Abstract
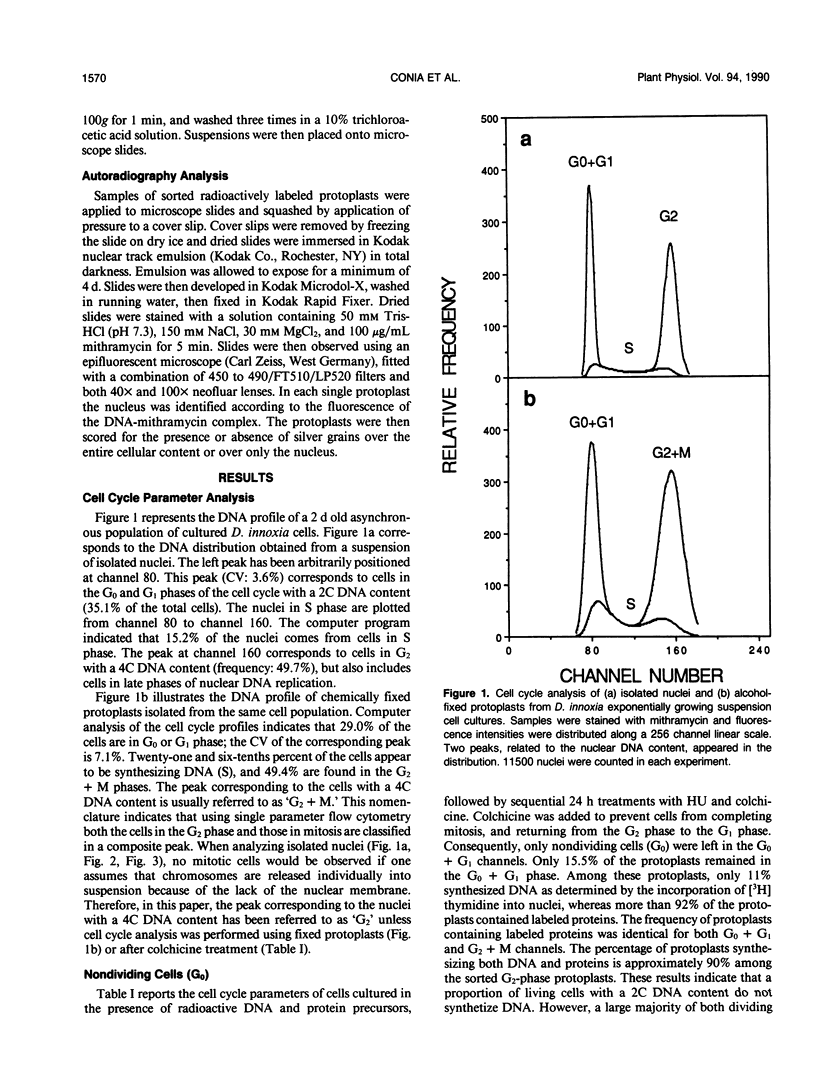
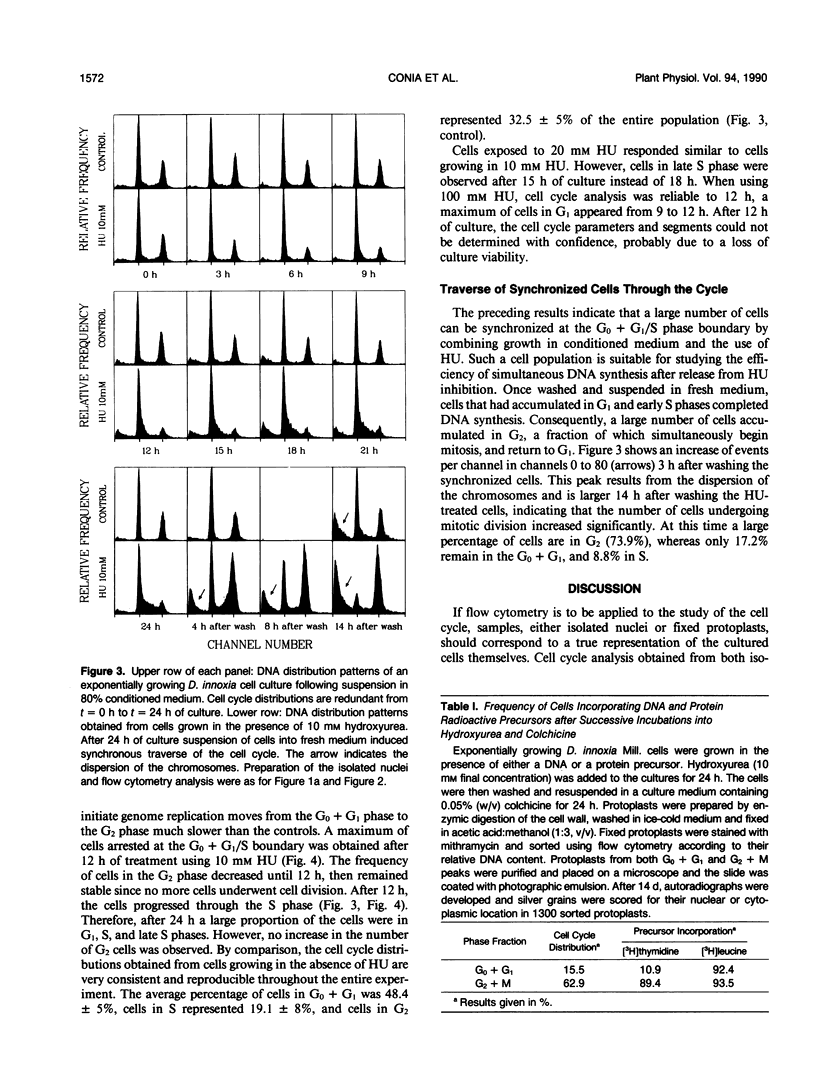
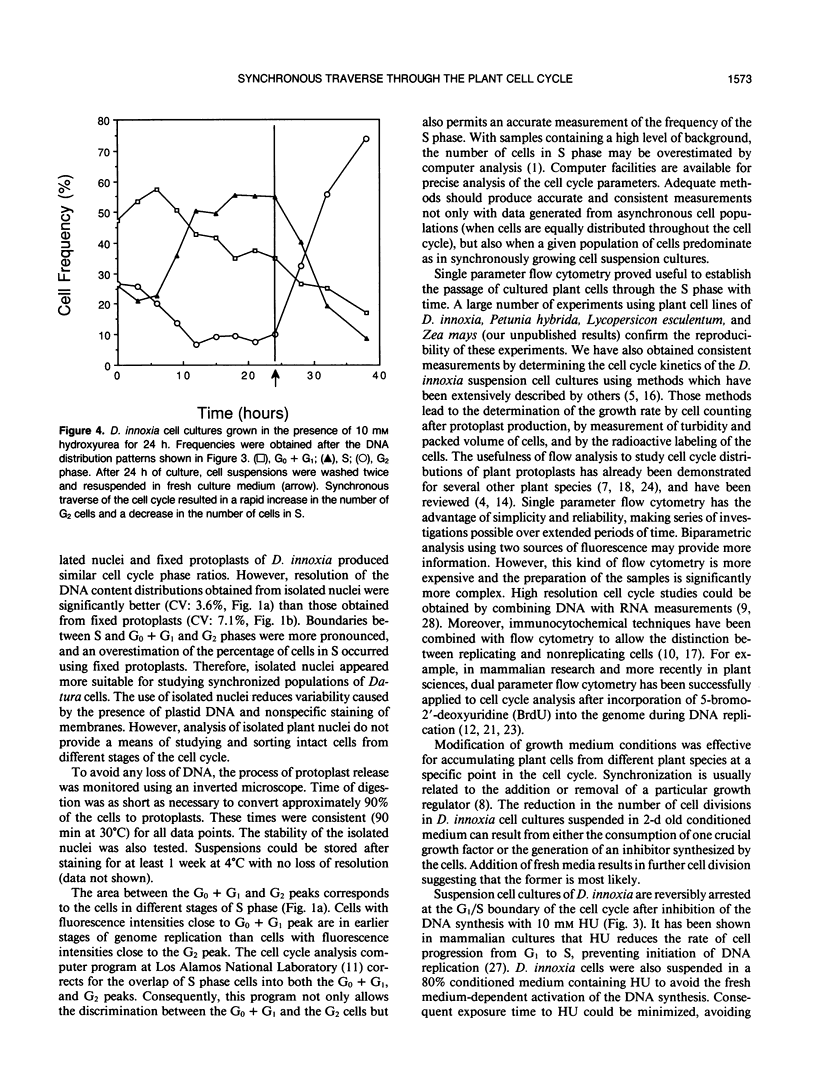
The induction of DNA synthesis in Datura innoxia Mill. cell cultures was determined by flow cytometry. A large fraction of the total population of cells traversed the cell cycle in synchrony when exposed to fresh medium. One hour after transfer to fresh medium, 37% of the cells were found in the process of DNA synthesis. After 24 hours of culture, 66% of the cells had accumulated in G2 phase, and underwent cell division simultaneously. Only 10% of the cells remained in G0 or G1. Transfer of cells into a medium, 80% (v/v) of which was conditioned by a sister culture for 2 days, was adequate to inhibit this simultaneous traverse of the cell cycle. A large proportion of dividing cells could be arrested at the G0 + G1/S boundary by exposure to 10 millimolar hydroxyurea (HU) for 12 to 24 hours. Inhibition of DNA synthesis by HU was reversible, and when resuspended into fresh culture medium synchronized cells resumed the cell cycle. Consequently, a large fraction of the cell population could be obtained in the G2 phase. However, reversal of G1 arrested cells was not complete and a fraction of cells did not initiate DNA synthesis. Seventy-four percent of the cells simultaneously reached 4C DNA content whereas the frequency of cells which remained in G0 + G1 phase was approximately 17%. Incorporation of radioactive precursors into DNA and proteins identified a population of nondividing cells which represents the fraction of cells in G0. The frequency of cells entering G0 was 11% at each generation. Our results indicate that almost 100% of the population of dividing cells synchronously traversed the cell cycle following suspension in fresh medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baisch H., Beck H. P., Christensen I. J., Hartmann N. R., Fried J., Dean P. N., Gray J. W., Jett J. H., Johnston D. A., White R. A. A comparison of mathematical methods for the analysis of DNA histograms obtained by flow cytometry. Cell Tissue Kinet. 1982 May;15(3):235–249. doi: 10.1111/j.1365-2184.1982.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Conia J., Bergounioux C., Perennes C., Muller P., Brown S., Gadal P. Flow cytometric analysis and sorting of plant chromosomes from Petunia hybrida protoplasts. Cytometry. 1987 Sep;8(5):500–508. doi: 10.1002/cyto.990080511. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Sharpless T., Staiano-Coico L., Melamed M. R. Subcompartments of the G1 phase of cell cycle detected by flow cytometry. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6696–6699. doi: 10.1073/pnas.77.11.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. N., Dolbeare F., Gratzner H., Rice G. C., Gray J. W. Cell-cycle analysis using a monoclonal antibody to BrdUrd. Cell Tissue Kinet. 1984 Jul;17(4):427–436. doi: 10.1111/j.1365-2184.1984.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbeare F., Gratzner H., Pallavicini M. G., Gray J. W. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5573–5577. doi: 10.1073/pnas.80.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Jackson P. J., Roth E. J., McClure P. R., Naranjo C. M. Selection, Isolation, and Characterization of Cadmium-Resistant Datura innoxia Suspension Cultures. Plant Physiol. 1984 Aug;75(4):914–918. doi: 10.1104/pp.75.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavicini M. G., Summers L. J., Dolbeare F. D., Gray J. W. Cytokinetic properties of asynchronous and cytosine arabinoside perturbed murine tumors measured by simultaneous bromodeoxyuridine/DNA analyses. Cytometry. 1985 Nov;6(6):602–610. doi: 10.1002/cyto.990060616. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Production and characterization of mammalian cells reversibly arrested in G1 by growth in isoleucine-deficient medium. Methods Cell Biol. 1973;6:67–112. doi: 10.1016/s0091-679x(08)60048-5. [DOI] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Van Dilla M. A., Trujillo T. T., Mullaney P. F., Coulter J. R. Cell microfluorometry: a method for rapid fluorescence measurement. Science. 1969 Mar 14;163(3872):1213–1214. doi: 10.1126/science.163.3872.1213. [DOI] [PubMed] [Google Scholar]
- van den Engh G. J., Trask B. J., Gray J. W. The binding kinetics and interaction of DNA fluorochromes used in the analysis of nuclei and chromosomes by flow cytometry. Histochemistry. 1986;84(4-6):501–508. doi: 10.1007/BF00482983. [DOI] [PubMed] [Google Scholar]


