Abstract
The long-term outcome of first-line moderate-intensity statin with ezetimibe combination therapy for secondary prevention after percutaneous coronary intervention in patients with acute coronary syndrome (ACS) compared to high-intensity statin monotherapy remains elusive. The objective of this study was to compare the effectiveness of moderate-intensity statin and ezetimibe combination therapy with high-intensity statin monotherapy. We conducted a nationwide, population-based, retrospective, cohort study of patients with ACS from 2013 to 2019. The patients using combination therapy were matched (1:1) to those using monotherapy. The primary outcome was a composite of myocardial infarction, stroke and all-cause mortality. We estimated the hazard ratios (HR) and 95% confidence intervals (CIs) using the Cox proportional hazards regression. After propensity score matching, 10,723 pairs were selected. Men accounted for 70% of the patients and 37% aged > 70 years. The primary endpoint occurred in 1297 patients (12.1%) in the combination group and in 1426 patients (13.3%) in the monotherapy group, and decreased risk (HR 0.85, 95% CI 0.78–0.92, P < 0.001) in the combination group. Among the patients with ACS, moderate-intensity statin with ezetimibe combination therapy was associated with decreased risk of adverse cardiovascular outcomes compared with high-intensity statin monotherapy in a nationwide population-based study representing routine clinical practice.
Subject terms: Cardiology, Diseases, Health care, Medical research
Introduction
The idea of ‘lower is better’ has become a central dogma in secondary prevention of coronary atherosclerotic disease1,2. Reducing low-density lipoprotein cholesterol by using high-dose 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, is one of the cornerstones in patient management after percutaneous coronary intervention3,4. Decreased low-density lipoprotein cholesterol levels with high-intensity statin therapy has been proven to prevent secondary atherosclerotic cardiovascular disease in a dose–response manner5,6. However, the evidence has been poorly translated into clinical practice. Many clinicians and patients alike are reluctant to initiate and maintain high-intensity statins for secondary prevention because of anxiety to a higher rate of adverse drug related events and psychological burden to high-intensity, even after percutaneous coronary intervention, and often require additional treatment that includes ezetimibe7,8.
After the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) demonstrated the superior efficacy of combined moderate-intensity statin and ezetimibe therapy compared with moderate-intensity statin monotherapy in hospitalized patients with acute coronary syndrome9, current guidelines recommend that in cases where the optimal low-density lipoprotein goal is not achieved, ezetimibe therapy should be added to the maximally tolerated statin dose10. However, this trial was conducted to assess the comparison between moderate-intensity statin monotherapy and combination therapy, therefore the effectiveness of moderate-intensity statin with ezetimibe combination therapy compared to of high-intensity statin monotherapy remains unclear.
The recent the randomized comparison of efficacy and safety of lipid lowering with statin monotherapy versus statin–ezetimibe combination for high-risk cardiovascular disease (RACING) trial demonstrated the comparable long-term efficacy and safety of high-intensity statin monotherapy versus moderate-intensity statin with ezetimibe combination therapy in patients with stable status of atherosclerotic cardiovascular disease11. However, patient compliance with the prescribed lipid-lowering therapy and the proportion of patients with low-density lipoprotein cholesterol concentration levels < 70 mg/dL was higher in the moderate-intensity statin with ezetimibe combination therapy group than in the high-intensity statin monotherapy group11. Nonetheless, there is still uncertainty about safety for using moderate-intensity statin with ezetimibe combination therapy instead of high-intensity monotherapy focused on the patients with acute coronary syndrome even in immediately after percutaneous coronary intervention.
This study aimed to compare the effectiveness to reduction of cardiovascular outcome and drug adherence of moderate-intensity statin with ezetimibe combination therapy with high-intensity statin monotherapy in patients who underwent percutaneous coronary intervention with acute coronary syndrome in routine clinical practice.
Materials and methods
Data sources
This study was a retrospective analysis based on the Korean National Health Claims database established by the National Health Insurance Service (NHIS). The NHIS, a single insurer run by the Korean government, includes 97.1% of Korean citizens, with the remaining 3% designated as those in need of medical assistance. This database includes comprehensive data on patient sociodemographic information, inpatient and outpatient services, drug prescriptions, procedures, and mortality12. Diagnostic variables are recoded according to the International Classification of Diseases, 10th revision (ICD‐10) codes, and data with anonymized identifiers were provided to the researchers.
Cohort design and study population
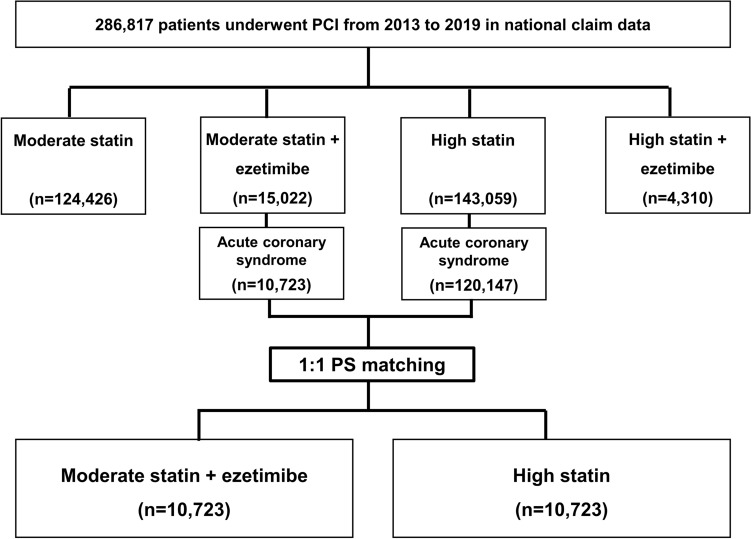
We conducted a nationwide, population-based, retrospective cohort study. We enrolled patients who underwent percutaneous coronary intervention with a diagnosis of acute coronary syndrome between January 1, 2013, and December 31, 2019 (Fig. 1). The lipid-lowering strategy was characterized at hospital discharge (based on the most frequently prescribed drug group during hospitalization) as follows: moderate-intensity statin monotherapy, moderate-intensity statin with ezetimibe combination therapy, high-intensity statin monotherapy, and high-intensity statin with ezetimibe combination therapy (Supplementary Table S1). The statin intensity was prescribed according to the 2013 American College of Cardiology and American Heart Association guidelines13. The moderate-intensity statin with ezetimibe combination therapy and high-intensity statin monotherapy groups were restricted to acute coronary syndrome and matched using 1:1 propensity score matching.
Figure 1.
Flow chart of the study population. PCI, percutaneous coronary intervention; PSM, propensity score matching.
Outcome and covariates
The primary outcome was a composite of myocardial infarction, ischemic stroke, and all-cause mortality (Supplementary Table S2)11. Secondary outcomes included individual outcomes of the primary outcome.
Deaths during hospitalization for percutaneous coronary intervention were excluded from the analysis. Myocardial infarction was defined as a case in which the cardiovascular procedure code and a new diagnosis of myocardial infarction as the main disease were recorded. Stroke was defined as both new hospitalization for and diagnosis of stroke as the main disease. Event definitions and covariates are presented in Supplementary Table S2.
Patient history was extracted from the date of percutaneous coronary intervention and backtracked up to 2 years prior to intervention. A clinical presentation was considered as newly diagnosed if the diagnosis was made within 1 week before the percutaneous coronary intervention hospitalization period. If there were two or more diagnoses, the more severe diagnosis was selected. Drug adherence was defined as patients who collected at least 80% of the prescribed statin monotherapy or combination therapy for 3 months after discharge for percutaneous coronary intervention.
Statistical analysis
The frequency and proportion of patients were reported according to baseline characteristics, comorbidity, revascularization, clinical presentation at index procedure, year of percutaneous coronary intervention, and drug adherence, which equal or exceeded 80% among the study and matched populations. Categorical variables were performed using the chi-square test and presented as means and standard deviations and were analyzed using the independent t-test and analysis of variance test. Propensity scores were calculated by logistic regression for baseline characteristics, the size of the hospital, potent P2Y12 inhibitor use and years of percutaneous coronary intervention where the patients received treatment. We considered 1:1 propensity score matching as the nearest neighbor with calipers of width of 0.2 standard deviations of the logit of the propensity score between the moderate-intensity statin with ezetimibe and high-intensity statin groups. A calculated standardized difference of < 0.1 was considered acceptable.
Survival analysis was conducted using the Kaplan–Meier method with log-rank test. The follow-up period was defined from the index date until the occurrence of the outcome, or the last date of the study (December 31, 2020), whichever came first. The proportional hazards assumption was tested using the by log− log survival plots, and the violations of the assumption were not found for the outcome. The hazard ratios (HR) and 95% confidence intervals (CIs) were assessed using the Cox proportional hazards regression model among the matched populations. We adjusted for drug adherence for statin monotherapy or combination therapy (≥ 80%) to control for confounders. Statistical significance was defined as a two-sided p-value < 0.05.
In the subgroup analysis, the risk according to age (≥ 70 years), sex, myocardial infarction history, diabetes mellitus, acute myocardial infarction, and drug adherence was calculated, and the interaction with statin therapy was analyzed using the p-value for interaction. As a sensitivity analysis, we used an inverse probability of treatment weighting approach based on baseline characteristics and the size of the hospital to calculate the risk of adverse outcomes on the weighted population. Database construction and statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). The study was performed in accordance with the Declaration of Helsinki and reported in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Ethical approval
This study was approved and the requirement for informed consent was waived because of the retrospective nature of the study by the Institutional Review Board of the National Health Insurance Service Ilsan Hospital (IRB No. 2021-03-024).
Results
Patient characteristics
We identified 286,817 patients who underwent percutaneous coronary intervention between 2013 and 2019; 124,426 patients (43.4%) received moderate-intensity statin, 15,022 patients (5.2%) received combined moderate-intensity statin with ezetimibe, 143,059 patients (49.9%) received high-intensity statin, and 4310 patients (1.5%) received combined high-intensity statin with ezetimibe (Fig. 1, Table 1). The combined use of moderate-intensity statins with ezetimibe has steadily increased in clinical practice. The 60–69-year-old group was the largest (28.7%), and 71.2% of the patients were male.
Table 1.
Baseline characteristics of patients with acute coronary syndrome who underwent percutaneous coronary intervention.
| Statin group | Moderate statin | Moderate statin + ezetimibe | High statin | High statin + ezetimibe |
|---|---|---|---|---|
| Participants | 124,426 (100.0) | 15,022 (100.0) | 143,059 (100.0) | 4310 (100.0) |
| Male sex | 84,940 (68.3) | 10,580 (70.4) | 105,590 (73.8) | 3228 (74.9) |
| Age groups | ||||
| ≤ 40 s | 10,420 (8.4) | 1319 (8.8) | 17,417 (12.2) | 570 (13.2) |
| 50 s | 26,287 (21.1) | 3424 (22.8) | 35,113 (24.5) | 1165 (27.0) |
| 60 s | 35,991 (28.9) | 4831 (32.1) | 40,130 (28.1) | 1248 (29.0) |
| 70 s | 35,671 (28.7) | 3863 (25.7) | 34,493 (24.1) | 894 (20.7) |
| ≥ 80 s | 16,057 (12.9) | 1585 (10.6) | 15,906 (11.1) | 433 (10.1) |
| Comorbidity and revascularization | ||||
| Diabetes mellitus | 46,553 (37.4) | 5658 (37.7) | 51,026 (35.7) | 1654 (38.4) |
| Hypertension | 106,925 (85.9) | 12,989 (86.5) | 119,945 (83.8) | 3441 (79.8) |
| MI | 33,956 (27.3) | 4166 (27.7) | 67,637 (47.3) | 1792 (41.6) |
| Heart failure | 30,431 (24.5) | 3082 (20.5) | 32,048 (22.4) | 1040 (24.1) |
| PAD | 9695 (7.8) | 1302 (8.7) | 8801 (6.2) | 295 (6.8) |
| ESRD | 4548 (3.7) | 369 (2.5) | 3747 (2.6) | 100 (2.3) |
| Prior PCI | 9830 (7.9) | 672 (4.5) | 12,252 (8.6) | 454 (10.5) |
| Prior CABG | 10,053 (8.1) | 691 (4.6) | 12,401 (8.7) | 456 (10.6) |
| Clinical presentation at index procedure | ||||
| STEMI | 25,820 (20.8) | 3124 (20.8) | 36,251 (25.3) | 1062 (24.6) |
| NSTEMI | 25,921 (20.8) | 3236 (21.5) | 47,975 (33.5) | 1294 (30.0) |
| Unstable angina | 31,894 (25.6) | 3750 (25.0) | 26,452 (18.5) | 881 (20.4) |
| Stable angina | 29,739 (23.9) | 3676 (24.5) | 20,150 (14.1) | 748 (17.4) |
| Others | 11,052 (8.9) | 1236 (8.2) | 12,231 (8.5) | 325 (7.5) |
| Potent P2Y12 inhibitor | 9515 (7.7) | 1386 (9.2) | 24,555 (17.2) | 666 (15.5) |
| Years of PCI | ||||
| 2013 | 20,137 (16.2) | 843 (5.6) | 12,587 (8.8) | 0 (0.0) |
| 2014 | 19,796 (15.9) | 739 (4.9) | 17,341 (12.1) | 0 (0.0) |
| 2015 | 17,898 (14.4) | 791 (5.3) | 19,183 (13.4) | 0 (0.0) |
| 2016 | 18,658 (15.0) | 1586 (10.6) | 21,190 (14.8) | 206 (4.8) |
| 2017 | 17,009 (13.6) | 3173 (21.1) | 22,601 (15.8) | 768 (17.8) |
| 2018 | 15,886 (12.8) | 3518 (23.4) | 24,504 (17.1) | 1248 (29.0) |
| 2019 | 15,042 (12.1) | 4372 (29.1) | 25,653 (18.0) | 2088 (48.4) |
Data are number (%).
MI myocardial infarction, PAD peripheral artery disease, ESRD end-stage renal disease, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, STEMI ST-elevation myocardial infarction, NSTEMI non-ST elevation myocardial infarction.
Patients in the high-intensity statin group were more likely to have myocardial infarction (47.3% vs. 27.7%), heart failure (22.4% vs. 20.5%), and revascularization (8.6% vs. 4.5% with percutaneous coronary intervention and 8.7% vs. 4.6% with coronary artery bypass graft) than the moderate-intensity statin with ezetimibe group (Table 1). Patients in the high-intensity statin group had higher prevalence of ST-elevation myocardial infarction (27.7% vs. 22.7%), non-ST-elevation myocardial infarction (36.7% vs. 23.4%), and potent P2Y12 inhibitor use (17.2% vs. 9.2%) than the moderate-intensity statin with ezetimibe group.
Using 1:1 propensity score matching, 10,723 pairs were selected among patients who were prescribed monotherapy high-intensity statin and combined moderate-intensity statin with ezetimibe therapy after percutaneous coronary intervention for acute coronary syndrome (Fig. 1, Table 2). After propensity score matching, maximum standardized mean difference was 0.07 (Table 2). The propensity score distribution is shown in Supplementary Fig. S1. The mean follow-up duration was 1028 days in the moderate-intensity statin with ezetimibe group and 1026 days in the high-intensity statin group (Table 2). Approximately 70% of the patients were male, more than one-third were ≥ 70 years of age, 12% were receiving potent P2Y12 inhibitors, and 58% were treated at tertiary hospitals. Of the clinical presentations at the index procedure, unstable angina was the most common, followed by non-ST-elevation myocardial infarction, and ST-elevation myocardial infarction. The proportion of patients undergoing percutaneous coronary intervention between 2013 and 2019 increased from 6.0 to 28.2% in the moderate-intensity statin with ezetimibe group and from 5.8 to 28.1% in the high-intensity statin group.
Table 2.
Baseline characteristics of the matched population.
| Statin group | Moderate Statin + Ezetimibe | High Statin | Standardized Difference |
|---|---|---|---|
| Participants | 10,723 (100.0) | 10,723 (100.0) | |
| Follow-up duration, days | 1028 ± 654 | 1026 ± 645 | |
| Male sex | 7544 (70.4) | 7590 (70.8) | − 0.01 |
| Age groups | 0.00 | ||
| ≤ 40 s | 1015 (9.5) | 994 (9.3) | |
| 50 s | 2489 (23.2) | 2459 (22.9) | |
| 60 s | 3307 (30.8) | 3311 (30.9) | |
| 70 s | 2714 (25.3) | 2734 (25.5) | |
| ≥ 80 s | 1198 (11.2) | 1225 (11.4) | |
| Comorbidity and revascularization | |||
| Diabetes mellitus | 3546 (33.1) | 3516 (32.8) | 0.01 |
| Hypertension | 9245 (86.2) | 9305 (86.8) | − 0.02 |
| Old MI | 3967 (37.0) | 3962 (37.0) | 0.00 |
| Heart failure | 1788 (16.7) | 1737 (16.2) | 0.01 |
| PAD | 891 (8.3) | 829 (7.7) | 0.02 |
| ESRD | 270 (2.5) | 167 (1.6) | 0.07 |
| Prior PCI | 507 (4.7) | 459 (4.3) | 0.02 |
| Prior CABG | 521 (4.9) | 462 (4.3) | 0.03 |
| Clinical presentation at index procedure | |||
| STEMI | 1828 (17.0) | 1851 (17.3) | − 0.01 |
| NSTEMI | 3076 (28.7) | 3089 (28.8) | 0.00 |
| Unstable angina | 4563 (42.6) | 4538 (42.3) | 0.00 |
| Others | 1256 (11.7) | 1245 (11.6) | 0.00 |
| Potent P2Y12 inhibitor | 1255 (11.7) | 1245 (11.6) | 0.03 |
| Tertiary general hospital | 6247 (58.3) | 6189 (57.7) | 0.01 |
| Years of PCI | 0.05 | ||
| 2013 | 645 (6.0) | 625 (5.8) | |
| 2014 | 562 (5.3) | 573 (5.3) | |
| 2015 | 578 (5.4) | 593 (5.5) | |
| 2016 | 1199 (11.2) | 1231 (11.5) | |
| 2017 | 2245 (20.9) | 2256 (21.0) | |
| 2018 | 2469 (23.0) | 2439 (22.8) | |
| 2019 | 3025 (28.2) | 3006 (28.1) | |
Data are number (%) or mean ± standard deviation (SD).
MI, myocardial infarction; PAD, peripheral artery disease; ESRD, end-stage renal disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction.
Comparative effectiveness
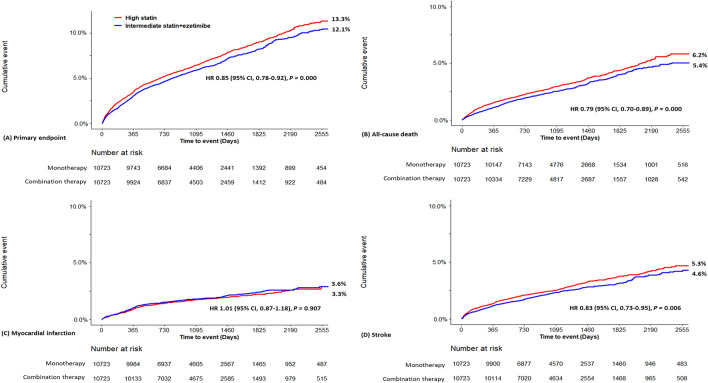
The cumulative incidence curves for the major adverse cardiovascular event are shown in Fig. 2. Among the matched patients, the primary endpoints, including myocardial infarction, ischemic stroke, and all-cause mortality, occurred in 1,297 patients (4.29 events per 100 person-year) in the moderate-intensity statin with ezetimibe combination group and in 1426 patients (4.73 events per 100 person-year) in the high-intensity statin group (Table 3). The risk and incidence of the primary outcome were significantly lower (HR 0.85, 95% CI 0.78–0.92) in the moderate-intensity statin with ezetimibe combination group. The risk of all-cause mortality (HR 0.79, 95% CI 0.70–0.89) and stroke (HR 0.83, 95% CI 0.73–0.95) were lower in the combination group. There was no difference in the risk of myocardial infarction (HR 1.01, 95% CI 0.87–1.18) between the two groups.
Figure 2.
Kaplan–Meier curves of the primary endpoint in matched population.
Table 3.
Risk of clinical outcomes and description of statin adherence in the matched population.
| Moderate Statin + Ezetimibe (n = 10,723) | High Statin (n = 10,723) | Hazard ratio | P value | |
|---|---|---|---|---|
| Primary endpoint—Composite of all-cause death, myocardial infarction, and stroke | 1297 (12.1) | 1426 (13.3) | 0.85 (0.78–0.92) | 0.000 |
| All-cause death | 574 (5.4) | 664 (6.2) | 0.79 (0.70–0.89) | 0.000 |
| Myocardial infarction | 382 (3.6) | 358 (3.3) | 1.01 (0.87–1.18) | 0.907 |
| Stroke | 493 (4.6) | 564 (5.3) | 0.83 (0.73–0.95) | 0.006 |
| Drug adherence (≥ 80%) | 7,276 (67.8) | 5,363 (50.0) | 0.000 |
Data are number (%).
Hazard ratio for moderate statin + ezetimibe group was derived from Cox proportional analysis.
Drug adherence was defined as patients who collected at least 80% of the prescribed statin monotherapy or combination therapy for 3 months after hospitalization for percutaneous coronary intervention.
Based on the 90-day statin prescription, statin compliance in the moderate-intensity statin with ezetimibe combination therapy group was higher than that in the high-intensity statin monotherapy group (67.8% vs. 50.0%, p < 0.001).
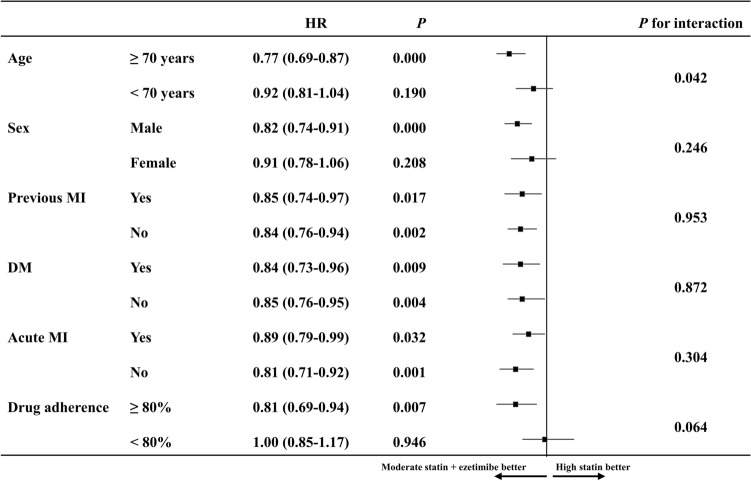
Subgroup analyses
A subgroup analysis was performed according to the primary endpoint of the matched population by age (≥ 70 years), sex, myocardial infarction history, diabetes mellitus, acute myocardial infarction, and drug adherence (Fig. 3). The risk of the primary endpoint showed consistent result by myocardial infarction history, diabetes mellitus and acute myocardial infarction. There was no interaction effect in any subgroup.
Figure 3.
Subgroups analysis for the primary endpoint in matched population. HR, Hazard ratio; MI, myocardial infarction; DM, diabetes mellitus.
Sensitivity analyses
The population also standardized differences between the matched groups were balanced for identified confounders, except for hospital size, after the inverse probability of treatment weighting (Supplementary Table S3). The risks of primary endpoint (HR 0.91, 95% CI 0.86–0.95) and all-cause mortality (HR 0.86, 95% CI 0.80–0.93) were lower in the combination group, and the results showed similar pattern with the propensity score matching (Supplementary Table S4). In the subgroup analysis, risk of the primary endpoint was decreased in the all subgroups excepts to female (HR 0.98, 95% CI 0.90–1.07) and low drug adherence (HR 0.97, 95% CI 0.90–1.04).
Discussion
In this study, we found that (i) the risks of major adverse cardiovascular event were decreased in the use of moderate-intensity statin with ezetimibe combination therapy compared to high-intensity statin monotherapy in patients who underwent percutaneous coronary intervention for acute coronary syndrome. The risk in all-cause mortality and stroke were also lower; (ii) the moderate-intensity statin with ezetimibe combination therapy group showed a higher rate of drug compliance for 90 days after discharge; (iii) regardless of myocardial infarction history, diabetes mellitus and acute myocardial infarction, the risks of cardiovascular composite outcomes decreased in the ezetimibe combination therapy, and there was no interaction between each subgroup and treatment strategies. To the best of our knowledge, this study enrolled the largest population representing nationwide clinical practice.
The results of this study are comparable to those of previous interventional and observational studies. A Chinese randomized clinical trial found superior efficacy of moderate-intensity statin with ezetimibe combination therapy over high-intensity statin monotherapy in decreasing low-density lipoprotein cholesterol levels in stable patients14. Observational studies, which enrolled patients with combination lipid-lowering therapy and included < 1000 patients, also found comparable effectiveness of the two therapy options after acute myocardial infarction or percutaneous coronary intervention15–17. The recent RACING trial demonstrated the non-inferiority of combination therapy compared with high-intensity statin in patients with stable atherosclerotic cardiovascular disease with a higher control rate of low-density lipoprotein cholesterol level and therapy adherence in the combination therapy group11. In this clinical trial, included subjects with various conditions for vascular disease which was not specified to patients underwent percutaneous coronary intervention. Therefore, although numerous studies have explored high-intensity monotherapy and moderate-intensity combination treatment to reduce lipid levels for secondary prevention, there remains insufficient evidence for the preemptive use of combination therapy in high-risk patients who have undergone stent implantation for acute coronary syndrome9,18. In this regard, we established evidence for the additional benefits of combining moderate-intensity statins with ezetimibe in acute coronary syndrome patients.
Current guidelines recommend initiating and maintaining high-potency statins, which can decrease low-density lipoprotein cholesterol levels by > 50%. As the benefit of statin therapy largely relies on individual absolute risks, it is important to reinforce prolonged statin treatment in high-risk patients, such as patients undergoing percutaneous coronary intervention5. Nonetheless, the progress toward improved lipid management in routine clinical practice has been slower than expected. Among Medicare beneficiaries hospitalized for myocardial infarction, less than two-thirds adhered to high-intensity statin therapy at 6 months (58.9%) and 2 years (41.6%) after discharge7.
Because of the high failure rate in achieving the low-density lipoprotein target in patients with atherosclerotic cardiovascular disease, the 2019 European Society of Cardiology guidelines recommend adding ezetimibe therapy to the maximally tolerated intensity of statin. However, this study, which represents routine clinical practice in Korea and was performed before the publication of the RACING trial, identified an increasing trend of favoring moderate-intensity statin with ezetimibe combination therapy over high-intensity statin monotherapy in patients undergoing percutaneous coronary intervention. This trend of favoring combination therapy has been previously demonstrated in the treatment of hypertension.
Since 2018, the European Society of Cardiology/European Society of Hypertension guidelines recommend initiating antihypertensive drug treatment with combination therapy rather than sequential monotherapy or the stepped-care strategy19. Of note, this recommendation was based on weak supporting evidence from a small-sized randomized controlled trial20 and large observational studies21,22, which reported that initial combination therapy is better in achieving target blood pressure than sequential monotherapy or stepped-care strategies without clear demonstration of hard clinical endpoints. The reasons for favoring initial combination therapy over add-on therapy for hypertension may include the following: (1) the well-established relationship between blood pressure control and clinical endpoints and additional evidence supporting the idea that ‘lower is better’ in blood pressure; (2) accumulated evidence of the importance of treatment adherence; (3) the reluctance of physicians to prescribe high-intensity antihypertensive medication; (4) physician or treatment inertia can lead to suboptimal completion of the treatment regimen; (5) the presence of a single-pill combination23. This hypertension management approach can be applied to the management of hyperlipidemia.
This study had several limitations. Although propensity scoring matched the well-balanced baseline characteristics of the two groups, the possibility of unadjusted bias cannot be ruled out. And, the size of the two groups was different. Therefore, we tried to reduce the discrepancy between the two groups by conducting 1:1 propensity score matching and inverse probability of treatment weighting approach. Second, validation of diagnosis was not performed to confirm the outcome in the study population during follow-up due to the use of a de-identified claim database. However, the accuracy in definitions of stroke and recurrent acute myocardial infarction was reported in previous studies with similar outcome incidence, with more than 90.5% and 92.0% positive predictive value in the NHIS database24,25. Third, since no laboratory data are available, it is unknown whether the low-density lipoprotein-lowering effect of combination therapy was greater than that of the high-intensity statin group. Fourth, the results of this study do not guarantee generalizability of finding beyond Korea.
Conclusions
In this observational study, moderate-intensity statin therapy with ezetimibe combination therapy provides a benefit over high-intensity statin monotherapy for the risk of adverse cardiovascular outcomes and all-cause mortality in the patients after percutaneous coronary intervention for acute coronary syndrome. These finding support a use of statin and ezetimibe combination therapy for patients with acute coronary syndrome.
Supplementary Information
Abbreviations
- RACING trial
Randomized comparison of efficacy and safety of lipid lowering with statin monotherapy versus statin–ezetimibe combination for high-risk cardiovascular disease trial
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- NHIS
National Health Insurance Service
- HR
Hazard ratio
- CI
Confidence interval
Author contributions
Conception/design: J.-Y.J., S.C.Y. and J.-S.K. Data curation: J.C. and S.C. Formal analysis: S.K. and S.C. Funding acquisition: J.-Y.J., S.C.Y. and J.-S.K. Investigation: J.-Y.J., S.K., S.C.Y. and J.-S.K. Methodology: J.-Y.J., S.K., J.C. and S.C.Y. Project administration: J.-Y.J., S.C.Y. and J.-S.K. Software: S.K., J.C. and S.C. Supervision: J.-Y.J., S.C.Y. and J.-S.K. Validation: J.-Y.J., S.K., S.C. and S.C.Y. Visualization: J.-Y.J., S.K. and S.C. Writing: J.-Y.J., S.K. and S.C.Y. Approval of final manuscript: all authors.
Funding
This research was supported by a Grant (22213MFDS486) from Ministry of Food and Drug Safety in 2022.
Data availability
The data used in this study are third-party data owned and operated by the Korean NHIS. Interested researchers can contact the NHIS to access the data (http://nhiss.nhis.or.kr/bd/ab/bdabd003cv.do).
Competing interests
Dr You reports being CTO of PHI Digital Healthcare. Dr Jang, Dr S Kim, Dr Cho, Dr Chun, and Dr and Dr J-S Kim reported no disclosures.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ji-Yong Jang and Seonji Kim.
Contributor Information
Seng Chan You, Email: chandryou@yuhs.ac.
Jung-Sun Kim, Email: kjs1218@yuhs.ac.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-51310-5.
References
- 1.de Lemos JA, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. Jama. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen TR, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: A randomized controlled trial. Jama. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Ballantyne CM, Petersen LA. Guideline-concordant statin therapy use in secondary prevention: Should the medical community wait for divine intervention? J. Am. Coll. Cardiol. 2022;79:1814–1817. doi: 10.1016/j.jacc.2022.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J. Am. Coll. Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 7.Colantonio LD, et al. Adherence to high-intensity statins following a myocardial infarction hospitalization among medicare beneficiaries. JAMA Cardiol. 2017;2:890–895. doi: 10.1001/jamacardio.2017.0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner Ž. Resistance and intolerance to statins. Nutr. Metab. Cardiovasc. Dis. 2014;24:1057–1066. doi: 10.1016/j.numecd.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 10.Collet J-P, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC) Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 11.Kim B-K, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): A randomised, open-label, non-inferiority trial. Lancet. 2022;400:380–390. doi: 10.1016/S0140-6736(22)00916-3. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Lee JS, Park S-H, Shin SA, Kim K. Cohort profile: The national health insurance service–national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 13.Stone NJ, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Qian J, et al. Efficacy and tolerability of ezetimibe/atorvastatin fixed-dose combination versus atorvastatin monotherapy in hypercholesterolemia: A phase III, randomized, active-controlled study in Chinese patients. Clin. Ther. 2022;44:1282–1296. doi: 10.1016/j.clinthera.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, et al. Moderate-intensity statins plus ezetimibe vs. high-intensity statins after coronary revascularization: A cohort study. Cardiovasc. Drugs Ther. 2021;37:141–150. doi: 10.1007/s10557-021-07256-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, et al. Comparison of the effects of high-intensity statin therapy with moderate-intensity statin and ezetimibe combination therapy on major adverse cardiovascular events in patients with acute myocardial infarction: A nationwide cohort study. J. Lipid Atheroscler. 2021;10:291–302. doi: 10.12997/jla.2021.10.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauriah M, et al. High-potency statin and ezetimibe use and mortality in survivors of an acute myocardial infarction: A population-based study. Heart. 2014;100:867–872. doi: 10.1136/heartjnl-2013-304678. [DOI] [PubMed] [Google Scholar]
- 18.Trialists CT. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. The Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 19.Williams B, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of cardiology (ESC) and the European society of hypertension (ESH) Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 20.Mourad J-J, et al. Comparison of different therapeutic strategies in hypertension: A low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approach. J. Hypertens. 2004;22:2379–2386. doi: 10.1097/00004872-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Egan BM, et al. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012;59:1124–1131. doi: 10.1161/HYPERTENSIONAHA.112.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mourad J-J, Nguyen V, Lopez-Sublet M, Waeber B. Blood pressure normalization in a large population of hypertensive patients treated with perindopril/indapamide combination: Results of the OPTIMAX trial. Vasc. Health Risk Manag. 2007;3:173–180. [PMC free article] [PubMed] [Google Scholar]
- 23.Persu A, Lopez-Sublet M, Algharably EAE-H, Kreutz R. Starting antihypertensive drug treatment with combination therapy: Controversies in hypertension-pro side of the argument. Hypertension. 2021;77:800–805. doi: 10.1161/HYPERTENSIONAHA.120.12857. [DOI] [PubMed] [Google Scholar]
- 24.You SC, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Cardiol. 2020;324:1640–1650. doi: 10.1001/jama.2020.16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, et al. Validation of diagnostic codes of major clinical outcomes in a national health Insurance database. Int. J. Arrhythm. 2019;20:1–7. doi: 10.1186/s42444-019-0005-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are third-party data owned and operated by the Korean NHIS. Interested researchers can contact the NHIS to access the data (http://nhiss.nhis.or.kr/bd/ab/bdabd003cv.do).