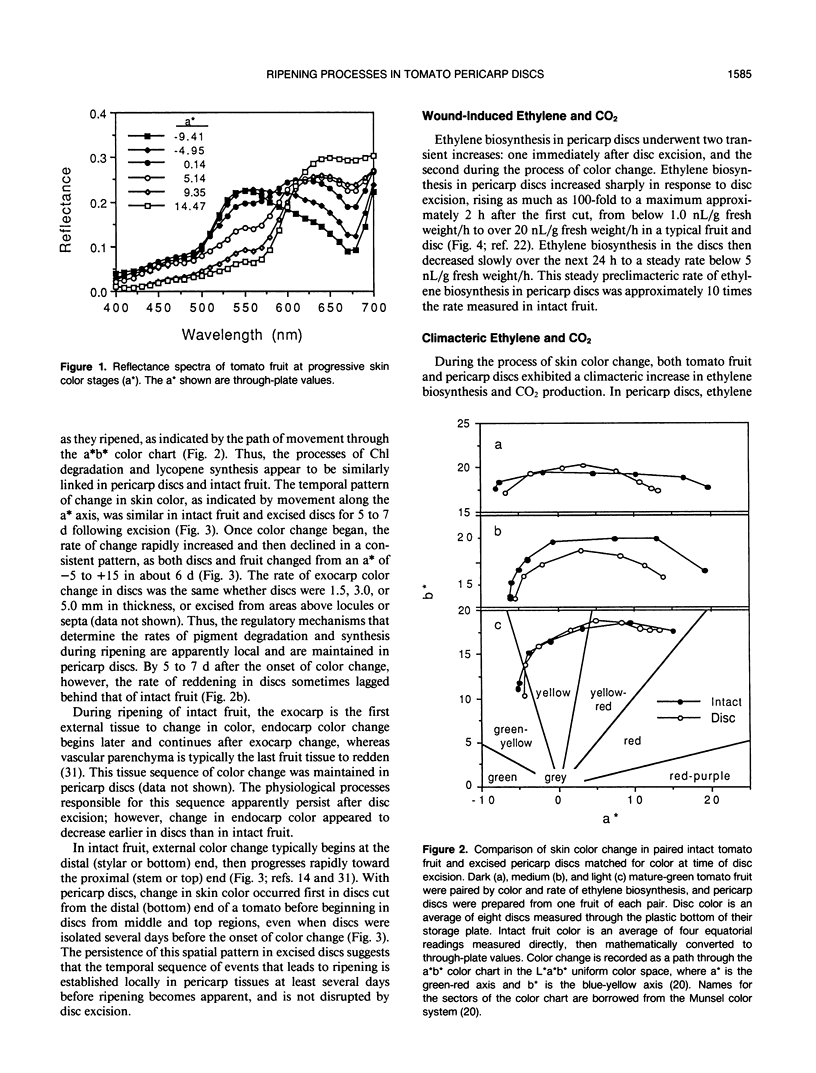
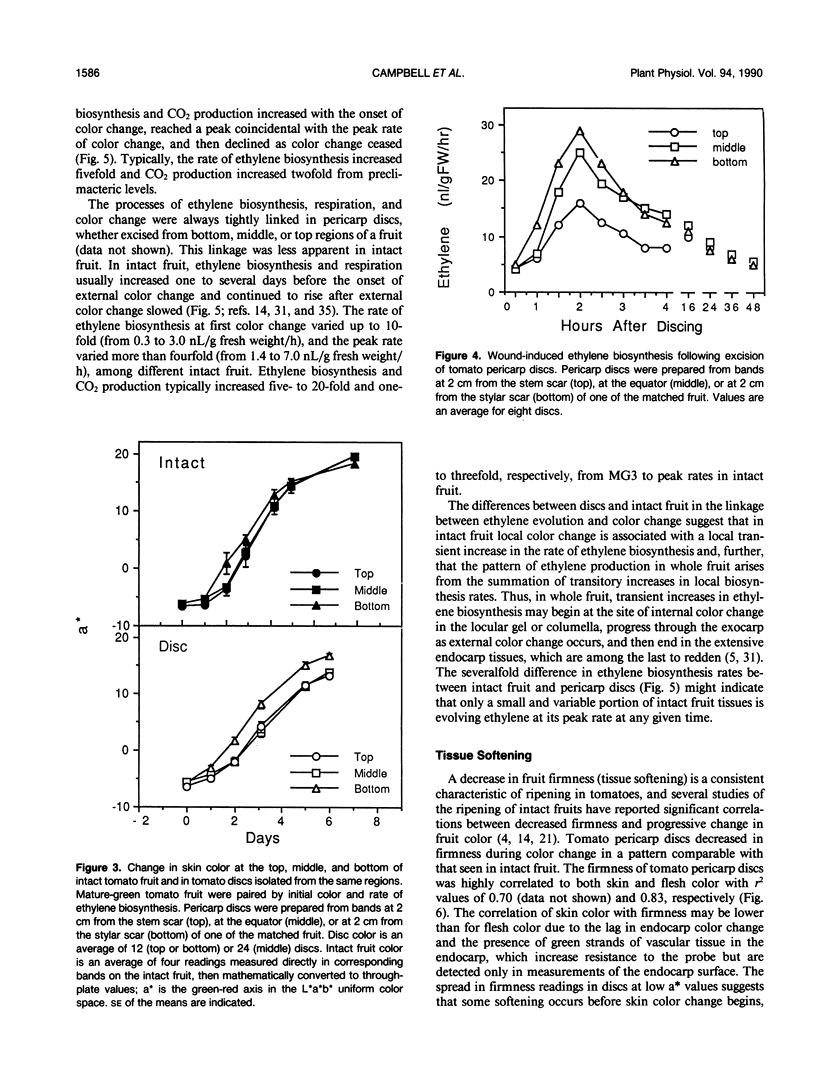
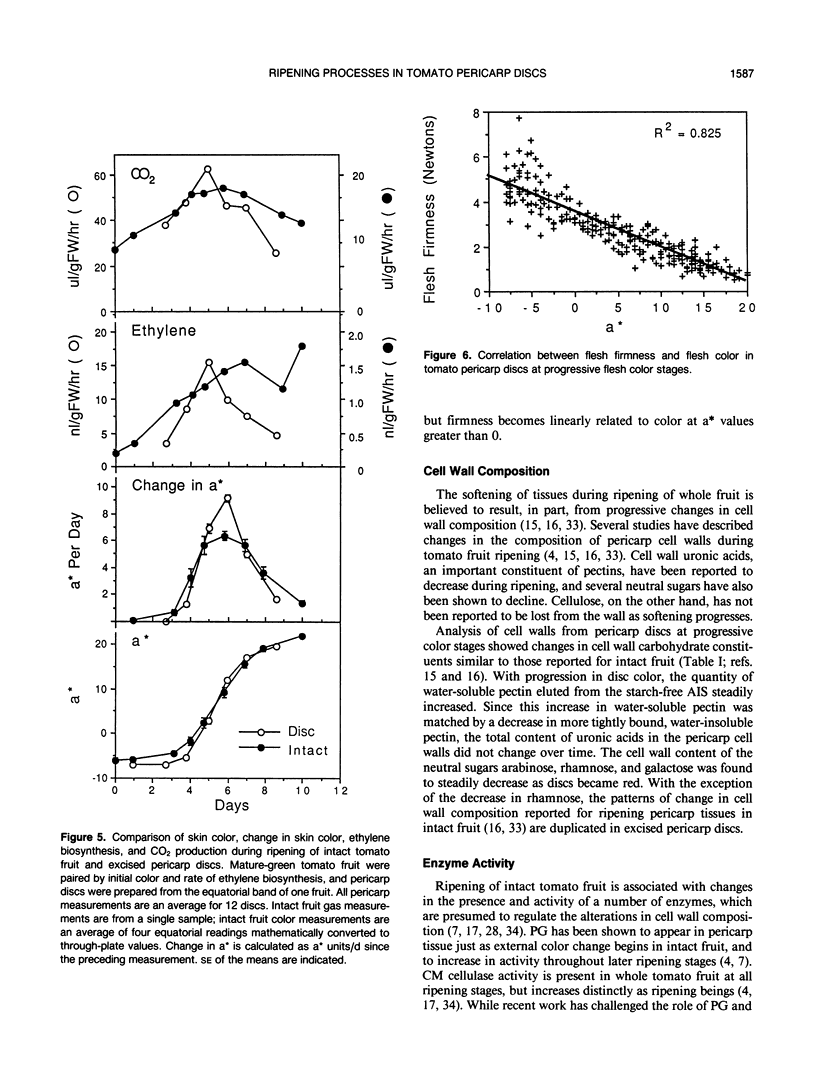
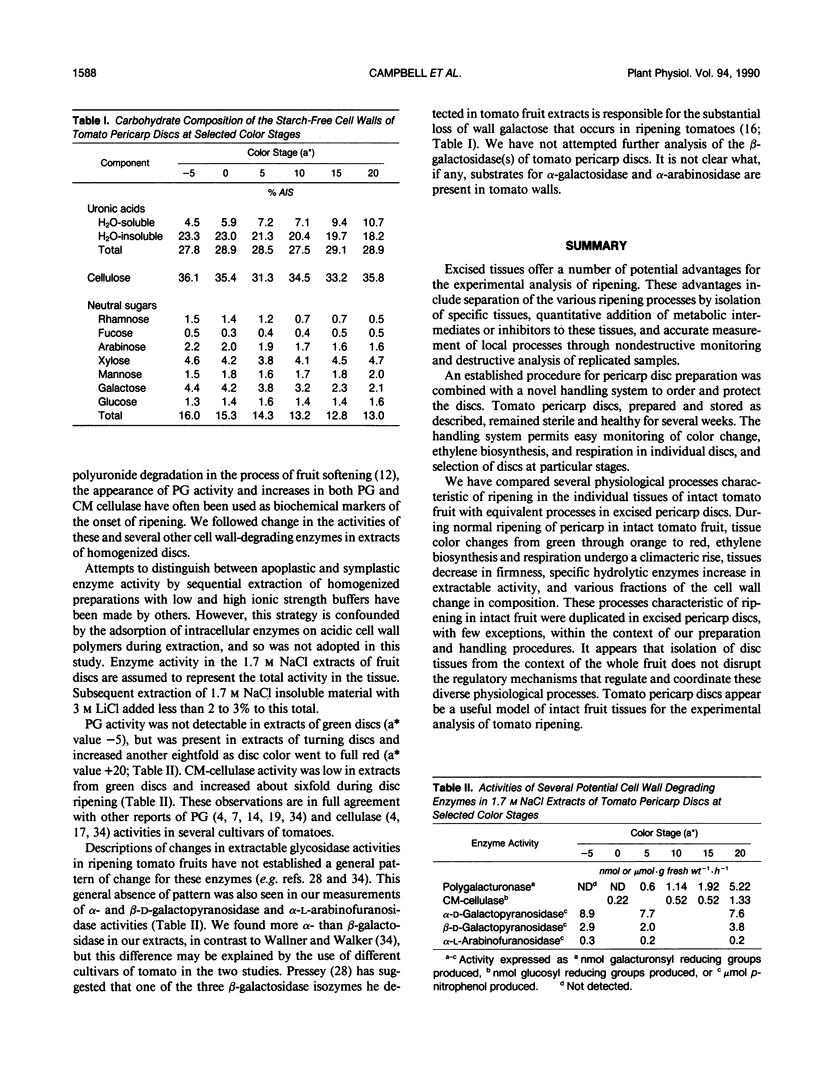
Abstract
Physiological processes characteristic of ripening in tissues of intact tomato fruit (Lycopersicon esculentum Mill.) were examined in excised pericarp discs. Pericarp discs were prepared from mature-green tomato fruit and stored in 24-well culture plates, in which individual discs could be monitored for color change, ethylene biosynthesis, and respiration, and selected for cell wall analysis. Within the context of these preparation and handling procedures, most whole fruit ripening processes were maintained in pericarp discs. Pericarp discs and matched intact fruit passed through the same skin color stages at similar rates, as expressed in the L*a*b* color space, changing from green (a* < −5) to red (a* > 15) in about 6 days. Individual tissues of the pericarp discs changed color in the same sequence seen in intact fruit (exocarp, endocarp, then vascular parenchyma). Discs from different areas changed in the same spatial sequence seen in intact fruit (bottom, middle, top). Pericarp discs exhibited climacteric increases in ethylene biosynthesis and CO2 production comparable with those seen in intact fruit, but these were more tightly linked to rate of color change, reaching a peak around a* = 5. Tomato pericarp discs decreased in firmness as color changed. Cell wall carbohydrate composition changed with color as in intact fruit: the quantity of water-soluble pectin eluted from the starch-free alcohol insoluble substances steadily increased and more tightly bound, water-insoluble, pectin decreased in inverse relationship. The cell wall content of the neutral sugars arabinose, rhamnose, and galactose steadily decreased as color changed. The extractable activity of specific cell wall hydrolases changed as in intact fruit: polygalacturonase activity, not detectable in green discs (a* = −5), appeared as discs turned yellow-red (a* = 5), and increased another eight-fold as discs became full red (a* value +20). Carboxymethyl-cellulase activity, low in extracts from green discs, increased about six-fold as discs changed from yellow (a* = 0) to red.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Dellapenna D., Kates D. S., Bennett A. B. Polygalacturonase Gene Expression in Rutgers, rin, nor, and Nr Tomato Fruits. Plant Physiol. 1987 Oct;85(2):502–507. doi: 10.1104/pp.85.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J. J., DellaPenna D., Bennett A. B., Fischer R. L. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989 Jan;1(1):53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. C., Wallner S. J. Degradation of Cell Wall Polysaccharides during Tomato Fruit Ripening. Plant Physiol. 1979 Jan;63(1):117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972 May;47(1):273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- Lincoln J. E., Cordes S., Read E., Fischer R. L. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci U S A. 1987 May;84(9):2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. beta-Galactosidases in Ripening Tomatoes. Plant Physiol. 1983 Jan;71(1):132–135. doi: 10.1104/pp.71.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltveit M. E. Effect of alcohols and their interaction with ethylene on the ripening of epidermal pericarp discs of tomato fruit. Plant Physiol. 1989 May;90(1):167–174. doi: 10.1104/pp.90.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendran R. R., O'Neill M. A. Isolation and analysis of cell walls from plant material. Methods Biochem Anal. 1987;32:25–153. doi: 10.1002/9780470110539.ch2. [DOI] [PubMed] [Google Scholar]
- Simons D. H., Bruinsma J. Effect of water on the ripening of pericarp disks from tomato fruits. Plant Physiol. 1973 Aug;52(2):132–136. doi: 10.1104/pp.52.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Bloom H. L. Characteristics of tomato cell wall degradation in vitro: implications for the study of fruit-softening enzymes. Plant Physiol. 1977 Aug;60(2):207–210. doi: 10.1104/pp.60.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Walker J. E. Glycosidases in Cell Wall-degrading Extracts of Ripening Tomato Fruits. Plant Physiol. 1975 Jan;55(1):94–98. doi: 10.1104/pp.55.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman M., Pratt H. K. Studies on the Physiology of Tomato Fruits. II. Ethylene Production at 20 degrees C as Related to Respiration, Ripening, and Date of Harvest. Plant Physiol. 1957 Jul;32(4):330–334. doi: 10.1104/pp.32.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]


