Abstract
Objective:
Anemia is prevalent in patients with gynecologic cancers and is associated with increased perioperative morbidity. We aim to characterize risk factors for preoperative anemia and describe outcomes among patients undergoing surgery by a gynecologic oncologist to identify potential areas for impactful intervention.
Methods:
We analyzed major surgical cases performed by a gynecologic oncologist in the National Surgical Quality Improvement Program (NSQIP) database from 2014 – 2019. Anemia was defined as hematocrit < 36. Demographic characteristics and perioperative variables for patients with and without anemia were compared using bivariable tests. Odds of perioperative complications in patients stratified by preoperative anemia were calculated using logistic regression models.
Results:
Among 60,017 patients undergoing surgery by a gynecologic oncologist, 23.1% had preoperative anemia. Women with ovarian cancer had the highest rate of preoperative anemia at 39.7%. Patients with advanced stage cancer had a higher risk of anemia than early-stage disease (42.0% vs 16.3%, p=<0.001). In a logistic regression model adjusting for potential demographic, cancer-related, and surgical confounders, patients with preoperative anemia had increased odds of infectious complications (OR 1.16, 95% CI = 1.07 – 1.26), thromboembolic complications (OR 1.39, 95% CI = 1.15 – 1.68), and blood transfusion (OR 5.78, 95% CI 5.34 – 6.26).
Conclusion:
There is a high rate of anemia in patients undergoing surgery by a gynecologic oncologist, particularly those with ovarian cancer and/or advanced malignancy. Preoperative anemia is associated with increased odds of perioperative complication. Interventions designed to screen for and treat anemia in this population have the potential for significant impact on surgical outcomes.
Keywords: preoperative care, surgery, postoperative complications, ovarian cancer, anemia
INTRODUCTION
Anemia is widespread among patients with solid malignancies and affects 20–90% of patients with gynecologic cancers, depending on the population and phase of treatment (1–4). In the preoperative setting, anemia has been shown to be associated with higher rates of morbidity and mortality for those undergoing major surgery (5, 6). Treating anemia with perioperative blood transfusion is associated with additional risks and does not mitigate the negative effects of anemia (7–10). For this reason, the Enhanced Recovery After Surgery (ERAS®) society has identified screening and alternative treatments for preoperative anemia, such as iron replacement, as important targets of future efforts to optimize patient outcomes (11).
In obstetrics and gynecology, previously published studies including large numbers of patients from national databases have similarly demonstrated an association between preoperative anemia and increased composite morbidity/mortality scores in mixed populations of patients (12–14). Compared to the gynecologic surgery population as a whole, patients receiving care from a gynecologic oncologist are likely to have poorer performance status and unique risk factors such as receipt of neoadjuvant chemotherapy (15). Preoperative anemia in gynecologic oncology patients has been shown to be associated with surgical site infection (16), but a more comprehensive evaluation of the relationship between preoperative anemia and morbidity in this population has not previously been performed. Using a national surgical database, we aim to identify the patients undergoing surgery by a gynecologic oncologist who are most at risk for preoperative anemia as well as the perioperative complications associated with anemia. We hypothesize that the rates of infectious and thromboembolic complications are higher among patients going to the OR with anemia based on previous data demonstrating a link between these complications and anemia in other populations (17, 18). Gynecologic oncologists may consider the results of this study when developing guidelines designed to improve perioperative outcomes.
We hypothesize that patients with ovarian cancer are at highest risk for preoperative anemia because of advanced age, medical comorbidities, and receipt of neoadjuvant chemotherapy for some. We analyze this subpopulation of patients and report the rate of preoperative anemia and perioperative complications. These patients may have more to gain from interventions designed to treat anemia, particularly those undergoing neoadjuvant chemotherapy with a longer time interval between diagnosis and surgery.
METHODS
Study Design and Participants
This retrospective cohort study was performed using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The database collects patient demographics, perioperative variables, and 30-day postoperative outcomes data for patients undergoing major surgical procedures at hospitals across the US. This information is collected by trained clinical reviewers at each institution and audited to ensure inter-rater reliability (19). The linked hysterectomy-specific and gynecology participant use files from 2014 – 2019 were used for this study. Our cohort included patients with “gynecologic oncologist” listed under gynecology or hysterectomy subspecialist. All patients with surgery performed by a gynecologic oncologist, including those with benign pathology, were included to reflect the entire clinical practice of the subspecialty (15, 20, 21). Patients were excluded from the analysis if there was no hematocrit value available within the 30 days before surgery. This study was reviewed by the Institutional Review Board at Northwestern University and declared exempt from formal review as a secondary analysis of a deidentified dataset. In accordance with the journal’s guidelines, we will provide our data for independent analysis by the Editorial Team for the purposes of additional data analysis or for the reproducibility of this study in other centers if such is requested.
Variables
The primary exposure in this study was preoperative anemia, defined as hematocrit lower than 36% in the 30 days prior to surgery. This was used to approximate the World Health Organization (WHO) definition of anemia that is based on hemoglobin (<12g/dl) because hemoglobin value is not available in the National Surgical Quality Improvement Program database (22). For patients with multiple laboratory draws leading up to surgery, the hematocrit value taken closest to the day of surgery was used. We compared demographic characteristics among patients with and without preoperative anemia including age, body mass index, race, and ethnicity. Race was categorized as White, Black, Asian, or none of the above, which included Native Hawaiian, Pacific Islander, American Indian, or Alaska Native. These groups were combined under none of the above due to small sample size. Baseline health characteristics were also compared including history of hypertension requiring medication, chronic obstructive pulmonary disease (COPD), diabetes, smoking history, and ASA class. Missing data for any of these characteristics was classified in a separate category. In the subset of patients in the cohort with a malignancy, we identified cancer site from the combined participant use file and determined whether the patient had advanced or local disease. Advanced disease was defined by Stage IIIA-IVB uterine cancer, Stage IIIA-IVB cervical cancer, or Stage IIA-IVB ovarian cancer; all other patients with cancer were characterized as having local or early-stage disease. Receipt of neoadjuvant chemotherapy was not reported because of high proportion of missing data. Surgical variables examined included mode of surgery as determined by CPT codes, operative time, and total relative value units (RVUs), which was used as a surrogate for surgical complexity (23).
The two primary outcome measures were odds of infectious complications and thromboembolic complications. Patients were identified as having had an infectious complication if they had one or more of the following complications: superficial wound infection, deep wound infection, organ space infection, pneumonia, urinary tract infection, sepsis, or septic shock. Thromboembolic complication similarly was a composite that included pulmonary embolism, deep vein thrombosis (DVT)/thrombophlebitis, or stroke. Secondary outcomes included any blood transfusion (administered intraoperatively or within 72 hours after surgery), length of hospital stay > 4 days, rate of readmission within 30 days, and individual infectious/thromboembolic complications. The number of units of blood transfused was not available in the surgical dataset during this study period, so transfusion was considered a binary variable.
Statistical Methods
Chi-squared analyses were used to compare categorical variables between the two groups, and Wilcoxon rank sum tests were used compare continuous variables. Associations between preoperative anemia and primary and secondary outcomes were described using raw data compared with Chi-squared analyses, as well as individual univariate logistic regression models for each outcome of interest. Covariates controlled for in the logistic regression models included age, body mass index, race, ethnicity, ASA class, hypertension requiring medication, diabetes, smoking status, local malignancy versus advanced malignancy versus benign pathology, mode of surgery, and surgical RVUs. All variables were categorical except for surgical RVUs, which was included as a continuous variable. Operative time and cancer site were not included in the models to avoid collinearity with mode of surgery, surgical RVUs, and local/advanced/benign pathology. Patients with missing values for the covariates of interest were excluded from the logistic regression analyses. Patients with ovarian cancer were analyzed as a subpopulation using unadjusted logistic regression models as well as adjusted regression analyses for each outcome of interest, with the same covariates as described above. All analyses were performed using STATA 17.0.
RESULTS
We identified 66,446 patients from the surgical database who had surgery by a gynecologic oncologist. 6,429 patients were excluded due to lack of available hematocrit data in the 30 days prior to surgery, with 60,017 patients included in the final analysis (Figure 1). There were 13,881 patients with anemia as defined as hematocrit <36%, representing 23.1% of the study population. Among patients with anemia, 10,233 (73.6%) had mild anemia (hematocrit 30–36%), 3,269 (23.6%) had moderate anemia (hematocrit 24–30%), and 389 (2.8%) had severe anemia (hematocrit <24%).
Figure 1.
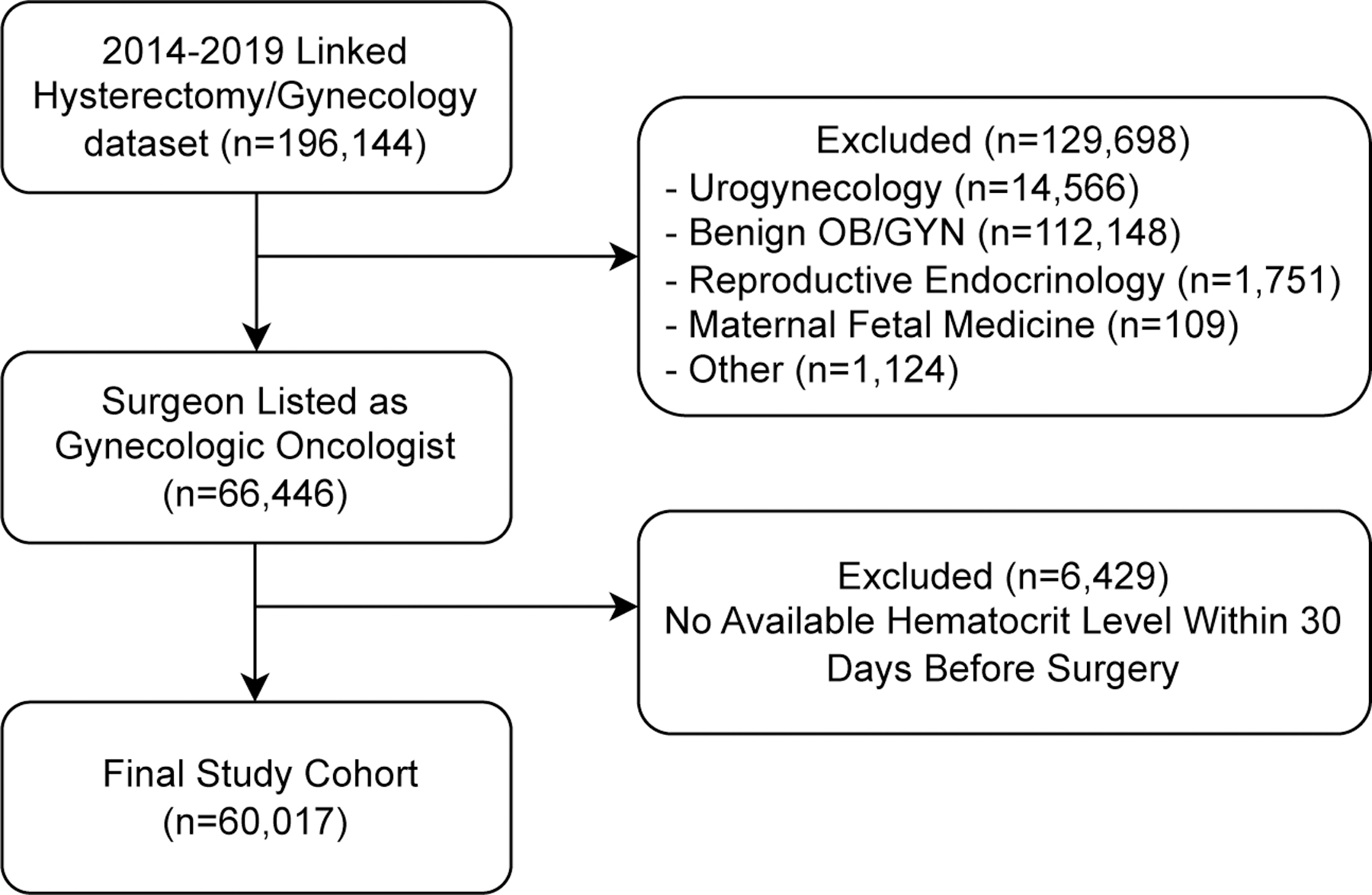
Cohort study enrollment diagram.
Patient characteristics are described in Table 1. Black race, ASA class III+, hypertension requiring medication, and diabetes were associated with a higher likelihood of preoperative anemia. With regards to cancer-related variables, patients with ovarian cancer had the highest rate of preoperative anemia at 39.7% compared to rates of 18–21% for uterine cancer, cervical cancer, and benign pathology. 42% of those with advanced stage disease had preoperative anemia compared to only 16.3% with local disease. Patients going into the operating room with preoperative anemia were more likely to have a laparotomy and had on average longer operative times (169.6 minutes vs 151.5 minutes, p <0.001) and more complex procedures as estimated by total RVUs (33.4 vs 27.7, p<0.001).
Table 1.
Association between patient and surgical characteristics and preoperative anemia.
| Not Anemic (n=46,136) |
Anemic (n=13,881) |
p-value | |
|---|---|---|---|
| Age (years) | <0.001 | ||
| ≤ 30 | 679 (1.5%) | 288 (2.1%) | |
| 31 – 50 | 12,216 (26.5%) | 4,871 (35.1%) | |
| 51 – 70 | 25,757 (55.8%) | 6,172 (44.5%) | |
| 71+ | 7,484 (16.2%) | 2,550 (18.4%) | |
| Body Mass Index (kg/m 2 ) | <0.001 | ||
| <18.5 | 434 (0.9%) | 239 (1.7%) | |
| 18.5 – 24.9 | 9,218 (20.0%) | 3,290 (23.7%) | |
| 25 – 29.9 | 10,872 (23.6%) | 3,280 (23.6%) | |
| 30 – 39.9 | 16,157 (35.0%) | 4,440 (32.0%) | |
| ≥ 40 | 9,315 (20.2%) | 2,532 (18.2%) | |
| Missing | 140 (0.3%) | 100 (0.7%) | |
| Race | <0.001 | ||
| White | 33,980 (73.7%) | 8,382 (60.4%) | |
| Black | 3,496 (7.6%) | 2,432 (17.5%) | |
| Asian | 1,733 (3.8%) | 678 (4.9%) | |
| None of the Above | 378 (0.8%) | 149 (1.1%) | |
| Missing | 6,549 (14.2%) | 2,240 (16.1%) | |
| Ethnicity | <0.001 | ||
| Hispanic | 2,714 (5.9%) | 1,066 (7.7%) | |
| Non-Hispanic | 38,285 (83.2%) | 11,142 (80.5%) | |
| Missing | 5,032 (10.9%) | 1,626 (11.8%) | |
| ASA Class | <0.001 | ||
| I | 1,536 (3.3%) | 283 (2.1%) | |
| II | 22,333 (48.4%) | 4,977 (35.9%) | |
| III | 21,095 (45.7%) | 7,595 (55.3%) | |
| IV or V | 1,159 (2.5%) | 942 (6.8%) | |
| Missing | 13 (0.03%) | 4 (0.03%) | |
| Hypertension Requiring Medication | <0.001 | ||
| Yes | 19,974 (43.3%) | 6,276 (45.2%) | |
| No | 26,162 (56.7%) | 7,605 (54.8%) | |
| Diabetes | <0.001 | ||
| Yes | 6,929 (15.0%) | 2,660 (19.2%) | |
| No | 39,207 (85.0%) | 11,221 (80.8%) | |
| COPD | 0.161 | ||
| Yes | 946 (2.1%) | 313 (2.3%) | |
| No | 45,190 (97.9%) | 13,568 (97.7%) | |
| Smoking History | 0.002 | ||
| Yes | 5,681 (12.3%) | 1,575 (11.4%) | |
| No | 40,455 (87.7%) | 12,306 (88.6%) | |
| Cancer Site | <0.001 | ||
| Cervix | 2,729 (5.9%) | 640 (4.6%) | |
| Uterus | 17,842 (38.7%) | 4,155 (29.9%) | |
| Ovary | 5,926 (12.8%) | 3,907 (28.2%) | |
| Other/Unknown | 888 (1.9%) | 322 (2.3%) | |
| Benign | 18,751 (40.6%) | 4,857 (35.0%) | |
| Local versus Advanced Malignancy | <0.001 | ||
| Local | 18,809 (40.8%) | 3,653 (26.3%) | |
| Advanced | 5,651 (12.3%) | 4,095 (29.5%) | |
| Benign | 18,751 (40.6%) | 4,857 (35.0%) | |
| Unknown | 2,925 (6.3%) | 1,276 (9.2%) | |
| Route of Surgery | <0.001 | ||
| Laparotomy | 14,754 (32.0%) | 8,069 (58.1%) | |
| Minimally-invasive | 30,629 (66.3%) | 5,488 (39.5%) | |
| Vulvectomy / Vaginectomy | 194 (0.4%) | 54 (0.4%) | |
| Other / Missing | 559 (1.2%) | 270 (2.0%) | |
| Operative Time (Min) | <0.001 | ||
| Mean +/− SD | 151.5 +/− 73.3 | 169.6 +/− 85.7 | |
| Median | 137 | 152 | |
| Missing | 7 | 6 | |
| Total RVUs | <0.001 | ||
| Mean +/− SD | 27.7 +/− 15.3 | 33.4 +/− 20.4 | |
| Median | 23.9 | 29.7 | |
| Total | 46,136 (76.9%) | 13,881 (23.1%) | 60,017 |
The unadjusted rates of primary and secondary outcomes stratified by preoperative anemia are described in Table 2. Patients with preoperative anemia had higher rates of infectious complication (9.9% vs 6.3%, p<0.001), thromboembolic complication (2.0% vs 0.9%, p<0.001), perioperative blood transfusion (25.9% vs 3.6%, p<0.001), length of stay >4 days (28.0% vs 8.8%, p<0.001), and readmission within 30 days (7.7% vs 4.3%, p<0.001) compared to patients without anemia. The unadjusted and adjusted odds ratios of primary and secondary outcomes from the logistic regression models are reported in Table 3. In the adjusted model, patients with anemia had an odds ratio of 1.16 (95% CI 1.07 – 1.26) for any infectious complication and 1.39 (95% CI 1.15 – 1.68) for any thromboembolic complication. For the secondary outcomes, preoperative anemia was associated with increased odds of blood transfusion (OR 5.78, 95% CI 5.34 – 6.26), length of stay > 4 days (OR 2.15, 95% CI 2.01 – 2.31), and readmission within 30 days of surgery (OR 1.30, 95% CI 1.18 – 1.42).
Table 2.
Unadjusted rates of infectious complication, thromboembolic complication, blood transfusion, length of stay >4 days, and 30-day readmission in anemic versus non-anemic patients.
| Not Anemic (n=46,136) |
Anemic (n=13,881) |
p-value | |
|---|---|---|---|
| Infectious Complications | |||
| Superficial wound infection | 889 (1.9%) | 388 (2.8%) | <0.001 |
| Deep wound infection | 118 (0.3%) | 76 (0.6%) | <0.001 |
| Organ space infection | 729 (1.6%) | 366 (2.6%) | <0.001 |
| Pneumonia | 227 (0.5%) | 163 (1.2%) | <0.001 |
| Urinary tract infection | 1,094 (2.4%) | 412 (3.0%) | <0.001 |
| Sepsis + Septic Shock | 460 (1.0%) | 352 (2.5%) | <0.001 |
| Any infectious complication | 2,908 (6.3%) | 1,374 (9.9%) | <0.001 |
| Thromboembolic Complications | |||
| Pulmonary embolism | 266 (0.6%) | 154 (1.1%) | <0.001 |
| DVT/thrombophlebitis | 158 (0.3%) | 132 (1.0%) | <0.001 |
| Stroke | 43 (0.1%) | 22 (0.2%) | 0.04 |
| Any thromboembolic complication | 427 (0.9%) | 282 (2.0%) | <0.001 |
| Blood Transfusion | <0.001 | ||
| Yes | 1,657 (3.6%) | 3,601 (25.9%) | |
| No | 44,479 (96.4%) | 10,280 (74.1%) | |
| Length of Stay > 4 Days | <0.001 | ||
| Yes | 4,039 (8.8%) | 3,890 (28.0%) | |
| No | 42,097 (91.2%) | 9,991 (72.0%) | |
| Median Length of Stay (Days) +/− SD | 1 +/− 2.87 | 3 +/− 4.87 | <0.001 |
| Readmission Within 30 Days | <0.001 | ||
| Yes | 1,995 (4.3%) | 1,066 (7.7%) | |
| No | 44,141 (95.7%) | 12,815 (92.3%) | |
| Total | 46,136 (76.9%) | 13,881 (23.1%) | 60,017 |
Table 3.
Unadjusted and adjusted odds ratios of perioperative complications in patients with anemia compared to those without anemia from logistic regression models.
| Unadjusted Odds Ratio (95% CI) | p-value | Adjusted Odds Ratio (95 % CI)* | p-value | |
|---|---|---|---|---|
| Infectious Complications | ||||
| Superficial wound infection | 1.46 (1.30 – 1.65) | <0.001 | 1.00 (0.86 – 1.16) | 0.965 |
| Deep wound infection | 2.15 (1.61 – 2.87) | <0.001 | 1.31 (0.92 – 1.86) | 0.139 |
| Organ space infection | 1.69 (1.49 – 1.92) | <0.001 | 1.19 (1.02 – 1.39) | 0.026 |
| Pneumonia | 2.40 (1.96 – 2.94) | <0.001 | 1.15 (0.89 – 1.48) | 0.281 |
| Urinary tract infection | 1.26 (1.12 – 1.41) | <0.001 | 1.05 (0.91 – 1.21) | 0.530 |
| Sepsis + Septic Shock | 2.58 (2.24 – 2.97) | <0.001 | 1.55 (1.31 – 1.84) | <0.001 |
| Any infectious complication | 1.63 (1.53 – 1.75) | <0.001 | 1.16 (1.07 – 1.26) | <0.001 |
| Thromboembolic Complications | ||||
| Pulmonary embolism | 1.93 (1.58 – 2.36) | <0.001 | 1.24 (0.97 – 1.60) | 0.089 |
| DVT/thrombophlebitis | 2.79 (2.22 – 3.52) | <0.001 | 1.79 (1.35 – 2.36) | <0.001 |
| Stroke | 1.70 (1.02 – 2.85) | 0.043 | 0.98 (0.53 – 1.82) | 0.944 |
| Any thromboembolic complication | 2.22 (1.91 – 2.58) | <0.001 | 1.39 (1.15 – 1.68) | 0.001 |
| Blood Transfusion | 9.40 (8.84 – 10.00) | <0.001 | 5.78 (5.34 – 6.26) | <0.001 |
| Length of Stay > 4 Days | 4.06 (3.86 – 4.26) | <0.001 | 2.15 (2.01 – 2.31) | <0.001 |
| Readmission Within 30 Days | 1.84 (1.70 – 1.99) | <0.001 | 1.30 (1.18 – 1.42) | <0.001 |
Covariates: age, body mass index, race, ethnicity, ASA class, hypertension requiring medication, diabetes, smoking status, local malignancy versus advanced malignancy versus benign pathology, mode of surgery, surgical RVUs.
The 9,833 patients with ovarian cancer were analyzed separately. The unadjusted and adjusted odds ratios of primary and secondary outcomes from logistic regression models are shown in Table 4. In the adjusted models, there was no statistically significant difference identified in infectious or thromboembolic complications or 30-day readmissions in patients with or without preoperative anemia. However, the odds of perioperative blood transfusion were significantly higher for patients with anemia (OR 4.32, 95% CI 3.80 – 4.92), as was the odds of length of stay > 4 days (OR 1.48, 95% CI 1.32 – 1.66). In this population, the rate of blood transfusion was 41.8% for patients going into the OR with anemia compared to 12.6% of those without anemia.
Table 4.
Unadjusted and adjusted odds ratios of perioperative complications for patients with ovarian cancer and anemia compared to those without anemia from logistic regression models.
| Unadjusted Odds Ratio (95% CI) | p-value | Adjusted Odds Ratio (95% CI)* | p-value | |
|---|---|---|---|---|
| Infectious Complications | ||||
| Superficial wound infection | 0.86 (0.68 – 1.09) | 0.218 | 0.77 (0.56 – 1.05) | 0.097 |
| Deep wound infection | 1.77 (0.94 – 3.33) | 0.075 | 2.16 (0.85 – 5.45) | 0.104 |
| Organ space infection | 1.00 (0.78 – 1.27) | 0.978 | 0.86 (0.64 – 1.14) | 0.293 |
| Pneumonia | 1.57 (1.13 – 2.17) | 0.007 | 0.99 (0.66 – 1.47) | 0.955 |
| Urinary tract infection | 1.04 (0.82 – 1.32) | 0.754 | 0.80 (0.58 – 1.09) | 0.162 |
| Sepsis + Septic Shock | 1.34 (1.04 – 1.74) | 0.025 | 1.05 (0.76 – 1.43) | 0.760 |
| Any infectious complication | 1.08 (0.95 – 1.24) | 0.239 | 0.91 (0.77 – 1.08) | 0.279 |
| Thromboembolic Complications | ||||
| Pulmonary embolism | 1.09 (0.78 – 1.52) | 0.589 | 0.73 (0.48 – 1.12) | 0.150 |
| DVT/thrombophlebitis | 1.70 (1.13 – 2.56) | 0.011 | 1.49 (0.91 – 2.42) | 0.113 |
| Stroke | 1.06 (0.40 – 2.79) | 0.903 | 0.66 (0.18 – 2.38) | 0.527 |
| Any thromboembolic complication | 1.30 (1.00 – 1.67) | 0.047 | 0.98 (0.71 – 1.35) | 0.908 |
| Blood Transfusion | 4.96 (4.49 – 5.48) | <0.001 | 4.32 (3.80 – 4.92) | <0.001 |
| Length of Stay > 4 Days | 1.97 (1.81 – 2.14) | <0.001 | 1.48 (1.32 – 1.66) | <0.001 |
| Readmission Within 30 Days | 1.11 (0.96 – 1.29) | 0.173 | 0.97 (0.81 – 1.16) | 0.771 |
Covariates: age, body mass index, race, ethnicity, ASA class, hypertension requiring medication, diabetes, smoking status, local malignancy versus advanced malignancy versus benign pathology, mode of surgery, surgical RVUs.
DISCUSSION
Summary of Main Results
Our results demonstrate that patients undergoing surgery by a gynecologic oncologist have a high rate of preoperative anemia (23.1% in this large national cohort). 35.4% of patients undergoing open abdominal surgery had anemia. The most notable demographic difference in the anemic and non-anemic populations was race; Black race was associated with a significantly higher rate of preoperative anemia compared to other racial groups. In a logistic regression model controlling for relevant demographic, cancer-related, and surgical characteristics, anemia was associated with higher odds of infectious and thromboembolic complications. There was also a significant increase in the odds of intraoperative blood transfusion for patients going into the operating room with anemia even when controlling for patient characteristics, surgical approach, and complexity, suggesting an independent effect that is not just driven by selection bias. Preoperative anemia was associated with a higher rate of hospital length of stay >4 days and 30-day readmissions, both variables relevant to patients as well as hospital systems looking to improve on trackable outcome metrics. Patients in this study with ovarian cancer had significantly higher rates of preoperative anemia when compared to other gynecologic malignancies or benign pathology. When controlling for demographic and operative characteristics, anemic ovarian cancer patients had over four times the odds of requiring perioperative blood transfusion.
Results in the Context of Published Literature
The high rate of preoperative anemia in this large national database population is similar to that published in previous institution-specific studies (1, 24, 25). Increased risk of anemia among Black patients in this cohort is consistent with published data demonstrating that Black American women are more likely to be anemic when compared to other racial groups (26). The reasons for this effect are incompletely understood, and further research is needed to clarify the role of structural racism in this finding, identify potential barriers in access to treatment for anemia on the basis of race, and develop processes to address this health inequity (27).
Prior studies from the general surgery and gynecology literature have demonstrated poorer surgical outcomes for patients with preoperative anemia (5, 6, 12–14, 16); we identify a similar effect in a population of patients taken care of by gynecologic oncologists. The higher rates of blood transfusion in patients with preoperative anemia in this cohort represents a clinically relevant finding given the independent association between blood transfusion and perioperative morbidity and mortality in this population (7, 10). In the ovarian cancer subpopulation, we hypothesize that neoadjuvant chemotherapy is one of the primary drivers of preoperative anemia and intraoperative transfusions based on previous studies demonstrating high rates of anemia and blood transfusion for patients undergoing interval cytoreduction (10, 28, 29). However, the database used is missing complete data regarding receipt of preoperative chemotherapy, limiting our ability to make conclusive statements about the role of neoadjuvant chemotherapy.
Strengths and Weaknesses
The major strength of this study is the large sample size with high quality perioperative laboratory and complication data available. There are some relevant factors that are not collected consistently by National Surgical Quality Improvement Program and therefore not included in this study, such as receipt of neoadjuvant chemotherapy and complication >30 days after surgery. Although blood loss is relevant to this study, estimated blood loss has been shown to be unreliable and inaccurate with wide variations based on the estimator, so we do not view the lack of data regarding this variable as a significant limitation (30). Around 10% of the patient population was excluded due to lack of 30-day preoperative lab values. Patients without preoperative labs may be less likely to have anemia, perhaps causing overestimation of anemia rates. In addition, we included patients who received a blood transfusion in the preoperative period. Preoperative transfusion may improve the hematocrit value listed in the database prior to surgery but comes with the complications associated with transfusion. This would lead to overestimation of complications in the non-anemic cohort and weaken the association between anemia and perioperative complication, so it does not threaten the study’s conclusions. Finally, far outliers with high blood transfusion requirements were not excluded from our analysis due to lack of data available in the National Surgical Quality Improvement Program database regarding number of units transfused. These outliers, more likely to fall in the preoperative anemia cohort, may have high rates of morbidity that could pull up the overall rates of complication. However, we suspect that the number of significant outliers requiring four or more units of blood is low and does not diminish our study’s conclusions.
Implications for Practice and Future Research
The risks of complication associated with both preoperative anemia and blood transfusion suggest need for alternative interventions to treat preoperative anemia. Iron deficiency is one of the most common causes of anemia in this population; rates have been shown to be as high as 43% in patients with solid tumors (31). Iron deficiency is modifiable with oral or IV iron supplementation. IV formulations deliver dosages more quickly, providing rapid availability for erythropoiesis (32). Based on the available data, international surgical guidelines recommend treatment of iron deficiency anemia in the preoperative setting (33). The Enhanced Recovery After Surgery (ERAS®) society has published on the importance of evaluation and treatment of preoperative anemia but has recommended further research to clarify the role of iron supplementation and the populations that may benefit (11). Vitamin B12 and folate deficiencies are less common but similarly targetable nutritional deficiencies that should be considered as part of treatment for preoperative anemia (1).
Gynecologic oncology patients, and particularly advanced ovarian cancer patients, may have more to gain from active management of preoperative anemia than the general abdominal surgery population. IV iron administration appears to improve hemoglobin levels for anemic patients but takes time (32, 34). Patients receiving neoadjuvant chemotherapy may have 2–3 months between diagnosis and surgery, giving them time to benefit from correction of nutritional deficiencies (29). Many patients undergoing surgery by a gynecologic oncologist will need adjuvant treatment, and stakes are high to avoid perioperative complication and maintain postoperative hemoglobin levels when compared to other surgical patients. For patients with ovarian cancer, delay in the initiation of adjuvant chemotherapy beyond 37 days after surgery has been associated with decreased survival (35, 36). While data supporting iron supplementation for all anemic preoperative patients is mixed, the results of our study and the unique needs of this patient population suggest that any opportunity to improve perioperative anemia should be considered.
CONCLUSIONS
Previous work has demonstrated an increased risk of perioperative morbidity in patients with anemia undergoing major abdominal surgery or benign gynecologic procedures (5, 12–14). This study adds to the literature by evaluating a national cohort of patients from gynecologic oncology practices as a separate population. These patients have distinct demographic characteristics and risk factors for both anemia and perioperative complications. Approaches to screening and treatment for anemia should be specific to their needs.
What is already known on this topic –
Preoperative anemia has been shown to be associated with poorer surgical outcomes for general surgery and benign gynecology patients.
What this study adds –
Patients undergoing surgery by a gynecologic oncologist have high rates of anemia, particularly those with ovarian cancer and/or advanced disease. Preoperative anemia is associated with infectious and thromboembolic complications in this specific population.
How this study might affect research, practice or policy –
These results inform future research to determine if screening for and treating preoperative anemia for patients seeing a gynecologic oncologist improves perioperative outcomes.
Acknowledgments
Dr. Barber received career development funds from the GOG Foundation and the NIA (1P30AG059988-01a1).
Footnotes
Competing Interests: The authors have no other competing interests.
REFERENCES
- 1.Hufnagel DH, Mehta ST, Ezekwe C, Brown AJ, Beeghly-Fadiel A, Prescott LS. Prevalence of Anemia and Compliance With NCCN Guidelines for Evaluation and Treatment of Anemia in Patients With Gynecologic Cancer. J Natl Compr Canc Netw 2021;19(5):513–20. [DOI] [PubMed] [Google Scholar]
- 2.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 2004;116 Suppl 7A:11S–26S. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40(15):2293–306. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Xu L, Page JH, Cannavale K, Sattayapiwat O, Rodriguez R, et al. Incidence of anemia in patients diagnosed with solid tumors receiving chemotherapy, 2010–2013. Clin Epidemiol 2016;8:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron DM, Hochrieser H, Posch M, Metnitz B, Rhodes A, Moreno RP, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 2014;113(3):416–23. [DOI] [PubMed] [Google Scholar]
- 6.Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015;102(11):1314–24. [DOI] [PubMed] [Google Scholar]
- 7.Prescott LS, Aloia TA, Brown AJ, Taylor JS, Munsell MF, Sun CC, et al. Perioperative blood transfusion in gynecologic oncology surgery: analysis of the National Surgical Quality Improvement Program Database. Gynecol Oncol 2015;136(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 2009;208(5):931–7, 7 e1–2; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 9.Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, et al. Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg 2013;206(6):1024–32; discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 10.Connor JP, O’Shea A, McCool K, Sampene E, Barroilhet LM. Peri-operative allogeneic blood transfusion is associated with poor overall survival in advanced epithelial ovarian Cancer; potential impact of patient blood management on Cancer outcomes. Gynecol Oncol 2018;151(2):294–8. [DOI] [PubMed] [Google Scholar]
- 11.Ljungqvist O, de Boer HD, Balfour A, Fawcett WJ, Lobo DN, Nelson G, et al. Opportunities and Challenges for the Next Phase of Enhanced Recovery After Surgery: A Review. JAMA Surg 2021;156(8):775–84. [DOI] [PubMed] [Google Scholar]
- 12.Richards T, Musallam KM, Nassif J, Ghazeeri G, Seoud M, Gurusamy KS, et al. Impact of Preoperative Anaemia and Blood Transfusion on Postoperative Outcomes in Gynaecological Surgery. PLoS One 2015;10(7):e0130861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murji A, Lam M, Allen B, Richard L, Shariff SZ, Austin PC, et al. Risks of preoperative anemia in women undergoing elective hysterectomy and myomectomy. Am J Obstet Gynecol 2019;221(6):629 e1–e18. [DOI] [PubMed] [Google Scholar]
- 14.Tyan P, Taher A, Carey E, Sparks A, Radwan A, Amdur R, et al. The effect of anemia severity on postoperative morbidity among patients undergoing laparoscopic hysterectomy for benign indications. Acta Obstet Gynecol Scand 2020;99(1):112–8. [DOI] [PubMed] [Google Scholar]
- 15.Barber EL, Rossi EC, Alexander A, Bilimoria K, Simon MA. Benign hysterectomy performed by gynecologic oncologists: Is selection bias altering our ability to measure surgical quality? Gynecol Oncol 2018;151(1):141–4. [DOI] [PubMed] [Google Scholar]
- 16.Mahdi H, Gojayev A, Buechel M, Knight J, SanMarco J, Lockhart D, et al. Surgical site infection in women undergoing surgery for gynecologic cancer. Int J Gynecol Cancer 2014;24(4):779–86. [DOI] [PubMed] [Google Scholar]
- 17.Chi G, Gibson CM, Hernandez AF, Hull RD, Kazmi SHA, Younes A, et al. Association of Anemia with Venous Thromboembolism in Acutely Ill Hospitalized Patients: An APEX Trial Substudy. Am J Med 2018;131(8):972 e1–e7. [DOI] [PubMed] [Google Scholar]
- 18.Grosso MJ, Boddapati V, Cooper HJ, Geller JA, Shah RP, Neuwirth AL. The Effect of Preoperative Anemia on Complications After Total Hip Arthroplasty. J Arthroplasty 2020;35(6S):S214–S8. [DOI] [PubMed] [Google Scholar]
- 19.American College of Surgeons. User guide for the 2020 ACS NSQIP participant use data file (PUF)
- 20.Aviki EM, Rauh-Hain JA, Clark RM, Hall TR, Berkowitz LR, Boruta DM, et al. Gynecologic Oncologist as surgical consultant: intraoperative consultations during general gynecologic surgery as an important focus of gynecologic oncology training. Gynecol Oncol 2015;137(1):93–7. [DOI] [PubMed] [Google Scholar]
- 21.Blank SV, Huh WK, Bell M, Dilley S, Hardesty M, Hoskins ER, et al. Doubling down on the future of gynecologic oncology: The SGO future of the profession summit report. Gynecol Oncol 2023;171:76–82. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO). Nutrional Landscape Information System (NLiS): Anaemia 2008. [Available from: https://www.who.int/data/nutrition/nlis/info/anaemia.
- 23.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg 2009;198(5 Suppl):S19–27. [DOI] [PubMed] [Google Scholar]
- 24.Spenard ELY, Covens A, Gien L, Vicus D. EPV269/#87 Preoperatve anemia in gynecologic oncology patients: are we optimizing our patients? International Journal of Gynecologic Cancer2021 [Google Scholar]
- 25.Jadunandan S, Tano R, Vicus D, Callum J, Lin Y. The incidence of perioperative anemia and iron deficiency in patients undergoing gyne-oncology surgery. Can Oncol Nurs J 2022;32(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le CH. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003–2012). PLoS One 2016;11(11):e0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igbinosa IL; S.A.; Noelette F; Mujahid M; Main EK; Lyell DJ Health Disparities in Antepartum Anemia: The Intersection of Race and Social Determinants of Health. Am J Obstet Gynecol 2022;226(1):S529–30. [Google Scholar]
- 28.McCool KW, Sampene E, Polnaszek B, Connor J, Medlin EE, Barroilhet L. Neoadjuvant chemotherapy is associated with a high rate of perioperative blood transfusion at the time of interval cytoreductive surgery. BMC Cancer 2018;18(1):1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shea A, McCool K, Harrison R, Sampene E, Connor J, Barroilhet L. Neoadjuvant chemotherapy is associated with more anemia and perioperative blood transfusions than primary debulking surgery in women with advanced stage ovarian cancer. Gynecol Oncol 2018;150(1):19–22. [DOI] [PubMed] [Google Scholar]
- 30.Rothermel LD, Lipman JM. Estimation of blood loss is inaccurate and unreliable. Surgery 2016;160(4):946–53. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig H, Muldur E, Endler G, Hubl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol 2013;24(7):1886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charytan C, Levin N, Al-Saloum M, Hafeez T, Gagnon S, Van Wyck DB. Efficacy and safety of iron sucrose for iron deficiency in patients with dialysis-associated anemia: North American clinical trial. Am J Kidney Dis 2001;37(2):300–7. [DOI] [PubMed] [Google Scholar]
- 33.Mueller MM, Van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019;321(10):983–97. [DOI] [PubMed] [Google Scholar]
- 34.Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Chau M, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet 2020;396(10259):1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmermans M, van der Aa MA, Lalisang RI, Witteveen PO, Van de Vijver KK, Kruitwagen RF, et al. Interval between debulking surgery and adjuvant chemotherapy is associated with overall survival in patients with advanced ovarian cancer. Gynecol Oncol 2018;150(3):446–50. [DOI] [PubMed] [Google Scholar]
- 36.Seagle BL, Butler SK, Strohl AE, Nieves-Neira W, Shahabi S. Chemotherapy delay after primary debulking surgery for ovarian cancer. Gynecol Oncol 2017;144(2):260–5. [DOI] [PubMed] [Google Scholar]


