Abstract
Neuronomodulation refers to the modulation of neural conduction and synaptic transmission (i.e., the conduction process involved in synaptic transmission) of excitable neurons via changes in the membrane potential in response to chemical substances, from spillover neurotransmitters to paracrine or endocrine hormones circulating in the blood. Neuronomodulation can be direct or indirect, depending on the transduction pathways from the ligand binding site to the ion pore, either on the same molecule, i.e. the ion channel, or through an intermediate step on different molecules. The major players in direct neuronomodulation are ligand-gated or voltage-gated ion channels. The key process of direct neuronomodulation is the binding and chemoactivation of ligand-gated or voltage-gated ion channels, either orthosterically or allosterically, by various ligands. Indirect neuronomodulation involves metabotropic receptor-mediated slow potentials, where steroid hormones, cytokines, and chemokines can implement these actions. Elucidating neuronomodulation is of great significance for understanding the physiological mechanisms of brain function, and the occurrence and treatment of diseases.
Keywords: Neuron, Membrane potential, Excitability, Chemoactivation, Neuronomodulation
Introduction
The term “neuronomodulation” was first introduced by Kupfermann in 1979 [1], where the focus was on the modulation of neurotransmission and the neuromodulator in synaptic transmission (Fig. 1, left panel). The author used the term "modulated transmission". Later, the term “neuromodulators” emerged accordingly.
Fig. 1.
Evolution of the concept from classical neuromodulation and ambient modulation to NRM. NRM includes the modulation of synaptic transmission and the ambient modulation of neuronal excitability. Upper, left panel, model of synaptic action: a, an electrically excitable element (neuron dendrite, soma, presynaptic terminal, or muscle); b, a conventional (mediating) synaptic terminal that releases a transmitter that interacts with a receptor ionophore complex associated with a voltage-independent channel (●); c, a modulatory terminal that also releases a neurotransmitter; the modulatory transmitter can alter the effect of the mediating transmitter at voltage-independent channels, either by altering the receptor associated with that channel (▬) or by altering the extracellular concentration of a mediating transmitter. Alternatively, modulatory transmitters can alter the activity of voltage-dependent channels (▄) that regulate the ion flow involved in the generation of spikes, the endogenous pacemaker of the cell, or, in the case of presynaptic nerve terminals and muscle, involved in transmitter release and contraction, respectively. The modulatory action can operate either extracellularly at a receptor associated with the voltage-dependent channel, or intracellularly by means of a specialized receptor whose activation alters the levels of intracellular messengers. Adapted with permission from Kupfermann et al. [1]. Upper, right panel, ambient modulation of neuronal excitability: synaptic transmission (①) can be modulated by neuromodulators released by neighboring presynaptic terminals (②), acting either presynaptically or postsynaptically. The ambient modulation of neuronal excitability (③) acts in three ways: spillover of neurotransmitters from the nearby synapse (a), hormones from the bloodstream (b), and paracrine secretions (c).
Adapted with permission from Chen [2].
In 2016, Chen proposed the concept of "ambient modulation of neuronal excitability" [2]. This view broadens the scope beyond substances that act on the synapse site and highlights the role of the extra-synaptic space surrounding the neuron in modulating neuronal excitability. This concept was based on the discovery made in 1995 regarding the tonic inhibition of extra-synaptic GABAA receptors and their effects [3], as well as the previously known rapid, non-genomic actions of some steroid hormones that can result in changes in neuronal excitability [4, 5] (Fig. 1, right panel). The ambient (pericellular) modulation of neuronal excitability described in the right panel of Figure 1 includes the following: (1) Tonic inhibition of GABA receptors, which is generated by neurotransmitter overflow from the synaptic area and acts on extra-synaptic (pericellular) GABA receptors. However, the actual situation may be more complicated and also involves the function of local transmitter transporter proteins. (2) Experimentally-perfused glucocorticoids or estradiol. (3) Neurosteroids produced by adjacent cells in a paracrine manner.
In 2022, Chen further extended the concept of neuronal excitability modulation and introduced the term "neuronomodulation (NRM)" [6]. NRM refers to the change in neuronal membrane potential in response to any chemical substances, from spillover neurotransmitters to paracrine or endocrine hormones circulating in the blood (Fig. 1). Literally, NRM can encompass many different types of change, including communication and metabolic changes in response to various exogenous stimuli such as mechanical, thermal, chemical, electrical, and light stimuli. However, for the purposes of this discussion, we will focus specifically on the chemical modulation of neuronal excitability, which involves alterations in the polarization state of the neuronal membrane. It is important to note that the concept of neuronomodulation, which is widely used in clinical neurology, should be distinguished from NRM. Neuronomodulation refers to the modulation of brain or neural function by any chemical agent (such as drugs, agonists, or antagonists) or physical methods (such as electrical stimulation) to alleviate clinical symptoms. On the other hand, NRM is limited to the cellular level and is restricted to the range of chemo-modulation of the neuronal membrane potential. In this review, we focus on neuronal excitability modulation including direct NRM involving Nav, Kv, and GABAA receptor-Cl− channels, as well as indirect NRM involving metabotropic receptor-mediated slow potentials. We also discuss how steroid hormones, cytokines, and chemokines can implement these actions of indirect NRM.
Electrical and Chemical Excitability of Neurons
Neurons are capable of detecting signals from their proximal environment through their essential sensors, which can recognize both chemical signals (such as neurotransmitters, hormones, odorants, and metabolites) and physical signals (including temperature, electrical fields and mechanical stress), and transduce them into neuronal responses. There are four major superfamilies of proteins that serve as these sensors: ligand-gated (LGICs) and voltage-gated ion channels (VGICs), G-protein-coupled receptors (GPCRs), steroid receptors, and receptor tyrosine kinases (RTKs). Except for VGICs, which are activated by physical stimuli such as voltage or temperature, the other canonical receptors utilize chemical ligands as the initiators of signal transduction [7].
Neurons can be excited by both electrical and chemical stimuli, resulting in electrical excitability and chemical excitability, respectively. One important parameter in measuring neuronal excitability is the membrane potential. The membrane potential is maintained or changed through the flux of ions across the plasma membrane channels or through electrogenic pump activity, although the role of the latter is less discussed here. In fact, the modulation of ion channels, known as NRM, is a crucial factor in regulating neuronal excitability.
Orthosteric Modulation and Allosteric Modulation
Since NRM is defined as the modulation of neuronal excitability by chemical agents extrinsic to the neuron, its molecular basis must be sought in the activity changes of ion channels resulting from the acceptance of chemical signals via the binding sites on the ion channels. From a structural biology perspective, NRM is essentially a process of allosteric or orthosteric modulation of ion channels.
Allosteric modulation was first discovered in the study of the inhibition of L-threonine deaminase by L-isoleucine in a bacterial biosynthetic pathway [7, 8]. Allosteric interactions are a different mode of ligand-protein interaction compared to classical molecular competition. Instead of mutual exclusion from a common binding site by steric hindrance, allosteric interactions are defined as indirect interactions between topographically distinct sites, mediated by a reversible alteration of the protein’s molecular structure.
The concept of allosteric interaction has been extended to the action of neurotransmission in the nervous system, where it has been found that ligands can have two modes of action on receptors. The first is orthosteric modulation, which refers to an active molecule that competes with the ligand for binding to the receptor, resulting in competitive modulation of receptor activity. The second mode is allosteric modulation, where an active molecule acts on a non-ligand binding site of the receptor in a non-competitive way to change the conformation of the receptor molecule, thereby modulating its activity. The enhancing effect of allosteric modulation is called positive allosteric modulation, while the weakening effect is called negative allosteric modulation.
NRM can involve either orthosteric or allosteric modulation in the case of LGICs. However, non-receptor-gated ion channels like VGICs lack intrinsic ligands and are also sensitive to exogenous substances such as drugs or venom toxins that activate them by binding to specific sites on the channel molecule. This type of modulation is inherently allosteric since VGICs do not have orthosteric binding sites. Therefore, it can be said that non-receptor-gated ion channel interactions are primarily allosteric in nature.
Direct and Indirect NRM and B-F Linking
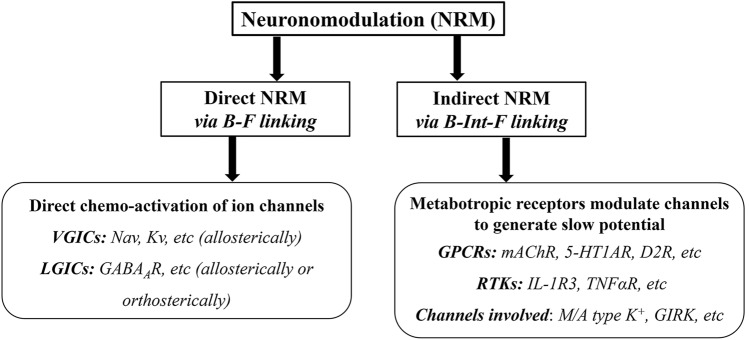
NRM is a process of chemoactivation of ion channels, and it involves the binding of a ligand to membrane receptors and the opening or closing of ion channels. We refer to this process as the B-F linking model, where B represents the binding site on the receptor (acceptor side) and F represents the functional component, i.e., the ion channel pore (effector side). NRM can be either a direct or indirect action, depending on whether there is a direct or indirect B-F linkage (Fig. 2).
Fig. 2.
Diagram summarizing the mechanisms of neuronal excitability modulation by NRM. NRM is the process of chemomodulation of ion channels, which leads to changes in membrane potential due to chemically active signal molecules. Depending on the transduction pathways from the ligand binding site (B) to the functional component (F), NRM can be direct or indirect, with the B-F linkage taking place on the same molecule (i.e., the ion channel) or through an intermediate step on different molecules (B-Int-F linkage), respectively. Direct NRM is primarily mediated by voltage-gated ion channels (VIGCs, e.g., Nav, Kv), which can be modulated allosterically, or ligand-gated ion channels (LIGCs, e.g., GABAAR), which can be modulated either allosterically or orthosterically. On the other hand, indirect NMR is implemented by various metabotropic receptors that are widely distributed throughout the CNS. These receptors can sense neurotransmitters, steroid hormones, cytokines, and chemokines in the extracellular fluid. GPCRs, G protein-coupled receptors; RTK, receptor tyrosine kinase; GIRK, G protein-regulated inwardly rectifying K+ channel.
Direct NMR operates in a direct B-F linking model, where the signal transduction pathways linking the ligand binding site to the target-ion channel are direct and on the same molecule, such as with VGICs and LGICs like NaV, KV, and extrasynaptic GABAA receptors. The essence of direct NRM is that when a ligand binds and acts on the ion channel, it immediately leads to an effect, i.e., the opening or closing of the ion pore. Therefore, the central theme of direct NRM is the conformational changes (orthosterically or allosterically) of the ion channel upon ligand binding within the same molecule.
Indirect NRM operates through an indirect B-F linking model, where the transduction pathways from the ligand binding site to the functional component (ion channel pore) are not on the same molecule but require an intermediate (Int) step on different molecules. For example, in a receptor binding−signal transduction−change of ion channel activity model, there is a B-Int-F linkage. The intermediate step may involve covalent modification of the ion channels or protein-channel interactions. Metabotropic receptors are major players in indirect NRM.
Direct NRM via Ion Channels
As discussed above, direct NRM refers to the modulation of ion channels of a neuron by chemical substances through a direct binding and activation process. This can include toxic substances such as poisonous toxins, as well as naturally-occurring ligands. Examples of ion channels that can be directly modulated include VGICs and LGICs such as the GABAA receptor. VGICs include sodium channels (Nav), potassium channels (Kv), and calcium channels (Cav). Cav channels primarily regulate the inflow of extracellular Ca2+ and intracellular processes, rather than directly affecting membrane potential changes. Therefore, the discussion of VGICs focuses here mainly on Nav and Kv channels.
The binding of drugs and toxins to Nav and Kv channels alters their activation, deactivation, and inactivation processes, potentially causing or alleviating the aberrant electrical excitability of neurons. Therefore, understanding the different binding sites is critical for drug development and pharmacovigilance. While the binding sites for these drugs and toxins are now well-defined and categorized within the Nav channel family, much less is known about the Kv channel family [9].
Chemo-activation of Nav Channels and NRM
Nav channels are essential for the initiation and propagation of action potentials in neurons. The Nav channel is composed of a large, channel-forming alpha subunit and one or two beta subunits. Nine alpha subunits (Nav1.1−Nav1.9) have been functionally characterized, and a tenth related isoform (Nax) may also function as a Na+ channel. The structure of the alpha subunit, which is ~2,000 amino-acid residues long, was revealed in the prokaryotic Na+ VGIC. The alpha subunit consists of four homologous domains, each containing six transmembrane segments (S1-S6) and an additional membrane re-entrant segment. Segments S1-S4 form the voltage-sensing module, while segments S5, S6, and the P loop between them form the pore. The central pore is surrounded by four independently folded S1-S4 domains, which contain voltage sensors and undergo substantial movement during gating [10]. The orthosteric activator for Nav is voltage itself, which modulates positive gating charges at intervals of three amino-acid residues in the S4 transmembrane segment, thus defined as the “orthosteric” site on these channels. In addition to this orthosteric site, there is a rich repertoire of neurotoxins and drugs that bind allosterically to Nav. To date, a total of 8 allosteric binding sites have been discerned in the structural schema of Nav [10], which confers chemo-activation of Nav by various ligands of toxins, such as tetrodotoxin (TTX), saxitoxin, µ-conotoxin, Asian scorpion BmKs, and spider venom-derived cysteine knot peptides, as well as local and general anesthetics and drugs that alleviate pain, epilepsy, ataxias and other pathophysiological conditions [11–13]. Local anesthetics bind within the central pore cavity as blockers, whereas TTX and µ-conotoxins bind to the outer pore opening [14]. Scorpion toxins modulate voltage-dependent gating by binding to the extracellular ends of the S3-S4 voltage sensor, while lipophilic toxins (e.g., batrachotoxin) bind within the transmembrane domain of the S5-S6 region [12]. Therefore, the pathway of action of modulators of VGICs can be roughly divided into two categories: blocking ionic pores and allosteric regulation.
Chemo-activation of Kv Channels and NRM
Voltage-gated K+ channels are transmembrane channels responsible for returning the depolarized cell to a resting state after each action potential. Kv channels can be divided into at least four types: slow-activating, Ca2+-activated, A-type, and M-type channels. KcsA (K channel of streptomyces A) is a homologous K+ channel found in the prokaryotic organism Streptomyces lividans, which forms a tetramer with a central ion conduction pore. The P-loop of each subunit contains the signature sequence TVGYGD, forming the selectivity filter [15]. Although many binding sites for drugs and toxins on Kv have been identified, allocating specific binding sites to the Kv structure is still rare. Some protein toxins from venomous organisms are known to commonly target the S1–S4 voltage-sensing domains of Kv [16]. For instance, tarantula venom toxin binds specifically to the voltage sensor of K+ channels and allosterically modulates the opening of K+-permeable pores. Therefore, it is the most selective inhibitor of Kv2 channels and is widely used to block or isolate endogenous Kv2 currents [17].
Regarding the NRM of Kv channels, two recent and impressive advances in Kv research are the discovery of the action of retigabine and the direct action of the neurotransmitter GABA on Kv7 (KCNQ) channels. Kv7.2 and Kv7.3 (KCNQ2/3) are important components of the slow voltage-gated M-channel, which plays a significant role in regulating neuronal excitability. Retigabine is an antiepileptic drug with a novel mechanism of action that involves the opening of neuronal KCNQ2-5 voltage-activated K+ channels, making it potentially useful in treating a wide range of disease states where neuronal hyperexcitability is a common factor. More intriguing is that it has been discovered that GABA as an endogenous neurotransmitter can directly bind and activate KCNQ channels [18]. A study by Manville et al. in 2018 found that GABA can activate endogenous neuronal M-type Kv channels, i.e., KCNQ3/5 channels, by binding to KCNQ3-W265 with an affinity similar to that of the most sensitive αxβ3γ2 GABAA receptors. This action of GABA is able to overcome muscarinic inhibition of KCNQ2/3 channels, while GABA analogs can competitively and differentially shift the voltage-dependence of KCNQ3 activation [17, 18].
Extra-synaptic GABAA Receptors and Tonic Inhibition
GABAA receptors are the predominant inhibitory neurotransmitter receptors in the central nervous system (CNS). They form a family of ligand-gated ion channels that have significant physiological and therapeutic implications. GABAA receptors are heteropentamers that form a chloride-ion-permeable channel. In humans, they are composed of 19 subunits: α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3. However, it is widely accepted that the main adult isoform is composed of α1, β2, and γ2 subunits which are arranged γ2β2α1β2α1 counterclockwise around a central pore as viewed from the cell exterior [19]. Besides GABA, researchers have identified several other ligands that can bind to different sites on the GABAA receptor and modulate its activity. These binding sites are specific to certain receptor subtypes, which in turn give rise to unique pharmacological profiles for the receptors.
GABAA receptors are localized to postsynaptic and extrasynaptic sites, the latter being highly involved in direct NRM. When the neurotransmitter GABA activates GABAA receptors at postsynaptic sites, it opens Cl− channels, which increases anion conductance for a short period (milliseconds). This leads to hyperpolarization of a depolarized membrane, a phenomenon referred to as phasic inhibition. In contrast, activation of GABAA receptors at extrasynaptic sites by low concentrations of GABA results in their opening for a longer period, termed tonic inhibition [3]. Initial investigations of extrasynaptic GABAA receptor-mediated tonic inhibition were conducted using whole-cell recording in rat cerebellar granule cells. It was found that a GABAA antagonist, bicuculline, blocks spontaneous inhibitory postsynaptic currents and markedly reduces background current noise. Further recordings were made in the presence of the Na+ channel blocker TTX, which blocked nearly all synaptic activity in these cells. The subsequent addition of bicuculline further reduced the background noise and caused a small outward shift in the mean current, revealing the presence of residual current fluctuations not blocked by TTX [3]. Following the initial study, evidence has been growing on the expression and molecular structure of extrasynaptic GABAA receptors in the CNS. It is now known that these receptors, with different subunit compositions, are widely expressed in various brain regions [20]. Extra-synaptic GABAA receptors can be either presynaptic receptors of the same neuron or on the terminals of adjacent neurons. Their widespread distribution throughout the brain confers an important role of tonic inhibition in various physiological and pathological conditions, such as sleep and wakefulness, the onset of epilepsy and psychiatric disorders, and some manifestations of alcoholism and drug abuse, as well as a treatment after a stroke and overuse of anesthesia [21]. Therefore, extrasynaptic GABAA receptor-mediated tonic inhibition, through direct NRM, has great value in the application of clinical pharmacology and therapeutics.
Indirect NRM via Metabotropic Receptors and Paracrine Actions
Metabotropic receptors can produce indirect NRM effects through both synaptic and extrasynaptic or non-synaptic transmission, and the latter can have a paracrine action. Not all neuronal membrane surfaces are equally electrically excitable, as the distribution of ion channels is uneven along different parts of the membrane. The postsynaptic plasma membrane of a neuron, which is equipped with ionotropic and/or metabotropic receptors, has the ability to combine with neurotransmitters and transduce the chemical signal into an electrical one. This patch of membrane is clearly both chemosensitive and electrically excitable. However, the rest of the membrane, which is much wider and extrasynaptic, has discretely distributed non-channel metabotropic receptors and is also chemosensitive. These receptors can sense chemical signals in the interstitial fluid in a paracrine manner and transduce them into membrane potential changes. Of special interest are those neurons in some brain areas without a blood-brain barrier, where the neuronal membrane is directly exposed to the action of active constituents of the cerebrospinal fluid such as steroid hormones, cytokines, and chemokines. In these cases, indirect NRM is also a common phenomenon.
The Metabotropic Receptor-Mediated Slow Potential is a Typical Form of Indirect NRM
In the mammalian brain, many neurotransmitter receptors and all neuropeptide receptors are metabotropic receptors. The two main types of metabotropic receptors are GPCRs and RTKs. GPCRs can powerfully regulate the activity of ion channels. The GPCR family includes receptors such as α- and β-adrenergic receptors, dopamine receptors, muscarinic acetylcholine receptors, GABAB receptors, metabotropic glutamate receptors, and metabotropic 5-HT receptors. Upon activation, GPCRs trigger a series of signal transduction events. Signaling molecules downstream of GPCRs produce diffusible second messengers that activate downstream molecules, which phosphorylate target molecules, including ion channels, through the action of enzymes. Alternatively, G proteins or second messengers directly act on ion channels. In addition, intracellular Ca2+ can be mobilized into the cytosol through these signaling pathways. On the other hand, RTKs contain protein kinase domains that phosphorylate various proteins, including ion channels, and alter their activity. Metabotropic receptors can activate diffusible second messengers and act on channels that are relatively distant from the receptors, unlike ionotropic receptors that are only limited to local canonical postsynaptic regions. This confers the ability to regulate nearly all types of ion channel, including resting channels, voltage-gated channels that generate action potentials or provide Ca2+ influx for neurotransmitter release, and ligand-gated channels. Thus, metabotropic receptors modulate the activity of ion channels and neuronal excitability through the B-Int-F linking model, which is an indirect NRM. The core step for this indirect NRM is the generation of a slow potential.
The slow potential refers specifically to a membrane potential change caused by metabotropic receptors; it is characterized by a slow onset (typically taking ~100 ms to develop) and long-lasting duration (ranging from hundreds of milliseconds to minutes, as it requires the coupling of multistep signaling cascades to reach the channel). This is in contrast to the so-called fast potential, which is triggered by ionotropic receptor channels and is characterized by a fast onset and brief duration (lasting only 10−50 ms, as the channel is directly gated by ligand binding). The slow potential is also relatively modest in magnitude compared to the fast potential. The slow potential can be either postsynaptic, which is initiated by fast transmitters (generally amino-acids), or non-synaptic, which is initiated by slow transmitters (non-amino-acid modulators, e.g., neuropeptides), while the fast potential is essentially restricted to postsynaptic regions. Furthermore, whereas a fast potential is always triggered by the increased opening of ion channels, a slow potential is more commonly triggered by the closing of channels. A slow potential can either excite/depolarize or inhibit/hyperpolarize the neuronal cell membrane.
There are various physiological scenarios of indirect NRM through the metabotropic receptor-implemented slow potential. One of the most well-studied examples is the Gq/11-coupled muscarinic acetylcholine (mACh) receptor-mediated modulation of muscarine-sensitive (or M-type) K+ channels in the hippocampus [22, 23]. In this case, a slow excitatory postsynaptic potential (sEPSP) is produced by activating mACh receptors, which stimulate phospholipase C to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2), yielding inositol triphosphate and diacylglycerol. The decrease in membrane PIP2 then causes the closure of the M-type delayed-rectifier K+ channel, resulting in a decrease in K+ efflux from the cell at the resting potential. Thus, K+ efflux no longer balances the Na+ influx through resting channels, and the net influx of Na+ induces depolarization, i.e., the sEPSP. In this way, mACh receptors implement an elegant indirect NRM to modulate the excitability state of neurons, which profoundly increases action potential firing in response to a fast excitatory input. As mACh receptors are widely distributed both synaptically and extrasynaptically throughout the CNS, diffuse or volume transmission, acting as a local hormone, seems to be the major mode of ACh action in the CNS. In fact, most fast neurotransmitters act as inducers of indirect NRM by interacting with their respective metabotropic receptors. Glutamate, for example, also induces sEPSPs through metabotropic glutamate receptors by inhibiting inwardly-rectifying K+ channel 3 (Kir 3.x, also known as GIRK channels because they require the binding of Gi/o beta-gamma subunits to activate) [24].
In addition to sEPSPs, slow inhibitory postsynaptic potentials (sIPSPs) can also be triggered by metabotropic receptors. For example, serotonin enhances the activity of GIRK channels and inhibits voltage-dependent Ca2+ channels through the activation of metabotropic 5-HT1A autoreceptors, both leading to the generation of sIPSPs [25, 26]. Dopamine triggers sIPSPs through its D2 receptors in the ventral tegmental area in mice, while noradrenaline does so via its α2 receptors in locus coeruleus neurons in rats [27]. Furthermore, in a specific class of projection neurons in the songbird forebrain, it has been found that the activation of GABAB receptors by GABA inhibits adenylyl cyclase and leads to the opening of GIRK channels, resulting in hyperpolarization and the generation of sIPSPs [28]. Interestingly, a recent spectroscopic study provided critical evidence for the existence of a GPCR/G protein/GIRK channel macromolecular signaling complex, supporting our suggested B-Int-F linkage model for indirect NRM [29].
As for the metabotropic receptors of RTKs, they can also indirectly modulate neuronal excitability through the generation of slow depolarizing potentials. The mitogen-activated protein kinase (MAPK) pathway is a well-known downstream signaling cascade that is activated by RTKs. MAPK activation can lead to the phosphorylation of an inactivating (A-type) K+ channel in the dendrites of hippocampal pyramidal neurons, reducing the K+ current and generating a slow depolarizing potential. This action enhances dendritic action potential firing [30].
Steroid Hormones, Cytokines, Chemokines, and Indirect NRM
Metabolic receptors located on neuronal membranes can also detect steroid hormones, cytokines, and other chemical factors present in the extracellular fluid. These molecular signals, whether circulating in the bloodstream or secreted by nearby cells, modulate neuronal excitability through an indirect NRM. It has long been recognized that effective communication between the neural and immune systems is crucial for maintaining homeostasis. However, it is only recently that evidence has emerged suggesting that many immune-reactive molecules directly influence the excitability of corresponding neurons.
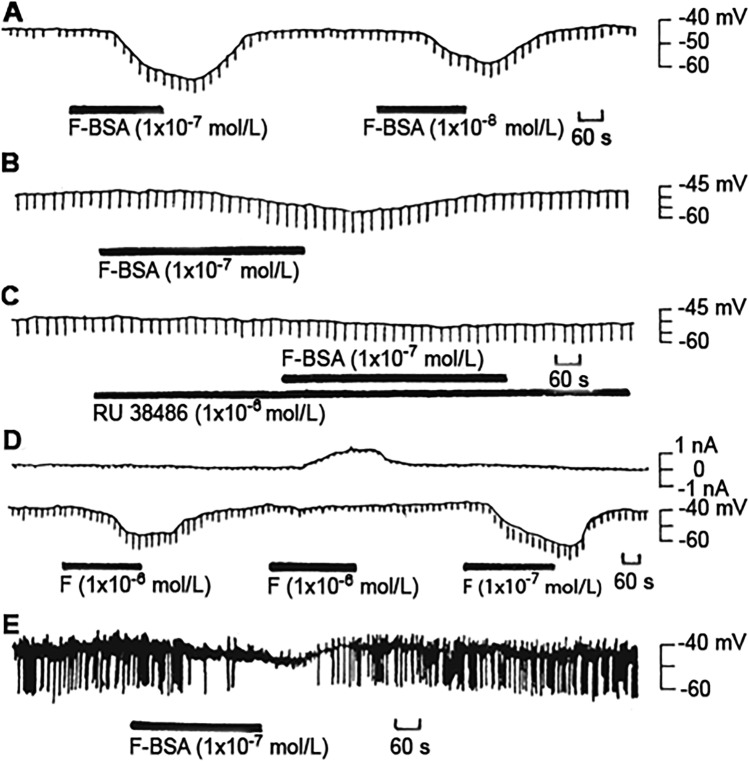
The non-genomic action of glucocorticoids (GCs) is perhaps the most well-known example of hormone-mediated NRM. In the 1980s, Chen and colleagues used intracellular recordings from isolated and superfused guinea pig coeliac ganglia neurons to demonstrate that cortisol succinate could hyperpolarize the membrane potential and change the cell's input resistance within 2 min. These effects persisted under low Ca2+/high Mg2+ conditions and were blocked by RU38486, a competitive antagonist of the GC cytosolic receptor. Interestingly, even the membrane-impermeable cortisol-21-bovine albumin conjugate had the same effect. These results suggest that GCs can act non-genomically and specifically through their membrane receptors on the neuronal surface. Furthermore, there may be a chemical similarity between the membrane receptor and the traditional cytosolic GC receptor (Fig. 3) [4]. Interestingly, twenty years later, through a cryo-electron microscopy study, Ping et al. identified the structures of GPR97-Go complexes that bound to GCs, revealing that GCs bind to a pocket within the transmembrane domain [31]. It remains to be investigated whether GPR97 is involved in GC-mediated NRM in ganglia neurons. In addition to GCs, estrogen and progesterone can also implement indirect NRM through non-genomic actions. Estrogen is known to hyperpolarize hypothalamic neurons and inhibit spontaneous activity [5]. Both progesterone and estradiol have non-genomic actions on the olfactory epithelium and olfactory receptor neurons to modulate odor responsiveness [32]. It should be noted that steroid hormones can also act as neurosteroids on VGICs or LGICs to implement direct NRM.
Fig. 3.
Membrane-receptor mediated effects of glucocorticoid on coeliac ganglion neurons and its significance. A, Bovine serum albumin-conjugated cortisol (F-BSA) hyperpolarizes a coeliac ganglion cell in a dose-dependent manner. B, C, The action of F-BSA is nearly completely blocked by RU38486. D, F-BSA apparently increases the membrane resistance during its hyperpolarizing effect, but when the membrane potential is manually clamped, it decreases its input resistance. The upper and lower traces are membrane current and potential, respectively. F, cortisol. E, In a spontaneously-discharging neuron, F-BSA inhibits the discharges of the neuron. With the pen recorder only postsynaptic potentials (upward deflections) and after-hyperpolarizations (downward deflections) are recorded, while the spikes of the action potentials are too rapid to be followed.
Reproduced with permission from Chen et al. [4].
Studies have shown that certain chemical factors and cytokines affect the membrane potential and discharge frequency of neurons. For example, interleukin 1β (IL-1β) increases voltage-gated K+ current by activating Akt kinase through interleukin (IL)-1 receptor member 3 [33], while tumor necrosis factor-alpha (TNF-α) modulates the voltage-gated transient Na+ current to cause significant changes in the membrane potential of subfornical organ neurons [34]. In a most recent study, three important pro-inflammatory cytokines (TNFα, IL-1β, and IL-6) have been shown to affect the membrane excitability of agouti-related peptide- or pro-opiomelanocortin-producing neurons[35].
Targets of NRM
Because NRM can impact both synaptic neurotransmission and the conduction of action potentials, it can be explained in terms of its effects on specific neural structures and processes, such as the axon and axon initial segment (AIS), as well as axonal conduction and the back-propagation of action potentials in the dendrite.
Since the genuine criteria for neuronal excitability are action potential initiation and propagation. It is interesting to examine the NRM under the "narrow sense of excitation"; the object of modulation naturally falls into the excitation initiation and conduction in parts of neurons. This is a classical NRM, where the outcome of modulation can be explicitly discerned and described. The axon initial segment is a special part of the neuron, which is devoid of myelination and therefore is liable to NRM. For example, activation of 5-HT1A receptors via the raphe-spinal pathway has been shown to decrease motoneuronal excitability [36]. This modulation specifically occurs at a "hot-spot" on the membrane, which has been identified as in the AIS [36]. In spinal motor neurons, the activation of 5-HT1A receptors inhibits Na+ channels, which are responsible for generating nerve impulses [37].
NRM can also modulate the conduction of action potentials in the axon through the activation of neurotransmitter receptors on the axonal membrane surface. Research has shown that the axon can express receptors of various neurotransmitters, including glutamate, GABA, ACh, and biogenic amines, which can alter the relative contribution of certain channels to axonal excitability. This renders the contribution of this compartment to neural coding conditional on the presence of neuromodulators. Both ionotropic and metabotropic receptors have been found in non-synaptic axonal membranes [38]. The majority of evidence for axonal NRM comes from experiments involving the application of chemicals and drugs.
Another example of indirect NRM of dendritic action potentials involves the regulation of tonic GABAergic inhibition through α5-subunit-containing GABAA receptors in adolescent CA1 hippocampal pyramidal neurons [39]. These receptors provide a tonic dendritic current that regulates the excitability of dendrites, thereby indirectly controlling the backpropagation of dendritic action potentials. Similarly, extracellular-regulated kinase-specific MAPK has been shown to play a crucial role in mediating the modulation of dendritic potentials [40]. Therefore, these indirect NRMs are particularly important in adolescent brain development.
The Significance and Prospects of NRM
The significance of NRM lies in the fact that changes in the concentration of endogenous bioactive molecules can cause NRM, which greatly impacts brain function. This has important implications for understanding the pathogenesis and occurrence of diseases. For example, when administering reagents under experimental or clinical conditions, changes in drug or reagent concentrations can result in NRM, which is important in interpreting and evaluating the effects of drugs used in experimental research and clinical treatment.
It is commonly believed that the functions of the brain, such as consciousness, cognition, and intelligence, are a result of the digital activity in neurons. However, neuronal excitability can also be modulated through NRM via non-digital activity. Thus, studying digital and non-digital signals and their importance in explaining brain function is a significant challenge, both currently and in the future.
Acknowledgements
This review article was supported by grants from the National Natural Science Foundation of China (31970913 and 32170957); the Natural Science Foundation of Guangdong Province (2021A1515012156); National Key Research and Development Program of China (2021ZD0201703), and the Key-Area Research and Development Program of Guangdong Province (2019B030335001).
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Yizhang Chen, Email: chenyizhang1927@163.com.
Lin Xiao, Email: liuyangxiaolin@aliyun.com.
Jian Qiu, Email: qiuj@ohsu.edu.
References
- 1.Kupfermann I. Modulatory actions of neurotransmitters. Annu Rev Neurosci. 1979;2:447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- 2.Chen YZ. Ambient modulation of neuronal excitability. Sheng Li Xue Bao. 2016;68:385–390. [PubMed] [Google Scholar]
- 3.Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485(Pt 2):419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YZ, Hua SY, Wang CA, Wu LG, Gu Q, Xing BR. An electrophysiological study on the membrane receptor-mediated action of glucocorticoids in mammalian neurons. Neuroendocrinology. 1991;53:25–30. doi: 10.1159/000125791. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH Neurons in the Guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen YZ. Neuronomodulation. In: Neuroscience. 4th ed. Peking University Medical Press, 2022: 1131–1149.
- 7.Changeux JP, Christopoulos A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell. 2016;166:1084–1102. doi: 10.1016/j.cell.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Changeux JP. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb Symp Quant Biol. 1961;26:313–318. doi: 10.1101/SQB.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Van Theemsche KM, Van de Sande DV, Snyders DJ, Labro AJ. Hydrophobic drug/toxin binding sites in voltage-dependent K+ and Na+ channels. Front Pharmacol. 2020;11:735. doi: 10.3389/fphar.2020.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lera Ruiz M, Kraus RL. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J Med Chem. 2015;58:7093–7118. doi: 10.1021/jm501981g. [DOI] [PubMed] [Google Scholar]
- 11.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng YJ, Feng Q, Tao J, Zhao R, Ji YH. Allosteric interactions between receptor site 3 and 4 of voltage-gated sodium channels: A novel perspective for the underlying mechanism of scorpion sting-induced pain. J Venom Anim Toxins Incl Trop Dis. 2015;21:42. doi: 10.1186/s40409-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso FC, Lewis RJ. Structure-function and therapeutic potential of spider venom-derived cysteine knot peptides targeting sodium channels. Front Pharmacol. 2019;10:366. doi: 10.3389/fphar.2019.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosti E, Boni R, Gallo A. µ-conotoxins modulating sodium currents in pain perception and transmission: A therapeutic potential. Mar Drugs. 2017;15:295. doi: 10.3390/md15100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DM, Nimigean CM. Voltage-gated potassium channels: A structural examination of selectivity and gating. Cold Spring Harb Perspect Biol. 2016;8:a029231. doi: 10.1101/cshperspect.a029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milescu M, Lee HC, Bae CH, Kim JI, Swartz KJ. Opening the shaker K+ channel with hanatoxin. J Gen Physiol. 2013;141:203–216. doi: 10.1085/jgp.201210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilley DC, Angueyra JM, Eum KS, Kim H, Chao LH, Peng AW, et al. The tarantula toxin GxTx detains K+ channel gating charges in their resting conformation. J Gen Physiol. 2019;151:292–315. doi: 10.1085/jgp.201812213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manville RW, Papanikolaou M, Abbott GW. Direct neurotransmitter activation of voltage-gated potassium channels. Nat Commun. 1847;2018:9. doi: 10.1038/s41467-018-04266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghit A, Assal D, Al-Shami AS, Eldin E Hussein D. GABA_A receptors: Structure, function, pharmacology, and related disorders. J Genet Eng Biotechnol. 2021;19:1–15. doi: 10.1186/s43141-021-00224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, Cao L, Yuan K, Shi J, Yan W, Lu L. Unique pharmacology, brain dysfunction, and therapeutic advancements for fentanyl misuse and abuse. Neurosci Bull. 2022;38:1365–1382. doi: 10.1007/s12264-022-00872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carver CM, Shapiro MS. Gq-coupled muscarinic receptor enhancement of KCNQ2/3 channels and activation of TRPC channels in multimodal control of excitability in dentate gyrus granule cells. J Neurosci. 2019;39:1566–1587. doi: 10.1523/JNEUROSCI.1781-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams PR, Jones SW, Pennefather P, Brown DA, Koch C, Lancaster B. Slow synaptic transmission in frog sympathetic Ganglia. J Exp Biol. 1986;124:259–285. doi: 10.1242/jeb.124.1.259. [DOI] [PubMed] [Google Scholar]
- 24.Correa AMB, Guimarães JDS, dos Santos e Alhadas E, Kushmerick C. Control of neuronal excitability by Group I metabotropic glutamate receptors. Biophys Rev. 2017;9:835–845. doi: 10.1007/s12551-017-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: Activation of an inwardly rectifying potassium conductance. J Neurophysiol. 1997;77:1349–1361. doi: 10.1152/jn.1997.77.3.1349. [DOI] [PubMed] [Google Scholar]
- 26.Courtney NA, Ford CP. Mechanisms of 5-HT1A receptor-mediated transmission in dorsal raphe serotonin neurons. J Physiol. 2016;594:953–965. doi: 10.1113/JP271716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtney NA, Ford CP. The timing of dopamine- and noradrenaline-mediated transmission reflects underlying differences in the extent of spillover and pooling. J Neurosci. 2014;34:7645–7656. doi: 10.1523/JNEUROSCI.0166-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutar P, Petrozzino JJ, Vu HM, Schmidt MF, Perkel DJ. Slow synaptic inhibition mediated by metabotropic glutamate receptor activation of GIRK channels. J Neurophysiol. 2000;84:2284–2290. doi: 10.1152/jn.2000.84.5.2284. [DOI] [PubMed] [Google Scholar]
- 29.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99:8366–8371. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ping YQ, Mao C, Xiao P, Zhao RJ, Jiang Y, Yang Z, et al. Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature. 2021;589:620–626. doi: 10.1038/s41586-020-03083-w. [DOI] [PubMed] [Google Scholar]
- 32.Kanageswaran N, Nagel M, Scholz P, Mohrhardt J, Gisselmann G, Hatt H. Modulatory effects of sex steroids progesterone and estradiol on odorant evoked responses in olfactory receptor neurons. PLoS One. 2016;11:e0159640. doi: 10.1371/journal.pone.0159640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian J, Zhu L, Li Q, Belevych N, Chen Q, Zhao F, et al. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc Natl Acad Sci USA. 2012;109:12189–12194. doi: 10.1073/pnas.1205207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson NJ, Ferguson AV. The proinflammatory cytokine tumor necrosis factor-α excites subfornical organ neurons. J Neurophysiol. 2017;118:1532–1541. doi: 10.1152/jn.00238.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaves FM, Mansano NS, Frazão R, Donato J., Jr Tumor necrosis factor α and interleukin-1β acutely inhibit AgRP neurons in the arcuate nucleus of the hypothalamus. Int J Mol Sci. 2020;21:8928. doi: 10.3390/ijms21238928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotel F, Exley R, Cragg SJ, Perrier JF. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci USA. 2013;110:4774–4779. doi: 10.1073/pnas.1216150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrier JF, Rasmussen HB, Jørgensen LK, Berg RW. Intense activity of the raphe spinal pathway depresses motor activity via a serotonin dependent mechanism. Front Neural Circuits. 2018;11:111. doi: 10.3389/fncir.2017.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballo AW, Nadim F, Bucher D. Dopamine modulation of Ih improves temporal fidelity of spike propagation in an unmyelinated axon. J Neurosci. 2012;32:5106–5119. doi: 10.1523/JNEUROSCI.6320-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groen MR, Paulsen O, Pérez-Garci E, Nevian T, Wortel J, Dekker MP, et al. Development of dendritic tonic GABAergic inhibition regulates excitability and plasticity in CA1 pyramidal neurons. J Neurophysiol. 2014;112:287–299. doi: 10.1152/jn.00066.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]