Abstract
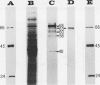
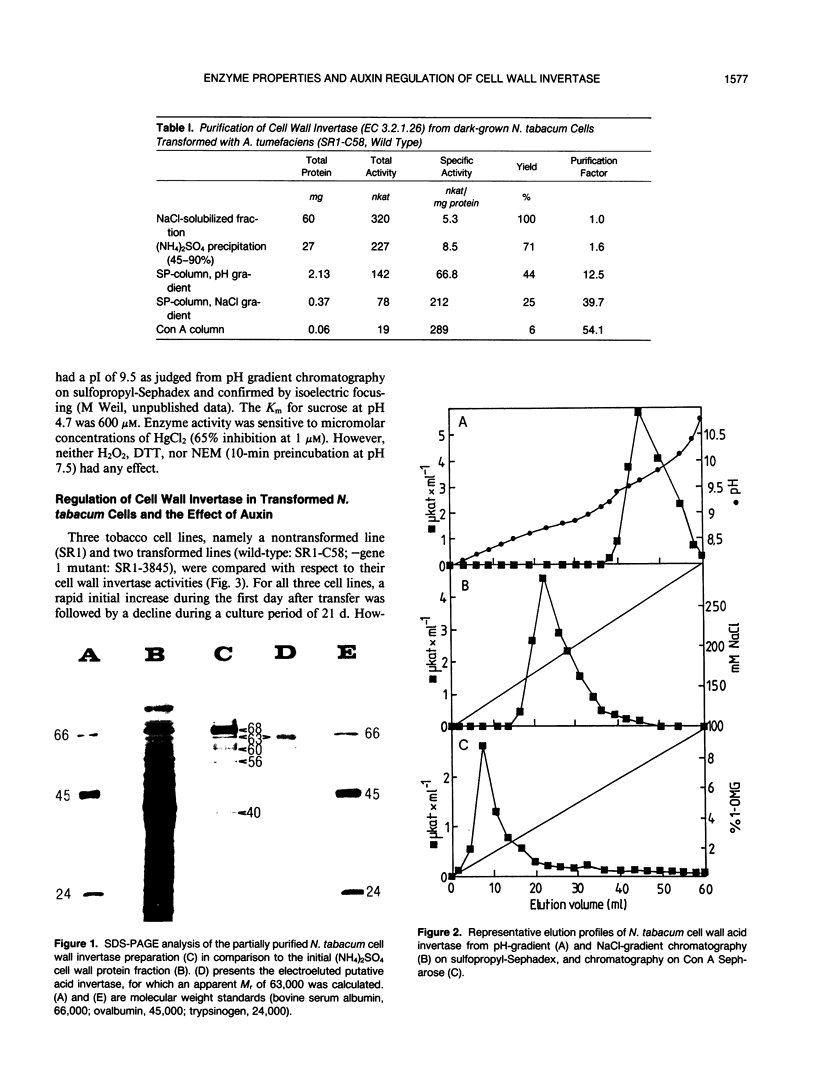
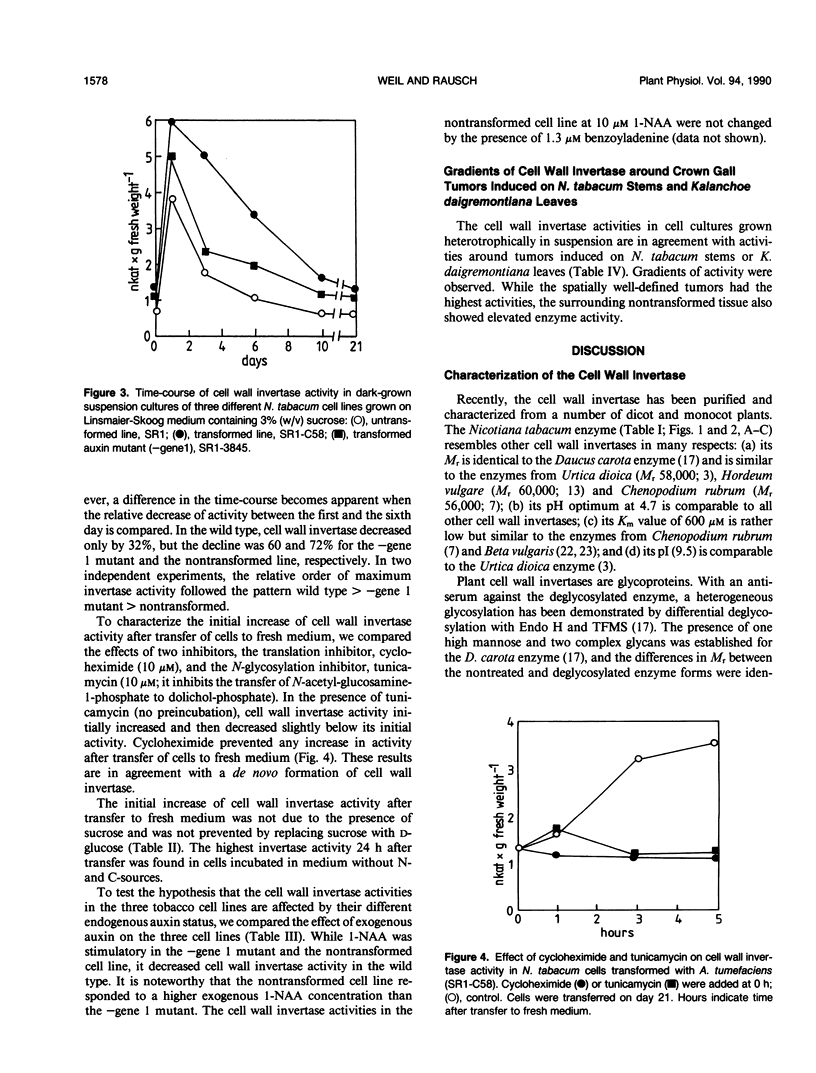
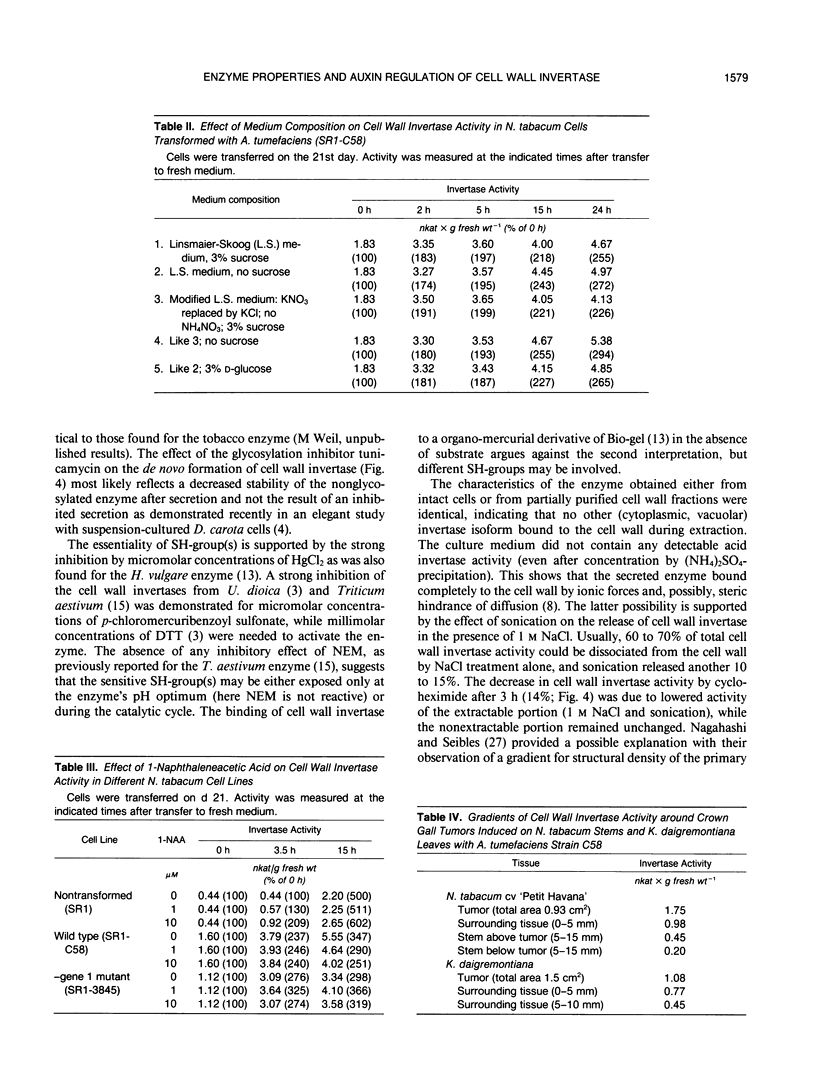
The cell wall invertase from an Agrobacterium tumefaciens-transformed Nicotiana tabacum cell line (SR1-C58) was purified. The heterogeneously glycosylated enzyme has the following properties: Mr 63,000, pH optimum at 4.7, Km sucrose 0.6 millimolar (at pH 4.7), pl 9.5. Enzyme activity is inhibited by micromolar concentrations of HgCl2 but is insensitive to H2O2, N-ethylmaleimide and dithiothreitol. Upon transfer of transformed cells from the stationary phase to fresh medium, a cycloheximide- and tunicamycin-sensitive de novo formation of cell wall invertase is demonstrated in the absence or presence of sucrose. While in an auxin mutant (lacking gene 1;SR1-3845) 1 micromolar 1-naphthaleneacetic acid led to a further increased activity, the wild-type transformed cell line (SR1-C58) responded with a decreased activity compared to the control. An analysis of cell wall invertase in and around tumors initiated with Agrobacterium tumefaciens (strain C58) on Nicotiana tabacum stem and Kalanchoë daigremontiana leaves revealed gradients of activity. The results indicate that the auxin-stimulated cell wall invertase is essential for the establishment of the tumor sink.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Apparent Inhibition of beta-Fructosidase Secretion by Tunicamycin May Be Explained by Breakdown of the Unglycosylated Protein during Secretion. Plant Physiol. 1989 Mar;89(3):845–851. doi: 10.1104/pp.89.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- Karuppiah N., Vadlamudi B., Kaufman P. B. Purification and characterization of soluble (cytosolic) and bound (cell wall) isoforms of invertases in barley (Hordeum vulgare) elongating stem tissue. Plant Physiol. 1989;91:993–998. doi: 10.1104/pp.91.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Blanchette J. T., Okita T. W. Wheat invertases : characterization of cell wall-bound and soluble forms. Plant Physiol. 1985 Jun;78(2):241–245. doi: 10.1104/pp.78.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurière C., Laurière M., Sturm A., Faye L., Chrispeels M. J. Characterization of beta-fructosidase, an extracellular glycoprotein of carrot cells. Biochimie. 1988 Nov;70(11):1483–1491. doi: 10.1016/0300-9084(88)90285-4. [DOI] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Sugawara S. Purification and Some Properties of Cell Wall-bound Invertases from Sugar Beet Seedlings and Aged Slices of Mature Roots. Plant Physiol. 1980 Jul;66(1):93–96. doi: 10.1104/pp.66.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Takahashi T., Sugawara S. Acid and alkaline invertases in suspension cultures of sugar beet cells. Plant Physiol. 1988 Jan;86(1):312–317. doi: 10.1104/pp.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Satoh S., Kamada H., Harada H., Fujii T. Auxin-controlled glycoprotein release into the medium of embryogenic carrot cells. Plant Physiol. 1986 Jul;81(3):931–933. doi: 10.1104/pp.81.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Van Lijsebettens M., Inzé D., Schell J., Van Montagu M. Transformed cell clones as a tool to study T-DNA integration mediated by Agrobacterium tumefaciens. J Mol Biol. 1986 Mar 20;188(2):129–145. doi: 10.1016/0022-2836(86)90299-8. [DOI] [PubMed] [Google Scholar]