Abstract
Gastrointestinal cancers are a public health problem that threatens the lives of human being. A good experimental model is a powerful tool to promote the uncovering pathogenesis and establish novel treatment methods. High-quality biomedical research requires experimental models to recapitulate the physiological and pathological states of their parental tissues as much as possible. Organoids are such experimental models. Organoids refer to small organ-like cellular clusters formed by the expansion and passaging of living tissues in 3D culture medium in vitro. Organoids are highly similar to the original tissues in terms of cellular composition, cell functions, and genomic profiling. Organoids have many advantages, such as short preparation cycles, long-term storage based on cryopreservation, and reusability. In recent years, researchers carried out the establishment of organoids from gastrointestinal mucosa and cancer tissues, and accumulated valuable experiences. In order to promote effective usage and further development of organoid-related technologies in the research of gastrointestinal diseases, this study proposes a benchmark based on utilization of available experimental consumables and reagents, which are involved in the key steps such as collection and pretreatment of biospecimen, organoid construction, organoid cryopreservation and recovery, growth status evaluation, and organoid quality control. We believe that the standard for the construction and preservation of organoids derived from human gastrointestinal epithelium and cancer tissues can provide an important reference for the majority of scientific researchers.
Keywords: Organoid construction, gastrointestinal epithelium, gastrointestinal carcinoma, methods
Introduction
The biomedicine has become one of the fastest-growing areas today. The development of high-level biomedicine, especially the diagnosis and treatment of cancers, has an increasing demand for experimental models. Traditionally-used cell line models have undergone selection pressure accompanying genomic variation in long-term in vitro culture, resulting in some cell lines that are difficult to reflect the biological behavior of the original cancer and the chemotherapeutic response. As a result, there have been cases where drug screening using cancer cell lines has been effective in vitro but has failed in subsequent clinical trials, at least in part because the cell lines are not representative of the biological characteristics of parental cancers. Animal models are another traditional model in cancer research, such as immunodeficiency mouse models and patient-derived xenograft (PDX) models. Although cancer tissues transplanted into immunodeficient mice can reflect the genotypic and phenotypic characteristics of parental cancers to some extent, and play a certain role in the studies of pathogenesis, molecular targets, and preclinical drug sensitivity evaluation, such models have the shortcomings of lacking complete immune system (1). In addition, the PDX model is relatively expensive and spends a long time to expand cancer cells. Organoids are a new experimental model that can be used to culture, cryopreservation, and passaging in vitro (2). Organoid models well retain the morphological phenotype, biological function, and genomic variation profiling of the original tissues, which can meet the demand of various biomedical researches. As a kind of renewable biospecimen to maintain cellular viability, organoids are known as “living biobanks” which is a key area of constructing next-generation biobanks (3). Since 2018, the authors’ team has initiated the construction of organoids in gastrointestinal epithelium and cancers. The work has been supported by the special fund for organoid construction of Municipal Science and Technology Commission of Shanghai. The team has published a number of high-level research papers based on organoid-related technologies (4-6), which have laid a solid foundation for the development of benchmarks for this area. The relevant achievements have been invited to be presented at international and domestic academic conferences for many times. In the discussion session, the most frequently asked questions by the audience are about the operation technology in the construction of organoids, as well as the related reagents. In order to promote the standardized construction and application of organoids in gastrointestinal diseases, especially cancer research, this study proposes the benchmark based on commercially available materials and reagents for sample collection and pretreatment, 3D culture, cryopreservation and recovery, passaging, cellular vitality evaluation, and quality control. It is believed that this benchmark can provide a valuable reference for organoids study of gastrointestinal epithelial and cancer.
General principles and terminology of organoid construction
This benchmark is suitable for scientific research of organoids culture, passaging, storage, and recovery. The basic principle of organoid construction is based on the pluripotent stem cells in captured epithelium and cancer tissues. The stem cells are induced to differentiate into tiny living tissue clusters that are composed of glandular structures similar to their parent epithelium or adenocarcinoma tissues in vitro 3D culture environment.
The benchmark is based on enzymatic digestion to prepare a single cell suspension of normal epithelium or adenocarcinoma tissues of the human gastrointestinal tract, and then mix the cells with a 3D culture scaffold (here refers to matrigel) to form 3D gel droplets, and then add commercially available complete medium for culture (specific medium for organoids refers to the growth factors, cytokines and small compounds required by cultured tissue cells). The purpose is to simulate the growth microenvironment of gastric epithelium, and promote the differentiation of gastrointestinal stem cells into various types of mucosal epithelial cells, and at the same time maintain the stemness of pluripotent stem cells, so as to achieve the possibility of long-term culture and passage in vitro. The donor source of biospecimen involved in organoid culture is based on the principle of voluntary consent to participate in scientific research, and is embodied in the signing of informed consent.
Experimental consumables and reagents
Experimental consumables
The main consumables include 24-well plates (Corning, NY, USA), 1.5 mL EP tubes, 15 mL centrifuge tubes, 50 mL centrifuge tubes, 10 mm dishes, pipettes, sterile tips, cryopreservation tubes, the pasteur pipettes, disposable sterile medical cotton ball, tweezers, tissue scissors, 70−100 μm cell strainer (Corning), and procedural freeze boxes, etc.
Reagents
The main reagents include animal-free recombinant trypsin (TrypLE™ Express Enzyme, Thermo Scientific, MA, USA), advanced DMEM/F12 medium (GIBCO, CA, USA), 1% penicillin/streptomycin (Beyotime, Shanghai, China), 1% primary cell antibiotic (Primocin, Invivogen, Hong Kong China), organoid cryopreservation solution CryoStor® CS10 (STEMCELL, VAN, Canada), collagenase type IV (Sigma-Aldrich, MO, USA), hyaluronidase (Sigma-Aldrich), and matrigel (Corning).
Organoid complete medium
The complete medium can not only induce the differentiation of pluripotent stem cells into various types of gastrointestinal epithelial cells, but also maintain the stemness of gastrointestinal stem continually, so as to achieve long-term survival of living organoids in vitro. The complete medium consists of a basal medium and some additional factors. The basal medium is based on advanced DMEM/F12 (GIBCO) and contains 1% penicillin/streptomycin, 1% primocin, 1 × 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES, Sigma-Aldrich, Mo, USA), 1 × alanine and L-glutamine condensed dipeptide medium (GlutaMAX, GIBCO, CA, USA), 1 × B27 serum-free supplement (GIBCO), 1 × N2 cell culture supplement (GIBCO), 10 nmol/L gastrin I (Sigma-Aldrich, MO, USA) and 1 mmol/L n-acetylcysteine (Sigma-Aldrich, MO, USA), and 4 mmol/L nicotinamide (Sigma-Aldrich). The growth factors and additional factors include: 50 ng/mL epidermal growth factor (EGF, Invitrogen, CA, USA), 200 ng/mL fibroblast growth factor 10 (FGF10, PeproTech, NJ, USA), 2 μmol/L transforming growth factor (TGF)-β inhibitor A-83-01 (Tocris, Bristol, UK), 100 ng/mL Wnt-3a (R&D Systems, MN, USA), 500 ng/mL R-Spondin 1 (PeproTech, NJ, USA), 100 ng/mL Noggin (PeproTech, NJ, USA), 5 μmol/L p38 MAPK inhibitor SB202190 (Sigma-Aldrich, MO, USA), 1 μmol/L prostaglandin E2 (Tocris, Bristol, UK). Whereas, 10 μmol/L RHOK inhibitor Y-27632 (Sigma-Aldrich, MO, USA) is taken as option (for primary extraction procedure, or when the organoids are in poor condition, as well as for organoid recovery) (7-9).
Experimental procedures
Collection of gastrointestinal epithelium and adenocarcinoma tissues
The surgically removed gastrointestinal epithelium and adenocarcinoma tissues are collected by a physician. Cancer tissues should be harvested as close as possible to the center of the tumor, removing black or green necrosis tissues on the surface of the tumor using scissors, and retaining the gray-white tissues inside. In order to minimize microbial contamination, scissors should be replaced for each part of the tissues. The size of the retained tissue block is greater than 5 mm × 5 mm, while the corresponding normal epithelium is collected from the cutting edge or 5 cm far from cancer. All tissues are harvested within 30 min after the gastrectomy.
The freshly harvested tissues are temporarily stored in advanced DMEM/F12 containing 1% penicillin/streptomycin and 100 μg/mL primocin for 6 h. If tissues need to be stored overnight, the tissues can be stored in advanced DMEM/F12 with 1% penicillin/streptomycin, 100 μg/mL primocin, and 10 μmol/L Y27632 at 4 °C for 24 h.
Tissue samples should be cleaned thoroughly. The initial cleaning step is to soak the tissue in no less than 5 mL of phosphate buffer saline (PBS) buffer containing 1% penicillin/streptomycin and 100 μg/mL primocin, wash every 5−10 min with shaking on a shaker, discard the dirty cleaning solution, and then add the aforementioned cleaning solution and continue to wash with shaking. The primary cleaning steps are no less than four times with the total duration no less than 30 min.
In the deep cleaning steps, firstly, the tissues are fully minced by ophthalmic scissors to a size of 0.5 mm × 0.5 mm in a 1.5 mL EP tube, and then soaking it in no less than 5 mL of PBS buffer containing 1% penicillin/streptomycin and 100 μg/mL primocin with shaking every 5−10 min. Then the soaking is centrifuged at 1,500 r/min for 5 min. After discarding the dirty cleaning solution, the aforementioned cleaning solution is added for washing again. The deep cleaning steps are no less than three times with total duration no less than 30 min.
Preparation of single-cell suspensions and plating
A 1 mL of collagenase type IV (1.5 mg/mL) and hyaluronidase (20 μg/mL) are added to the tissue pellet, and incubated in a 37 °C water bath for digestion with shaking for 15−30 s every 30 min, until the tissue pieces are digested into a flocculent viscous solution. The digestion time is about 1.5−2.0 h. The digested solution is collected into a 15 mL sterile tube, and added 5 mL of advanced DMEM/F12 medium with gently pipetting several times. According to needs, the above liquid is filtered with a 70−100 μm cell strainer to remove the undigested residues. The filtrates are transferred into a new 15 mL sterile centrifuge tube for centrifuge (1,500 r/min × 5 min) (note: this step depends on the needs, if no cell filtration is performed, the macroscopically undigested tissue residues can be aspirated away use a dropper). After centrifugation, the supernatant is discarded, and the pellet is retained.
As to plating, the cellular suspension is mixed with matrigel according to the ratio of 1:1. Generally, the amount of advanced DMEM/F12 medium is depended on the amount of cellular pellet. For example, if 50−80 μL of above medium is added, the equal amount (50−80 μL) of matrigel should be added, and then mixed well. In this step, it must be carefully to avoid a large number of bubbles generated, because air bubbles can cause the matrigel-medium mixture to fall off the bottom of the 24-well wall, resulting in a failed experiment. Before plating, all manipulations should be performed on an ice surface to avoid solidification of matrigel at room temperature. A 50−80 μL of the mixed liquid is droplet to 24-well plate. In primary plating, a total of 2−3 replicate wells could be generated. The 24-well plate is incubated at 37 °C incubator for 30 min for accelerating the solidification of matrigel. After the matrigel is fully solidified, a 500−800 μL of complete medium is added. An appropriate amount of PBS buffer is added to surrounding blank wells of the 24-well plate to maintain proper humidity during the incubation in 37 °C incubator.
The 24-well plate is observed and photographed every two days. In general, organoid spheroids can be observed under an inverted microscope at the second day of culture. The organoid spheroids will grow larger as the culture time is extended. At the same time, pay close attention to whether there is contamination. If the matrigel is cloudy within 3 d of incubation, it indicates that there may be fungi or bacterial contamination. The contaminated organoids should be discarded in time. In addition, mycoplasma contamination may also occur during the culture of organoids. The mycoplasma also grows in a clumpy and spherical shape on the medium, which is easy to be confused with organoids, but under a high-powered microscope (400×), the mycoplasma does not have tissue structure of epithelium. In cellular suspension digested by enzymes, the mycoplasma does not show cellular morphology. The mycoplasma remover (Yeasen, Shanghai, China) can be added for rescuing, which can inhibit and eliminate the growth of mycoplasma. If the mycoplasma contamination cannot be removed, the organoids will be discarded.
Passaging and cryopreservation of organoids
When the organoids are cultured to a certain number and size (about 2 weeks), the passage and cryopreservation of the organoids can be carried out. First, the complete medium is aspirated off from the 24-well plate. A 1 mL of PBS is used for cleaning once. After removing PBS, a 1 mL of animal-free recombinant tryptase (TrypLE™ ExpressEnzyme) is added with pipetting to break up the matrigel to facilitate full digestion of organoids by the protease. The organoids will be digested into single cells suspension by 1−2 h incubation at 37 °C incubator with pipetting once every 10−15 min. A 1 mL of advanced DMEM/F12 medium is added and then centrifuged (1,500 r/min × 5 min). After centrifugation, the supernatant is removed. The 1/3 of the pellet is used for passaging and 2/3 of the pellet is used for cryopreservation. Regarding to the passaging, the pellet is resuspended with 50−80 μL of advanced DMEM/F12 medium, and mixed with 50−80 μL of matrigel thoroughly. In this step, it must be carefully to avoid a large number of bubbles generated, because air bubbles can cause the matrigel-medium mixture to fall off the bottom of the 24-well wall, resulting in a failed experiment. A 50−80 μL of the mixture is pipetted into the 24-well plate. A total of 2−3 replicate wells could be passaged. As to the remaining 2/3 pellet for cryopreservation, a 1 mL of organoid cryopreservation solution (CryoStor® CS10) is added to form a cellular suspension, and then taking 500 μL of each into 2 cryopreservation tubes separately. The cryopreservation tubes are put into a programmed cryovial box. The programmed cryovial box is placed in a −80 °C deep freezer, and then transferred into a liquid nitrogen tank for a long-term storage at the next day.
Recovery of organoids
In this step, the water bath should be turned on in advance and set the temperature to 37 °C. The cryopreservation tubes are removed from the liquid nitrogen tank and quickly placed into a 37 °C water bath with shaking to thaw them as quickly as possible. After thawing, removing the liquid into a 15 mL sterile centrifuge tube and mixed with the 10 times volume of advanced DMEM/F12 medium. The mixed solution is centrifuged (1,500 r/min × 5 min). The supernatant is discarded and the pellet is retained.
The pellet is resuspended with 50−80 μL of advanced DMEM/F12 medium, and mixed with 50−80 μL of matrigel thoroughly. In this step, it must be carefully to avoid a large number of bubbles generated, because air bubbles can cause the matrigel-medium mixture to fall off the bottom of the 24-well wall, resulting in a failed experiment. A 50−80 μL of the mixture is pipetted into the 24-well plate. A total of 2−3 replicate wells could be plated. The 24-well plate is incubated at 37 °C incubator for 30 min for accelerating the solidification of matrigel. After the matrigel is fully solidified, a 500−800 μL of complete medium is added. An appropriate amount of PBS buffer is added to surrounding blank wells of the 24-well plate to maintain proper humidity during the incubation in 37 °C incubator.
The 24-well plate is observed and photographed every two days. In general, organoid spheroids can be observed under an inverted microscope at the second day of culture. The organoid spheroids will grow larger as the culture time is extended. In general, single cells with a bright and smooth surface under the inverted microscope are viable cells, while single cells with an opaque or rough surface are cells with lower viability.
When the organoids grow to a certain number and size (about 2 weeks), the passage and cryopreservation could be carried out. Generally, the subsequent scientific experiments should be done within 10 generations, which is considered as early passaging organoids. When the organoids are passaged more than 10 generations and less than 20 generations, they could be considered as mid-passage organoids. The organoids that passaged more than 20 generations are categorized into late passaging organoids (6,10). Researchers can choose organoids at different stages according to the experimental purpose. Since gastrointestinal epithelium will undergo senescence after 5 consecutive passages, it is recommended to use early passaging organoids for scientific experiments in normal epithelial organoids. The organoids from gastrointestinal cancers can be passaged differently depending on the purity of tumor cells or biological behaviors. It is recommended that experiments of molecular biology should be carried out with the organoids within 10 generations (6).
Evaluation of growth status and quality control of organoids
When the organoids are cultured for 7−10 d, the numbers and diameters can be calculated under the inverted microscope. In general, under a 4× objective, the number of organoids is no less than 60/field, and the diameter of organoids is 160−200 μm. Sometimes, the size of individual organoids is too large (up to 1,000 μm in diameter), which suggests that the passaging should be carried out, because the large organoids consume too much nutrients in the culture medium, and the matrigel scaffold is difficult to support its growth.
Assessing cellular viability by trypan blue
The trypan blue staining is a traditional method for quality control of living cells (11). For trypan blue staining, the cellular pellet is resuspended with 100 μL of advanced DMEM/F12 medium and mixed well. An 18 μL of the mixture is transferred into in a 1.5 mL EP tube, and then 2 μL of 0.4 % trypan blue staining solution is added to the EP tube, and stained for 3 min. A 10 μL of the cellular suspension is flushed into the hemocytometer and observed by the inverted microscope. The total number of unstained blue and blue-stained cells in the four squares are counted separately and recorded. The total number of cells as well as the cellular viability per 100 μL volume is calculated as the following formula:
The total number = [(unstained cell counting + blue cell counting)/4] × 103 × 1.11.
Cellular vitality = unstained cell counting/(blue cell counting + unstained cell counting) × 100%.
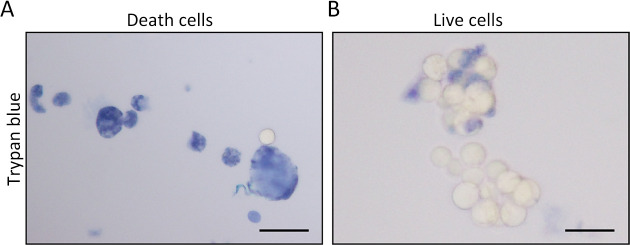
Regarding to trypan blue staining, the cell membrane of dead cells could be penetrated by the dye, thereby coloring DNA of dead cells. Under the microscope, the dead cells are stained blue (Figure 1A). On the other hand, the unstained cells are living cells, showing a translucent morphology under the microscope (Figure 1B). When the cellular viability is more than 80%, it indicates that the organoid is well grown, and suitable for subsequent experiments.
Figure 1.
Cellular vitality of organoids is evaluated by trypan blue staining. (A) Cells are considered as dead when they are stained blue; (B) Cells are considered as living cells when they are unstained and translucent in morphology. Scale bar 30 μm.
Tissue section preparation and hematoxylin-eosin (HE) staining
The organoid tissue sections can be prepared based on both cryo-embedding method and paraffin-embedding method. As to cryo-embedding method, the medium of the organoids is aspirated off. The matrigel containing organoid spheroids is scraped and put into the optimal cutting temperature compound (OCT) embedding medium by a blade. The OCT block is cryopreserved at −80 °C for 12 h, and then cut frozen sections as 6 μm. Regarding to the paraffin-embedding method, the organoids should be blowed away, and then centrifuged (1,500 r/min × 5 min). The supernatant is taken away. The pellet is fixed with 4% paraformaldehyde for 30 min. Then the pellet is enriched with 10% agarose, and then embedded in paraffin. The paraffin section is cut as a thickness of 8 μm for subsequent staining.
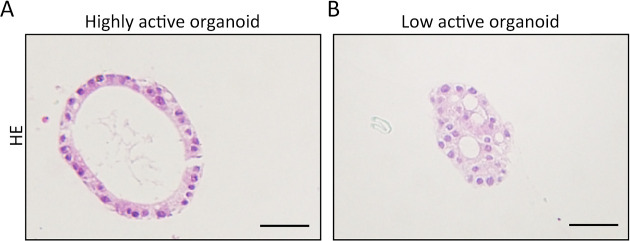
In HE staining, the slides are stained in hematoxylin solution for 5 min. Then the slides are rinsed in 0.5% hydrochloric acid alcohol for 30 s. The slides are washed in running water for 0.5 h. The slides are stained in eosin for 3 min, and then washed with running water for 30 min. Finally, the slides are dehydrated, transparent and covered. By HE-stained organoids, the cells with high-viability are arranged regularly. For example, the normal organoids of gastrointestinal epithelium show a single-layer glandular structure with a polarity (Figure 2A). The cells with poor viability show a disordered arrangement and intracytoplasmic vacuoles (Figure 2B).
Figure 2.
Cellular vitality of organoids is evaluated by HE staining. (A) High-viability organoids from normal gastrointestinal epithelium exhibit a single-layer glandular structure with a polarity; (B) Cells of low-viability organoids are disordered with vacuoles appearance in the cytoplasm. The cellular polarity is lost. Scale bar 60 μm. HE, hematoxylin-eosin.
Senescence-associated β-galactosidase (SA-β-Gal) staining
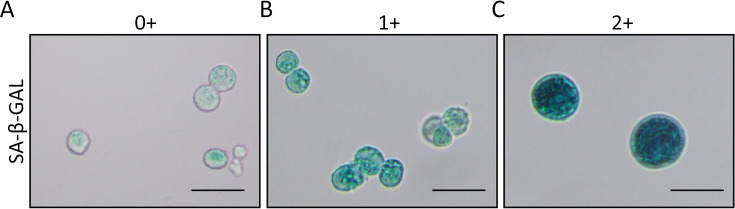
The SA-β-Gal is a reliable parameter for evaluation of cellular senescence (12,13). Therefore, organoids can be evaluated by SA-β-Gal staining. In this detection, the organoids should be digested enzymatically for 0.5−1.0 h. The single cell suspension of organoid is fixed with SA-β-Gal fixative solution for 15 min at room temperature. After fixation, the cells are washed with PBS for 3 times, and then stained with SA-β-Gal solution according to manufacturer’ instruction (Beyotime). The staining is carried out at 37 °C overnight. At the next day, the stained results are observed and photographed under a microscope (BX50, OLYMPUS, Tokyo, Japan). The senescent cells are stained green by SA-β-Gal. The positive cells stained by SA-β-Gal are counted for 10 medium-power fields (200×). Cells that are not stained are defined as negative (0+) (Figure 3A). Cells stained with light green are defined as 1+ (Figure 3B), while cells are stained as dark green is defined as 2+ (Figure 3C). If the proportion of 2+ cells is greater than 80%, it is judged as highly senescence, which is difficult for passaging or subsequent experiments, and should be discarded (6).
Figure 3.
Cellular vitality of organoids is evaluated by SA-β-Gal staining. (A) Cells that are not stained are defined as negative (0+); (B) Cells stained as light green are defined as 1+; (C) Cells stained as dark green are defined as 2+. Scale bar 30 μm. SA-β-Gal, senescence-associated β-galactosidase.
Artificial intelligence (AI)-assisted assessment of organoid viability
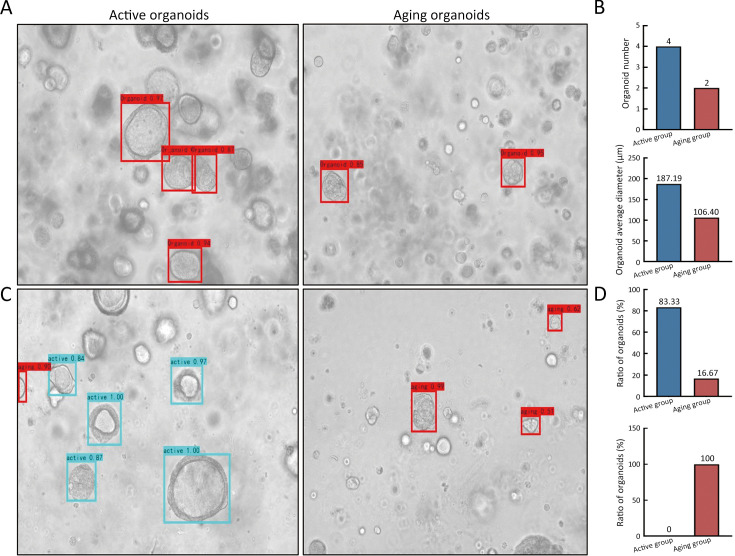
AI has recently been introduced to assist endoscopists or pathologists in the diagnosis of gastric neoplasia (14). Recently, a reliable evaluating approach for organoids vitality integrated phenotypic parameters with AI algorithm (CBAM-YOLOv3) was developed (6). Since the AI algorithm can evaluate aging degree of organoids only based on bright field images, it greatly shortened the time for preparing single-cell suspension and SA-β-Gal staining, thereby saving the time of quality control, and improving working procedure for living biobank administrator. Regarding to AI-assisted assessment, the bright field images of organoid at the culture of d 7 are captured under an inverted microscope with 10× objective. The organoid images are input into the CBAM-YOLOv3, an AI-assisted organoid phenotypic evaluation program. The CBAM-YOLOv3 program automatically output the parameters of the number of organoids, the average diameter of organoids (Figure 4A,B). The proportion of active organoid and aging organoids is also outputted (Figure 4C,D). If the proportion of aging organoids is greater than 80%, it indicates that the organoids are in a highly senescent condition, which is difficult to carry out passaging, and is no longer suitable for the subsequent experiments.
Figure 4.
Cellular vitality of organoids is evaluated by AI-assisted algorithm. (A) Light field images of active organoids and aging organoids are evaluated by AI algorithm. The corresponding organoids are marked by boxes; (B) Output parameters include organoid number and organoid average diameter; (C) As to viability evaluation of organoids, the active organoids and aging organoids can be marked by boxes on the output images; (D) Output parameters include the proportion of active organoids and aging organoids. AI, artificial intelligence.
The codes for this study are available at https://github.com/ruixinyang08/CBAM-YOLOV3-gastric-organoid and https://github.com/ruixinyang08/CBAM-YOLOV3-organoid-active-and-aging.
Biomarker examination of gastrointestinal epithelium
Frozen sections or paraffin-embedded sections of the aforementioned organoids are used for biomarker detection based on Immunofluorescence or immunohistochemistry methods. The antibodies used as gastric biomarkers include CK19, CK20, CDH1, or H+/K+ ATPase (15-18). The antibodies used as colorectal biomarkers include CK7, CK20, CDX2, MUC2, CEA or TFF3 (19-24). The alpha-SMA parameter is used as a negative control, because epithelial cells hardly express alpha-SMA (25,26).
Conclusions
Based on a large number of practical experiences in the past, this study proposed a basic benchmark for the construction of organoids from gastrointestinal epithelium and cancer tissues. In the current stage, this standard does not involve in the co-culture of epithelial cells with microenvironment components, and also does not relate to the preparation of organoid-on-chip.
Acknowledgements
This study was supported by Shanghai Science and Technology Committee (No. 20DZ2201900); National Natural Science Foundation of China (No. 82072602); Innovation Foundation of Translational Medicine of Shanghai Jiao Tong University School of Medicine (No. TM202001) and Collaborative Innovation Center for Clinical and Translational Science by Chinese Ministry of Education & Shanghai (No. CCTS-2022202 and No. CCTS-202302).
Contributor Information
Hengjun Gao, Email: hengjun_gao@shbiochip.com.
Yingyan Yu, Email: yingyan3y@sjtu.edu.cn.
References
- 1.Lau HCH, Kranenburg O, Xiao H, et al Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol. 2020;17:203–22. doi: 10.1038/s41575-019-0255-2. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, Vries RG, Snippert HJ, et al Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Liu H, Chen K Living biobank-based cancer organoids: prospects and challenges in cancer research. Cancer Biol Med. 2022;19:965–82. doi: 10.20892/j.issn.2095-3941.2021.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang R, Yu Y Patient-derived organoids in translational oncology and drug screening. Cancer Lett. 2023;562:216180. doi: 10.1016/j.canlet.2023.216180. [DOI] [PubMed] [Google Scholar]
- 5.Xiang Z, Zhou Z, Song S, et al Dexamethasone suppresses immune evasion by inducing GR/STAT3 mediated downregulation of PD-L1 and IDO1 pathways. Oncogene. 2021;40:5002–12. doi: 10.1038/s41388-021-01897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang R, Du Y, Kwan W, et al A quick and reliable image-based AI algorithm for evaluating cellular senescence of gastric organoids. Cancer Biol Med. 2023;20:519–36. doi: 10.20892/j.issn.2095-3941.2023.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015;148:126-36.e6.
- 8.Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 2018;23:882-97.e11.
- 9.Seidlitz T, Merker SR, Rothe A, et al Human gastric cancer modelling using organoids. Gut. 2019;68:207–17. doi: 10.1136/gutjnl-2017-314549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan HHN, Siu HC, Ho SL, et al Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut. 2020;69:2165–79. doi: 10.1136/gutjnl-2019-320019. [DOI] [PubMed] [Google Scholar]
- 11.Fauque P, Ben Amor A, Joanne C, et al Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7. doi: 10.1016/j.fertnstert.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 12.Sharpless NE, Sherr CJ Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 13.Mohamad Kamal NS, Safuan S, Shamsuddin S, et al Aging of the cells: Insight into cellular senescence and detection methods. Eur J Cell Biol. 2020;99:151108. doi: 10.1016/j.ejcb.2020.151108. [DOI] [PubMed] [Google Scholar]
- 14.Ho KY Beyond images: Emerging role of Raman spectroscopy-based artificial intelligence in diagnosis of gastric neoplasia. Chin J Cancer Res. 2022;34:539–42. doi: 10.21147/j.issn.1000-9604.2022.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita Y, Terashima M, Hoshino Y, et al Detection of cancer cells disseminated in bone marrow using real-time quantitative RT-PCR of CEA, CK19, and CK20 mRNA in patients with gastric cancer. Gastric Cancer. 2006;9:308–14. doi: 10.1007/s10120-006-0398-z. [DOI] [PubMed] [Google Scholar]
- 16.Sun GR, Dong XY, He QS, et al Expression and clinical significance of CK19 and CK20 expressions in transverse mesocolon biopsies from patients with gastric carcinoma. Cell Biochem Biophys. 2012;62:361–4. doi: 10.1007/s12013-011-9293-2. [DOI] [PubMed] [Google Scholar]
- 17.Zeng W, Zhu J, Shan L, et al The clinicopathological significance of CDH1 in gastric cancer: a meta-analysis and systematic review. Drug Des Devel Ther. 2015;9:2149–57. doi: 10.2147/DDDT.S75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrovic S, Wang Z, Ma L, et al Colocalization of the apical Cl-/HCO3- exchanger PAT1 and gastric H-K-ATPase in stomach parietal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1207–16. doi: 10.1152/ajpgi.00137.2002. [DOI] [PubMed] [Google Scholar]
- 19.Omiya M, Murata T, Sawaki A, et al. Cutaneous metastasis of transverse colon cancer with an aberrant pattern of CK7/CK20/CDX2 and high microsatellite instability. Intern Med 2023;62:3625-30.
- 20.Qualtrough D, Hinoi T, Fearon E, et al Expression of CDX2 in normal and neoplastic human colon tissue and during differentiation of an in vitro model system. Gut. 2002;51:184–90. doi: 10.1136/gut.51.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cecchini MJ, Walsh JC, Parfitt J, et al CDX2 and Muc2 immunohistochemistry as prognostic markers in stage II colon cancer. Hum Pathol. 2019;90:70–9. doi: 10.1016/j.humpath.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Lindmark G, Olsson L, Sitohy B, et al qRT-PCR analysis of CEACAM5, KLK6, SLC35D3, MUC2 and POSTN in colon cancer lymph nodes-An improved method for assessment of tumor stage and prognosis. Int J Cancer. 2024;154:573–84. doi: 10.1002/ijc.34718. [DOI] [PubMed] [Google Scholar]
- 23.Yusufu A, Shayimu P, Tuerdi R, et al TFF3 and TFF1 expression levels are elevated in colorectal cancer and promote the malignant behavior of colon cancer by activating the EMT process. Int J Oncol. 2019;55:789–804. doi: 10.3892/ijo.2019.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John R, El-Rouby NM, Tomasetto C, et al Expression of TFF3 during multistep colon carcinogenesis. Histol Histopathol. 2007;22:743–51. doi: 10.14670/HH-22.743. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Han S, Xu K, et al. α-SMA+ cancer-associated fibroblasts increased tumor enhancement ratio on contrast-enhanced multidetector-row computed tomography in stages I-III colon cancer. J Gastroenterol Hepatol 2023;38:2111-21.
- 26.Kulsum S, Raju N, Raghavan N, et al Cancer stem cells and fibroblast niche cross talk in an in-vitro oral dysplasia model. Mol Carcinog. 2019;58:820–31. doi: 10.1002/mc.22974. [DOI] [PubMed] [Google Scholar]