Abstract
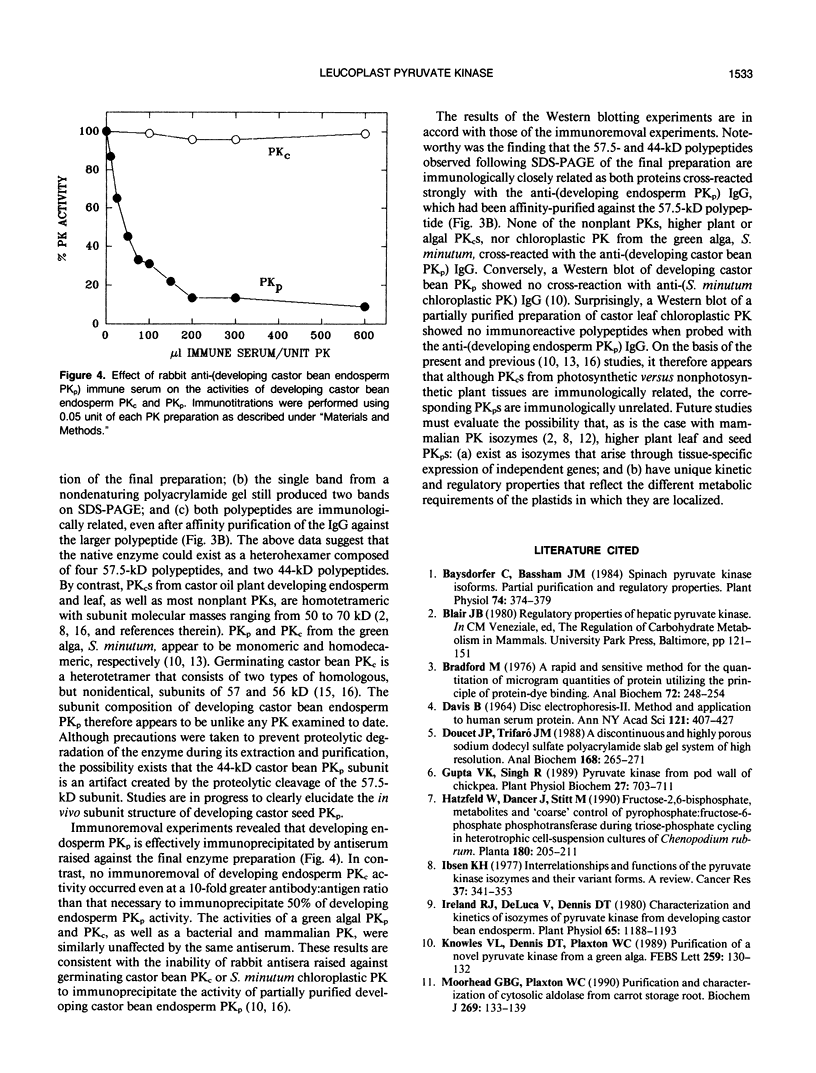
Leucoplast pyruvate kinase from endosperm of developing castor oil seeds (Ricinus communis L.; cv Baker) has been purified 1370-fold to a specific activity of 41.1 micromoles pyruvate produced per minute per milligram protein. Nondenaturing polyacrylamide gel electrophoresis of the purified enzyme resulted in a single protein staining band that co-migrated with pyruvate kinase activity. However, following sodium dodecyl sulfate polyacrylamide electrophoresis, two major protein staining bands of 57.5 and 44 kilodaltons, which occurred in an approximate 2:1 ratio, respectively, were observed. The native molecular mass was approximately 305 kilodaltons. Rabbit antiserum raised against the final enzyme preparation effectively immunoprecipitated leucoplast pyruvate kinase. The 57.5- and 44-kilodalton polypeptides are immunologically related as both proteins cross-reacted strongly on Western blots probed with the rabbit anti-(developing castor seed endosperm leucoplast pyruvate kinase) immunoglobulin that had been affinity-purified against the 57.5-kilodalton polypeptide. In contrast, pyruvate kinases from the following sources showed no immunological cross-reactivity with the same immunoglobulin: the cytosolic enzyme from developing or germinating castor bean endosperm; chloroplastic pyruvate kinase from expanding leaves of the castor oil plant; chloroplastic or cytosolic pyruvate kinase from the green alga, Selenastrum minutum; and mammalian or bacterial pyruvate kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Ibsen K. H. Interrelationships and functions of the pyruvate kinase isozymes and their variant forms: a review. Cancer Res. 1977 Feb;37(2):341–353. [PubMed] [Google Scholar]
- Ireland R. J., De Luca V., Dennis D. T. Characterization and kinetics of isoenzymes of pyruvate kinase from developing castor bean endosperm. Plant Physiol. 1980 Jun;65(6):1188–1193. doi: 10.1104/pp.65.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. II. Kinetic and regulatory properties. Arch Biochem Biophys. 1989 Feb 15;269(1):228–238. doi: 10.1016/0003-9861(89)90104-5. [DOI] [PubMed] [Google Scholar]
- Moorhead G. B., Plaxton W. C. Purification and characterization of cytosolic aldolase from carrot storage root. Biochem J. 1990 Jul 1;269(1):133–139. doi: 10.1042/bj2690133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead H. Isoenzymes of pyruvate kinase. Biochem Soc Trans. 1990 Apr;18(2):193–196. doi: 10.1042/bst0180193. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989 May 1;181(2):443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C. Purification of pyruvate kinase from germinating castor bean endosperm. Plant Physiol. 1988 Apr;86(4):1064–1069. doi: 10.1104/pp.86.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D. H., Botha F. C., Smith R. G., Feil R., Horsey A. K., Vanlerberghe G. C. Regulation of Carbon Partitioning to Respiration during Dark Ammonium Assimilation by the Green Alga Selenastrum minutum. Plant Physiol. 1990 May;93(1):166–175. doi: 10.1104/pp.93.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]