Abstract
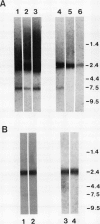
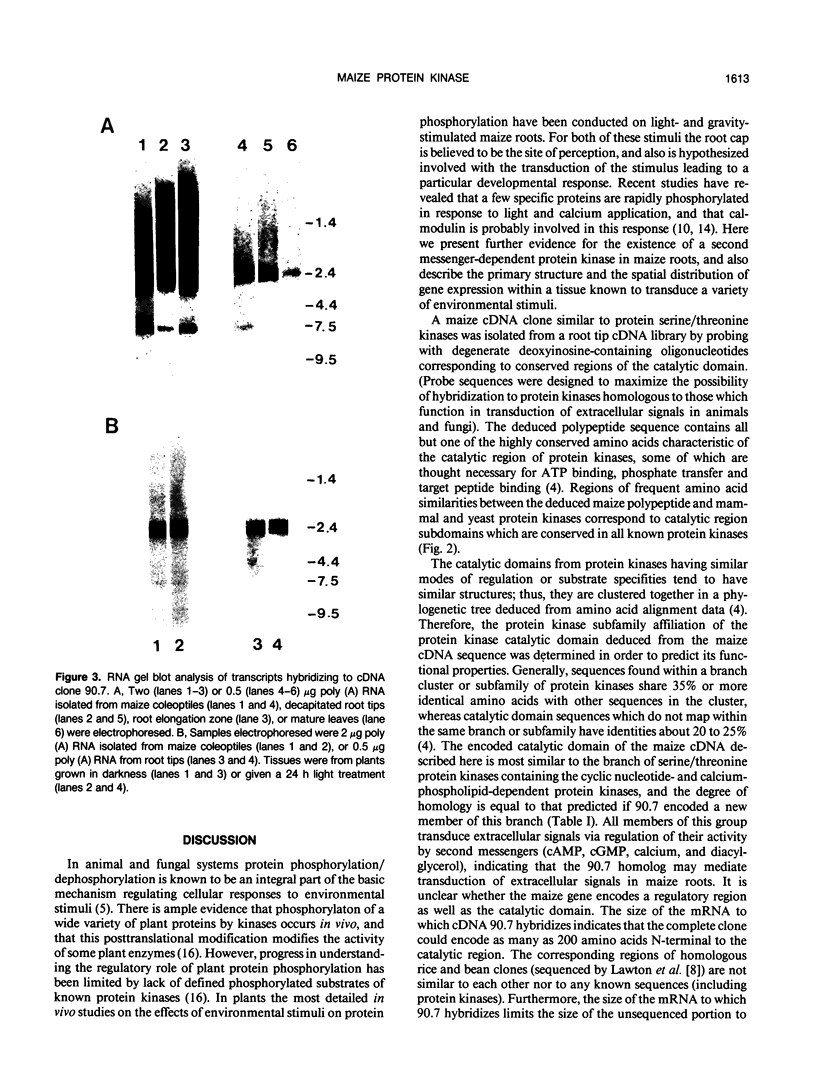
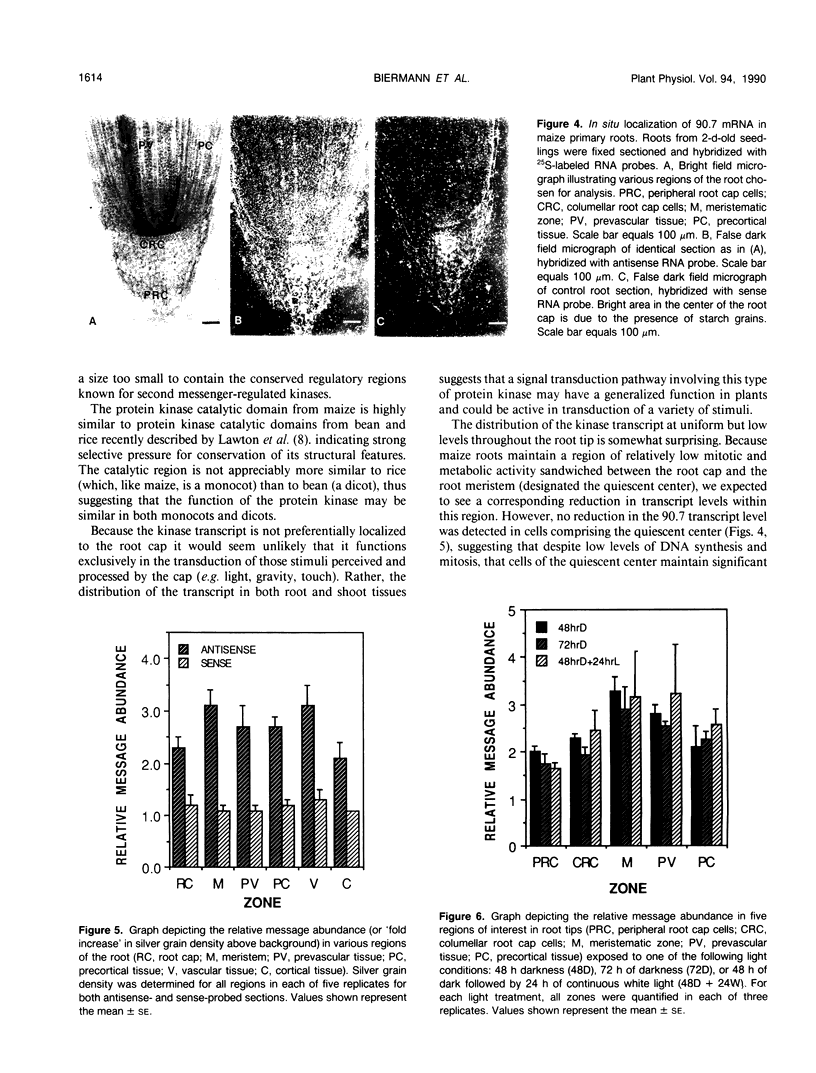
Maize (Zea mays) roots respond to a variety of environmental stimuli which are perceived by a specialized group of cells, the root cap. We are studying the transduction of extracellular signals by roots, particularly the role of protein kinases. Protein phosphorylation by kinases is an important step in many eukaryotic signal transduction pathways. As a first phase of this research we have isolated a cDNA encoding a maize protein similar to fungal and animal protein kinases known to be involved in the transduction of extracellular signals. The deduced sequence of this cDNA encodes a polypeptide containing amino acids corresponding to 33 out of 34 invariant or nearly invariant sequence features characteristic of protein kinase catalytic domains. The maize cDNA gene product is more closely related to the branch of serine/threonine protein kinase catalytic domains composed of the cyclic-nucleotide- and calcium-phospholipid-dependent subfamilies than to other protein kinases. Sequence identity is 35% or more between the deduced maize polypeptide and all members of this branch. The high structural similarity strongly suggests that catalytic activity of the encoded maize protein kinase may be regulated by second messengers, like that of all members of this branch whose regulation has been characterized. Northern hybridization with the maize cDNA clone shows a single 2400 base transcript at roughly similar levels in maize coleoptiles, root meristems, and the zone of root elongation, but the transcript is less abundant in mature leaves. In situ hybridization confirms the presence of the transcript in all regions of primary maize root tissue.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. J., Piechulla B., Sun P. S. Light-regulated protein and mRNA synthesis in root caps of maize. Plant Mol Biol. 1988;11:27–34. [PubMed] [Google Scholar]
- Feldman L. J. Regulation of root development. Annu Rev Plant Physiol. 1984;35:223–242. doi: 10.1146/annurev.pp.35.060184.001255. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Yamamoto R. T., Hanks S. K., Lamb C. J. Molecular cloning of plant transcripts encoding protein kinase homologs. Proc Natl Acad Sci U S A. 1989 May;86(9):3140–3144. doi: 10.1073/pnas.86.9.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Poovaiah B. W. Rapid changes in protein phosphorylation associated with light-induced gravity perception in corn roots. Plant Physiol. 1988;86:332–334. doi: 10.1104/pp.86.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., McFadden J. J., Reddy A. S. The role of calcium ions in gravity signal perception and transduction. Physiol Plant. 1987;71:401–407. doi: 10.1111/j.1399-3054.1987.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Raghothama K. G., Reddy A. S., Friedmann M., Poovaiah B. W. Calcium-regulated in vivo protein phosphorylation in Zea mays L. root tips. Plant Physiol. 1987;83:1008–1013. doi: 10.1104/pp.83.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K. T., Fink J. S., Gilman M. Z., Walsh D. A., Goodman R. H., Feramisco J. R. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988 Nov 3;336(6194):83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Roux S. J., Serlin B. S. Cellular mechanisms controlling light-stimulated gravitropism: role of calcium. CRC Crit Rev Plant Sci. 1987;5(3):205–236. doi: 10.1080/07352688709382240. [DOI] [PubMed] [Google Scholar]
- Shoji S., Ericsson L. H., Walsh K. A., Fischer E. H., Titani K. Amino acid sequence of the catalytic subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1983 Jul 19;22(15):3702–3709. doi: 10.1021/bi00284a025. [DOI] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]