the free dye in solution (grey) or the dye–protein conjugate (coloured). Spectra are the mean from three independent experiments. Scale bars, 15 µm. c, Normalized absorbance and fluorescence spectra of Cy3 19, Cy5 15 and Cy7 20 measured in ethanol + 0.1% TFA. Spectra represent single measurements. d, Brightness of SNAP-tag adducts in PBS, pH 7.4 and photobleaching curves measured for SNAP-tag adducts at a concentration of 50 nM in PBS following irradiation at the maximum excitation wavelength with monochromatic light (1.2 mW power). Photobleaching curves represent the mean from three independent experiments. e, Multicolour no-wash imaging in live HeLa cells co-transfected with H2B-SNAPf-mTurquoise2 and TUBB5-Halo, and incubated with 15 (50 nM) and JF549-Halo (50 nM). Scale bar, 15 µm. f, Total intensity measured in a cell co-transfected with H2B-SNAPf-mTurquoise2 and TUBB5-Halo and incubated with 19 (50 nM) and JF549-Halo (10 nM). Scale bar, 20 µm. g, Fluorescence lifetime multiplexing of the cell shown in f. Scale bar, 20 µm. All micrographs are representative of three independent biological samples measured over three separate imaging sessions.
Extended Data Fig. 4. Additional cellular targeting of probes 15, 19 and 20.
No-wash confocal microscopy of live HeLa cells transfected with SNAPf-mTurquoise2-KDEL, SNAPf-mTurquoise2 or LifeAct-SNAPf-mTurquoise2 and incubated with 15 (50 nM), 19 (50 nM), or 20 (250 nM). The overlay shows the signal of the reference fluorescent protein mTurquoise2 and that of the indicated compound linked to SNAP-tag. Scale bars: 15 µm. Micrographs are representative of three independent biological samples measured over three separate imaging sessions.
Finally, we applied our probes to multiplexing experiments. First, we performed multicolour imaging experiments using the fluorogenic Cy5 dye 15 in combination with the widely used JF549-Halo dye. We confirmed the orthogonality of our fluorogenic probe with rhodamine-HaloTag conjugates by imaging the 15/JF549-Halo pair in live HeLa cells co-transfected with H2B-SNAPf-mTurquoise2 and TUBB5-HaloTag (Fig. 4e). We next explored whether the fluorescence signals of the Cy3 derivative 19 and JF549-Halo—which have nearly identical excitation and emission wavelengths—could be separated by their excited-state lifetimes. We co-transfected HeLa cells with H2B-SNAPf-mTurquoise2 and TUBB5-HaloTag, and performed fluorescence lifetime imaging (FLIM). Although the signals could not be separated by their wavelength (Fig. 4f), they could be easily distinguished by their average excited-state lifetimes (2 ns for 19 and 4 ns for JF549-Halo, Fig. 4g) using phasor plot analysis.
Conclusion
We presented a general strategy to impart fluorogenicity to polymethine dyes via a 5-exo-trig ring-closure approach. These dyes, regardless of their excitation wavelength, can be readily synthesized in two high-yielding steps and are easy to derivatize by varying the indoleninium building blocks or the ring-closing moiety. We have illustrated the potential of our fluorogenic polymethine scaffold by generating a spontaneously blinking Cy5 dye, fluorogenic Cy3 and Cy5 dyes, and a bright and photostable near-infrared fluorogenic Cy7. Cy7 derivative probe 20 is particularly interesting due to its high brightness (εφ: product of extinction coefficient and quantum yield) and long emission wavelength (εϕ = 27,300 M–1 cm–1 and λem = 784 nm), particularly when compared with fluorescent proteins in the same spectral range (for example, miRFP718nano, εϕ = 4,500 M–1 cm–1 and λem = 718 nm; ref. 49). Furthermore, we showed that these probes can be used for no-wash confocal live-cell microscopy and SMLM; it can also be used in combination with the widely used rhodamine-HaloTag conjugates in multicolour and multiplexed lifetime imaging experiments.
We demonstrated the versatility of our turn-on strategy by using the self-labelling protein tag SNAP-tag, as well as jasplakinolide or Hoechst 33342 dye, to drive fluorescence turn-on. The mechanism of fluorescence turn-on of 5-exo-trig to such varied macromolecular targets remains to be fully elucidated. Preliminary modelling results suggest that ring-opening could be triggered by specific interactions (for example, hydrogen bonds) between the lactam rings and ubiquitous amides in proteins or phosphate groups in nucleic acids (Supplementary Discussion 2). More detailed studies including molecular dynamics simulations and site-directed mutagenesis could shed further light on the mechanism of fluorescence turn-on for specific 5-exo-trig polymethine dyes.
Given the high modularity of polymethine dyes, the spectral range can be further extended into the green (for example, Cy1 dyes50) as well as into the shortwave infrared (for example, Cy9 dyes51) wavelengths. Furthermore, the photophysical properties and fluorogenicity of polymethine dyes could be further tuned by varying the substituents on the indolenines or the linker13. We envision that this simple, yet general, method will be used to develop improved fluorogenic probes, facilitating new bioimaging experiments.
Methods
General remarks
All reagents were purchased from commercial sources and used as received. Anhydrous solvents were procured from Acros Organics and used as received. Nuclear magnetic resonance spectra were acquired on a Bruker 400 or 600 instrument using TopSpin 4.2.0 and analysed with MestreNova 14.2. The 1H NMR chemical shifts are reported in parts per million relative to SiMe4 (δ = 0) and were referenced internally with respect to residual protons in the solvent (δ = 7.26 for CDCl3, δ = 1.94 for CD3CN, δ = 3.31 for CD3OD, and δ = 2.50 for (CD3)2SO). Coupling constants are reported in hertz. The 13C NMR chemical shifts are reported in parts per million relative to SiMe4 (δ = 0) and were referenced internally with respect to solvent signal (δ = 77.16 for CDCl3, δ = 1.32 for CD3CN, δ = 49.00 for CD3OD and δ = 39.52 for (CD3)2SO). High-resolution mass spectra were acquired on a timsTOF Pro TIMS-QTOF LC/MS spectrometer (Bruker Daltonics GmbH) by using electrospray ionization and analysed using Xcalibur v.4.2 software. IUPAC names of all compounds are provided and were determined using CS ChemBioDrawUltra v.16.0.
Computational modelling
All calculations were performed using Gaussian 09 at the B3LYP/DGTZVP level of theory as well as at the M06-2X/DGTZVP level. An implicit solvation model (IEFPCM) was used to simulate the effect of an aqueous environment. All stationary states were characterized by harmonic analysis at the same level of theory. All minima displayed zero imaginary frequencies and all transition states gave one imaginary frequency along the C–O bond elongation coordinate. Energies were corrected by zero-point energy.
Optical spectroscopy
Stock solutions were prepared in DMSO (spectrophotometric grade >99.9%) at concentrations of 5 mM, 1 mM and 50 μM and stored at –20 °C. Spectroscopic measurements were conducted in PBS. Ultraviolet–visible spectra were acquired using a Multiskan SkyHigh Microplate Spectrophotometer (ThermoFisher Scientific) and quartz cuvettes from ThorLabs (10 mm path length).
Buffered aqueous solutions in the pH range of 2 to 8 were prepared by mixing citric acid (0.1 M) and sodium dihydrogen phosphate NaH2PO4 (0.2 M) in ultrapure water. Buffered aqueous solutions in the pH range of 9 to 11 were prepared by mixing sodium bicarbonate (0.1 M) and sodium carbonate (0.1 M) in ultrapure water. Buffered aqueous solutions in the pH range of 12 to 13 were prepared by mixing potassium chloride KCl (0.2 M) and sodium hydroxide (0.2 M); 5 μM solutions of the dyes in the buffered aqueous solutions were prepared and the absorbance spectra were recorded after 1.5 h in triplicates using 96-well plates (Corning) on the MultiSkan SkyHigh microplate reader. The obtained ultraviolet–visible spectra were background corrected and the absorbance maxima of each pH value were normalized. Normalized absorbance values were plotted against the pH value and fitted (non-linear fit, sigmoidal, 4PL) using Prism 9.
Solutions of different dielectric constant were prepared by making water-dioxane mixtures containing 10% (ε = 72.02), 20% (ε = 63.50), 30% (ε = 54.81), 40% (ε = 45.96), 50% (ε = 36.89), 60% (ε = 28.09), 70% (ε = 19.73), 80% (ε = 12.19) or 90% (ε = 6.23) dioxane (by volume) in water52.
We prepared 10 μM solutions of the dyes in the dioxane–water mixtures and the absorbance spectra were recorded in triplicates using 96-well plates (Corning) on a MultiSkan SkyHigh microplate reader. The obtained ultraviolet–visible spectra were background-corrected and the absorbance maxima of each dioxane-water mixture were normalized. Normalized absorbance values were plotted against the dielectric constant and fitted (non-linear fit, sigmoidal, 4PL) using Prism 9.
Extinction coefficients were obtained by measuring the absorbance spectra at various concentrations between 1 µM and 15 µM. The absorbance maxima were plotted against the corresponding concentration and fitted (simple linear regression) using Prism 9.
Fluorescence spectra were acquired using an FS5 Spectrofluorometer operated with Fluoracle software (Edinburgh Instruments) equipped with an SC-25 cuvette holder or SC-40 plate reader. Absolute fluorescence quantum yields were determined using 1 µM solutions employing an integrating sphere (SC-30, Edinburgh instruments). All spectroscopic measurements were performed in triplicate and at room temperature.
Photobleaching experiments were performed using a FS5 Spectrofluorometer equipped with an SC-25 cuvette holder. Protein adducts were measured at a 50 nM concentration and the emission slit width was adjusted individually for each dye to achieve a power of 1.2 mW (547 nm, 12 nm; 646 nm and 654 nm, 22.5 nm; 751 nm, 29.9 nm). The emission slit width was set to 0.75 nm and the fluorescence was measured every second for 10 min while keeping the shutter always open. The power was measured at the cuvette holder using a PMD100D compact power and energy meter console equipped with a S120VC standard photodiode power sensor (UV-Extended Si, 200–1,100 nm, 50 mW, Thorlabs GmbH).
Cloning
All plasmids were cloned by Gibson assembly. DNA encoding SNAPf or H2B-SNAPf was amplified from pSNAPf-H2B control plasmid (Addgene no. 101124) to generate an insert. To generate a backbone containing an organelle-specific targeting group and a fluorescent protein, the plasmids pmTurquoise2-ER (Addgene no. 36204) and pmTurquoise2-Golgi (Addgene no. 36205) were used. Primers for amplification (minimum 15 overlapping base pairs) were generated using SnapGene and modified manually to minimize secondary structures, self-dimers and repeated motifs. The designed primers were supplied by Microsynth AG (Switzerland) and are reported in Supplementary Tables 2 and 3. Vector and insert fragments were amplified by PCR using a Phusion High-Fidelity PCR Master Mix with HF buffer from New England Biolabs (NEB). The fragments were analysed by agarose gel electrophoresis and template DNA was digested using DpnI. PCR fragments were purified using a QIAquick PCR purification kit (Qiagen) according to the manufacturer’s instructions. The Gibson assembly reaction was performed using the Gibson Assembly Master Mix (NEB) according to the manufacturer’s protocol. DH5α competent Escherichia coli cells (NEB, C2987I) were transformed with the assembly product by heat shock following the manufacturer’s instructions, streaked onto lysogeny broth (LB) agar plates containing kanamycin (50 μg ml–1), and incubated at 37 °C for 24 h. Single colonies were selected and grown in LB liquid medium containing 50 μg ml–1 kanamycin at 37 °C for 16 h. Plasmid DNA was isolated using the QIAprep spin miniprep kit (Qiagen) according to the manufacturer’s instructions. The correct sequence of the gene of interest was verified by the Sanger sequencing service of Microsynth using the standard sequencing primers CMV-F, SV40pA-R, and EGFP-C. All plasmids produced in this paper are available on Addgene (no. 197494–98).
Expression and purification of fSNAP protein
pET24b-6His-fSNAP (Addgene no. 106999) was transformed into E. coli strain Rosetta2(DE3) (Merck, 71397) and streaked on an agar plate with kanamycin and chloramphenicol resistance. A single colony was picked, inoculated in 80 ml LB cultures containing 50 μg ml–1 kanamycin and 25 μg ml–1 chloramphenicol, and grown at 37 °C for 24 h. We innoculated 40 ml of the starter culture into 4 l of medium and shaken at 37 °C to an optical density at 600 nm (OD600) of 0.8. Expression was induced by the addition of 0.5 mM isopropyl β-d-thiogalactopyranoside, and cells were grown at 18 °C for 16 h. Cells were harvested by centrifugation, resuspended in HEPES buffer (300 mM NaCl, 20 mM HEPES, pH = 7.5), and supplemented with turbonuclease (20 μl) and a protease inhibitor cocktail tablet (Roche). Cells were lysed by sonication (70% power, 10 s pulse/10 s pulse off for 2 min, 30 s) and the lysate was cleared by centrifugation. The protein was purified by Ni-His-affinity column chromatography and the fractions were analysed by SDS–PAGE. The fractions containing the protein were concentrated using Amicon Ultra-4 and Ultra-15 centrifugal filter units (MWCO 10 kDa) and further purified by size-exclusion chromatography. The correct size and purity of the protein were verified by SDS–PAGE analysis. Purified SNAP protein was stored in aliquots in a buffer containing 150 mM NaCl, 20 mM HEPES, pH 7.4, 1 mM DTT at −80 °C.
Protein-binding turn-on assays
Prior to biological assays with purified SNAP-tag protein, the buffer was exchanged to PBS using Zebra Spin Desalting Columns (Thermo Scientific) according to the manufacturer’s protocol. SNAP-dyes (1 mM in DMSO) were either added to PBS alone or to a 5 μM SNAP-protein solution in PBS. The final concentration of SNAP-dyes was 2.5 μM and the resulting mixtures were incubated at 37 °C for 1.5 h.
The turn-on assay with actin was performed according to a published procedure45. Actin ligand 17-actin (1 mM in DMSO) was added to either supplemented actin buffer alone or in the presence of 0.4 mg ml−1 G-actin (catalogue no. AKL99, Cytoskeleton). The final ligand concentration was 2.5 μM. The supplemented actin buffer contained 5 mM Tris-HCl pH 8.0, 0.2 mM CaCl2 (from General Actin Buffer, catalogue no. BSA01, Cytoskeleton), 50 mM KCl, 2 mM MgCl2, 5 mM guanidine carbonate and 1.2 mM ATP (from Actin Polymerization Buffer, catalogue no. BSA02, and ATP, catalogue no. BSA04, Cytoskeleton). Samples were incubated at 37 °C for 2 h.
The DNA-binding assay was performed according to a published procedure47. The hairpin-forming 28-bp DNA oligonucleotide was purchased from Microsynth (5’-CGCGAATTCGCGTTTTCGCGAATTCGCG-3’) and dissolved in Tris-buffered saline (TBS, 50 mM Tris-HCl, 150 mM NaCl, pH 7.4) at 1 mM concentration. The folding of the DNA into the secondary hairpin structure was achieved by heating the DNA at 75 °C for 2 min followed by slowly cooling to 25 °C. Nucleus ligand 18-DNA (1 mM in DMSO) was incubated with either TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) alone or in the presence of 50 μM hairpin DNA at room temperature for 1 h. The final ligand concentration was 1 μM.
Absorbance spectra were recorded in 96-well plates (Corning) on the Multiskan SkyHigh microplate reader and fluorescence measurements were performed on the FS5 Spectrofluorometer (Edinburgh Instruments) equipped with a SC-40 plate holder. All spectroscopic measurements were carried out in triplicates and at room temperature.
Cell culture and fluorescence imaging
HeLa cells (CLS, 300194CP5) were grown in Dulbecco’s Modified Eagle Medium supplemented with foetal bovine serum (10%) and penicillin-streptomycin (1%) at 37 °C in a 95% humidity atmosphere under a 5% CO2 environment. The cells were grown to 90% confluency before seeding at a density of 15–20 000 cells per millilitre onto Ibidi μ-Slide 8-well glass-bottom plates 48 h before the imaging experiment. Cells were transfected with the plasmids H2B-SNAPf-mTurquoise2 (Addgene no. 197494), LifeAct-SNAPf-mTurquoise2 (no. 197497), TOM20-SNAPf-mTurquoise2 (no. 197496), SNAPf-mTurquoise2-KDEL (no. 197498) or SNAPf-mTurquoise2 (no. 197495) using jetPrime according to the manufacturer’s protocol 24 h before imaging. Cells were incubated with the respective probes in FluoroBrite Dulbecco’s Modified Eagle Medium for 1.5 h and imaged directly.
Confocal microscopy
Confocal imaging was performed with a Nikon W1 spinning disk microscope operated with NIS Elements AR software equipped with a CMOS camera (Photometrix). Brightfield imaging was performed with a white LED. Laser lines and filters were set up for the appropriate channel as described in Supplementary Table 5.
Images were collected using a CFI Plan Apochromat Lambda D oil immersion objective (60×, NA = 1.4). Channels were imaged sequentially. The microscope was operated using NIS Elements. Imaging experiments were performed at 37 °C in a 5% CO2 environment. Images were analysed by Fiji/ImageJ.
Determination of the absolute configuration of (+)−6 and (–)−6
Circular dichroism spectra were measured with a Chirascan V100 CD spectrometer (Applied Photophysics) and operated using the Chirascan v.4.2 software. For circular dichroism measurements, 60 μM solutions of (+)-6 and (–)-6 in PBS were used. Optical rotation measurements were performed with a Jasco P-2000 polarimeter and operated using the Spectra Manager CFR software.
For TD-DFT calculations, we first performed a systematic conformational search at the B3LYP/DGTZVP level of theory with an implicit solvation model (IEFPCM) by varying all rotatable bonds in 60° steps. We then applied the Boltzmann distribution to the set of low-energy minima obtained by using the free energy differences, and considered the structures above the 0.1% population threshold for the TD-DFT calculation. Time-dependent density functional theory calculations were performed at the CAM-B3LYP/DGTZVP level of theory with IEFPCM as the solvation model.
Fluorescence lifetime imaging
Fluorescence lifetime imaging was performed on a Leica SP8 inverse FALCON confocal laser scanning microscope and operated using the Leica LAS X Navigator. Images were collected using an HC PL APO corr CS2 oil objective (63×, NA = 2.4) and the RHOD fluorescence filter set (excitation = 546/10, emission = 585/40). Phasor analysis of the FLIM data was performed with the Leica LAS X Phasor License.
Single-molecule localization microscopy
HeLa cells (CLS, 300194CP5) were transfected with β-tubulin-SNAP plasmid using jetPrime according to the manufacturer’s protocol 48 h before imaging. Cells were incubated with probe 11 (100 nM) for 14 h, then detached and transferred to a new Ibidi slide. Cells were left for 10 h to adhere to the glass surface, and the medium was exchanged to FluoroBrite prior to imaging. Single-molecule imaging was performed on a Ti2 eclipse inverted microscope (Nikon) equipped with a water-cooled iXon 888 Ultra EMCCD camera (Andor) at 37 °C in a 5% CO2 environment. Acquisitions were performed at 638 nm (90 mW, 30 ms) and a single band 708/75 emission filter was used. 2000 frames were acquired. Acquisitions were collected using a NIKON 100x TIRF Apo Plan SR oil objective (NA = 1.49). All laser and camera shutters were controlled by a NIDAQ oscilloscope (National Instruments) controller unit. The single-molecule signal was fitted with 2D Gaussian point spread functions using ThunderSTORM. The localization threshold was set to 1.1*std(Wave.F1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-023-01367-y.
Supplementary information
Supplementary Discussions 1 and 2, Figs. 1–69, Tables 1–5, synthetic procedures and plasmid maps.
Source data
pH profiles, absorbance and fluorescence spectra, and SMLM pixel value data from line profiles.
Absorbance and fluorescence data for macromolecule-binding turn-on experiments.
Absorbance and fluorescence spectra, SNAP-tag binding turn-on experiments and photobleaching data.
Circular dichroism, absorbance and fluorescence spectra following SNAP-tag binding.
Acknowledgements
This work was supported by EPFL (SViPhD internal grant, P.R.-F.), University of Zurich, and the European Research Council (ERC Starting Grant HDPROBES no. 801572 to P. R.-F.). We thank S. Emmert for assistance with FLIM, L. Blatti (University of Zurich) for the synthesis of some indoleninium building blocks, K. Gademann for access to a polarimeter, and P. Gönczy and L. Reymond for valuable discussions. This work made use of infrastructure services provided by SCITAS, the Scientific IT and Application Support of EPFL, and the Service and Support for Science IT (S3IT) at the University of Zurich. Samples of jasplakinolide and Hoechst 33342 ligands were donated by Spirochrome AG (https://spirochrome.com/). FLIM was performed at the Center for Microscopy and Image Analysis of the University of Zurich. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Extended data
Author contributions
A.M. and P.R.-F. conceived the method, wrote the manuscript, perfomed computational modelling and analysed the results. A.M. performed all of the experiments. P. R.-F. acquired funding and supervised the project.
Peer review
Peer review information
Nature Chemistry thanks Kouichi Ohe, Francisco Raymo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All data supporting this paper, including coordinates for all calculated structures, are available through Zenodo53. X-ray crystallographic datasets used for modelling are available from the PDB under accession nos. 6Y8P and 1DNH. Samples of small-molecule probes are available from the authors on reasonable request. Source Data are provided with this paper.
Competing interests
EPFL and University of Zurich jointly filed a patent application (EP23153834.9) protecting the invention disclosed in this paper. A. M. and P. R.-F. are listed as co-inventors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41557-023-01367-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-023-01367-y.
References
- 1.Grimm JB, Lavis LD. Caveat fluorophore: an insiders’ guide to small-molecule fluorescent labels. Nat. Methods. 2022;19:149–158. doi: 10.1038/s41592-021-01338-6. [DOI] [PubMed] [Google Scholar]
- 2.Los GV, et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 3.Keppler A, et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 4.Lavis LD. Teaching old dyes new tricks: biological probes built from fluoresceins and rhodamines. Annu. Rev. Biochem. 2017;86:825–843. doi: 10.1146/annurev-biochem-061516-044839. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Frei MS, Salim A, Johnsson K. Small-molecule fluorescent probes for live-cell super-resolution microscopy. J. Am. Chem. Soc. 2018;141:2770–2781. doi: 10.1021/jacs.8b11134. [DOI] [PubMed] [Google Scholar]
- 6.Urano Y, et al. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J. Am. Chem. Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 7.Lukinavičius G, et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013;5:132–139. doi: 10.1038/nchem.1546. [DOI] [PubMed] [Google Scholar]
- 8.Grimm JB, et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods. 2015;12:244–250. doi: 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, et al. A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy. Nat. Chem. 2020;12:165–172. doi: 10.1038/s41557-019-0371-1. [DOI] [PubMed] [Google Scholar]
- 10.Grimm JB, et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods. 2017;14:987–994. doi: 10.1038/nmeth.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm JB, et al. A general method to optimize and functionalize red-shifted rhodamine dyes. Nat. Methods. 2020;17:815–821. doi: 10.1038/s41592-020-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorka AP, Nani RR, Schnermann MJ. Harnessing cyanine reactivity for optical imaging and drug delivery. Acc. Chem. Res. 2018;51:3226–3235. doi: 10.1021/acs.accounts.8b00384. [DOI] [PubMed] [Google Scholar]
- 13.Štacková L, Štacko P, Klán P. Approach to a substituted heptamethine cyanine chain by the ring opening of Zincke salts. J. Am. Chem. Soc. 2019;141:7155–7162. doi: 10.1021/jacs.9b02537. [DOI] [PubMed] [Google Scholar]
- 14.Altman RB, et al. Cyanine fluorophore derivatives with enhanced photostability. Nat. Methods. 2011;9:68–71. doi: 10.1038/nmeth.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gidi Y, et al. Unifying mechanism for thiol induced photoswitching and photostability of cyanine dyes. J. Am. Chem. Soc. 2020;142:12681–12689. doi: 10.1021/jacs.0c03786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ros-Lis JV, et al. Squaraines as fluoro-chromogenic probes for thiol-containing compounds and their application to the detection of biorelevant thiols. J. Am. Chem. Soc. 2004;126:4064–4065. doi: 10.1021/ja031987i. [DOI] [PubMed] [Google Scholar]
- 17.Ajayaghosh A. Chemistry of squaraine-derived materials: near-IR dyes, low band gap systems, and cation sensors. Acc. Chem. Res. 2005;38:449–459. doi: 10.1021/ar0401000. [DOI] [PubMed] [Google Scholar]
- 18.Karpenko IA, et al. Fluorogenic squaraine dimers with polarity-sensitive folding as bright far-red probes for background-free bioimaging. J. Am. Chem. Soc. 2015;137:405–412. doi: 10.1021/ja5111267. [DOI] [PubMed] [Google Scholar]
- 19.Cosco ED, et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 2020;12:1123–1130. doi: 10.1038/s41557-020-00554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosco ED, et al. Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection. J. Am. Chem. Soc. 2021;143:6836–6846. doi: 10.1021/jacs.0c11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujioka H, et al. Activatable fluorescent probes for hydrolase enzymes based on coumarin–hemicyanine hybrid fluorophores with large Stokes shifts. Chem. Commun. 2020;56:5617–5620. doi: 10.1039/d0cc00559b. [DOI] [PubMed] [Google Scholar]
- 22.Wrobel AT, Johnstone TC, Liang AD, Lippard SJ, Rivera-Fuentes P. A fast and selective near-infrared fluorescent sensor for multicolor imaging of biological nitroxyl (HNO) J. Am. Chem. Soc. 2014;136:4697–4705. doi: 10.1021/ja500315x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tirla A, Rivera-Fuentes P. Development of a photoactivatable phosphine probe for induction of intracellular reductive stress with single-cell precision. Angew. Chem. Int. Ed. 2016;55:14709–14712. doi: 10.1002/anie.201608779. [DOI] [PubMed] [Google Scholar]
- 24.Deniz E, Sortino S, Raymo FM. Fluorescence switching with a photochromic auxochrome. J. Phys. Chem. Lett. 2010;1:3506–3509. [Google Scholar]
- 25.Alander JT, et al. A review of Indocyanine Green fluorescent imaging in surgery. Int. J. Biomed. Imag. 2012;2012:1–26. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lelek, M. et al. Single-molecule localization microscopy. Nat. Rev. Methods Primer1, 39 (2021). [DOI] [PMC free article] [PubMed]
- 27.Demeter O, et al. Bisazide cyanine dyes as fluorogenic probes for bis-cyclooctynylated peptide tags and as fluorogenic cross-linkers of cyclooctynylated proteins. Bioconjug. Chem. 2017;28:1552–1559. doi: 10.1021/acs.bioconjchem.7b00178. [DOI] [PubMed] [Google Scholar]
- 28.Knorr G, et al. Bioorthogonally applicable fluorogenic cyanine-tetrazines for no-wash super-resolution imaging. Bioconjug. Chem. 2018;29:1312–1318. doi: 10.1021/acs.bioconjchem.8b00061. [DOI] [PubMed] [Google Scholar]
- 29.Usama SM, Inagaki F, Kobayashi H, Schnermann MJ. Norcyanine-carbamates are versatile near-infrared fluorogenic probes. J. Am. Chem. Soc. 2021;143:5674–5679. doi: 10.1021/jacs.1c02112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usama SM, et al. Targeted fluorogenic cyanine carbamates enable in vivo analysis of antibody–drug conjugate linker chemistry. J. Am. Chem. Soc. 2021;143:21667–21675. doi: 10.1021/jacs.1c10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miki K, et al. pH-Responsive near-infrared fluorescent cyanine dyes for molecular imaging based on pH sensing. Chem Comm. 2017;53:7792–7795. doi: 10.1039/c7cc03035e. [DOI] [PubMed] [Google Scholar]
- 32.Oe M, et al. pH-Responsive Cy5 dyes having nucleophilic substituents for molecular imaging. Tetrahedron Lett. 2018;59:3317–3321. [Google Scholar]
- 33.Mu H, et al. pH-Activatable cyanine dyes for selective tumor imaging using near-infrared fluorescence and photoacoustic modalities. ACS Sens. 2021;6:123–129. doi: 10.1021/acssensors.0c01926. [DOI] [PubMed] [Google Scholar]
- 34.Oe M, Miki K, Masuda A, Nogita K, Ohe K. An activator-induced quencher-detachment-based turn-on probe with a cationic substrate moiety for acetylcholinesterase. Chem. Commun. 2022;58:1510–1513. doi: 10.1039/d1cc05132f. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin, J. Rules for ring closure. J. Chem. Soc. 734–736 (1976).
- 36.Uno SN, et al. A spontaneously blinking fluorophore based on intramolecular spirocyclization for live-cell super-resolution imaging. Nat. Chem. 2014;6:681–689. doi: 10.1038/nchem.2002. [DOI] [PubMed] [Google Scholar]
- 37.Hall LM, Gerowska M, Brown T. A highly fluorescent DNA toolkit: synthesis and properties of oligonucleotides containing new Cy3, Cy5 and Cy3B monomers. Nucleic Acids Res. 2012;40:1–10. doi: 10.1093/nar/gks303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf N, Kersting L, Herok C, Mihm C, Seibel J. High-yielding water-soluble asymmetric cyanine dyes for labeling applications. J. Org. Chem. 2020;85:9751–9760. doi: 10.1021/acs.joc.0c01084. [DOI] [PubMed] [Google Scholar]
- 39.Kim IK, Erickson KL. Models for uleine-alkaloid biogenesis. Tetrahedron. 1971;27:3979–3991. [Google Scholar]
- 40.Wang L, et al. Novel asymmetric Cy5 dyes: synthesis, photostabilities and high sensitivity in protein fluorescence labeling. J. Photochem. Photobiolol. A. 2010;210:168–172. [Google Scholar]
- 41.Frei MS, et al. Engineered HaloTag variants for fluorescence lifetime multiplexing. Nat. Methods. 2022;19:65–70. doi: 10.1038/s41592-021-01341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautier, A. et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 15, 128–136 (2008). [DOI] [PubMed]
- 43.Meng G, et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature. 2019;574:86–89. doi: 10.1038/s41586-019-1589-1. [DOI] [PubMed] [Google Scholar]
- 44.Pescitelli G, Bruhn T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality. 2016;28:466–474. doi: 10.1002/chir.22600. [DOI] [PubMed] [Google Scholar]
- 45.Lukinavičius G, et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods. 2014;11:731–733. doi: 10.1038/nmeth.2972. [DOI] [PubMed] [Google Scholar]
- 46.Belov VN, et al. Synthesis of fluorescent Jasplakinolide analogues for live-cell STED microscopy of actin. J. Org. Chem. 2020;85:7267–7275. doi: 10.1021/acs.joc.0c00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukinavičius G, et al. SiR–Hoechst is a far-red DNA stain for live-cell nanoscopy. Nat. Commun. 2015;6:8497. doi: 10.1038/ncomms9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper, M. et al. Cy3BTM: improving the performance of cyanine dyes. J. Fluoresc.14, 145–150 (2004). [DOI] [PubMed]
- 49.Oliinyk OS, et al. Deep-tissue SWIR imaging using rationally designed small red-shifted near-infrared fluorescent protein. Nat. Methods. 2023;20:70–74. doi: 10.1038/s41592-022-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ficken, G. E. & Kendall, J. D. The reactivity of the alkylthio-group in nitrogen ring compounds. Part II. Cyanine bases from 3,3-dimethyl-2-methylthio-3H-indole. J. Chem. Soc. 1529–1536 (1960).
- 51.Bandi VG, et al. Targeted multicolor in vivo imaging over 1,000 nm enabled by nonamethine cyanines. Nat. Methods. 2022;19:353–358. doi: 10.1038/s41592-022-01394-6. [DOI] [PubMed] [Google Scholar]
- 52.Critchfield FE, Gibson JA, Jr., Hall JL. Dielectric constant for the dioxane–water system from 20 to 35 °C. J. Am. Chem. Soc. 1953;75:1991–1992. [Google Scholar]
- 53.Martin, A. & Rivera-Fuentes, P. A General Strategy to Develop Fluorogenic Polymethine Dyes for Bioimaging (Zenodo, 2023); 10.5281/zenodo.7588846 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Discussions 1 and 2, Figs. 1–69, Tables 1–5, synthetic procedures and plasmid maps.
pH profiles, absorbance and fluorescence spectra, and SMLM pixel value data from line profiles.
Absorbance and fluorescence data for macromolecule-binding turn-on experiments.
Absorbance and fluorescence spectra, SNAP-tag binding turn-on experiments and photobleaching data.
Circular dichroism, absorbance and fluorescence spectra following SNAP-tag binding.
Data Availability Statement
All data supporting this paper, including coordinates for all calculated structures, are available through Zenodo53. X-ray crystallographic datasets used for modelling are available from the PDB under accession nos. 6Y8P and 1DNH. Samples of small-molecule probes are available from the authors on reasonable request. Source Data are provided with this paper.