Abstract
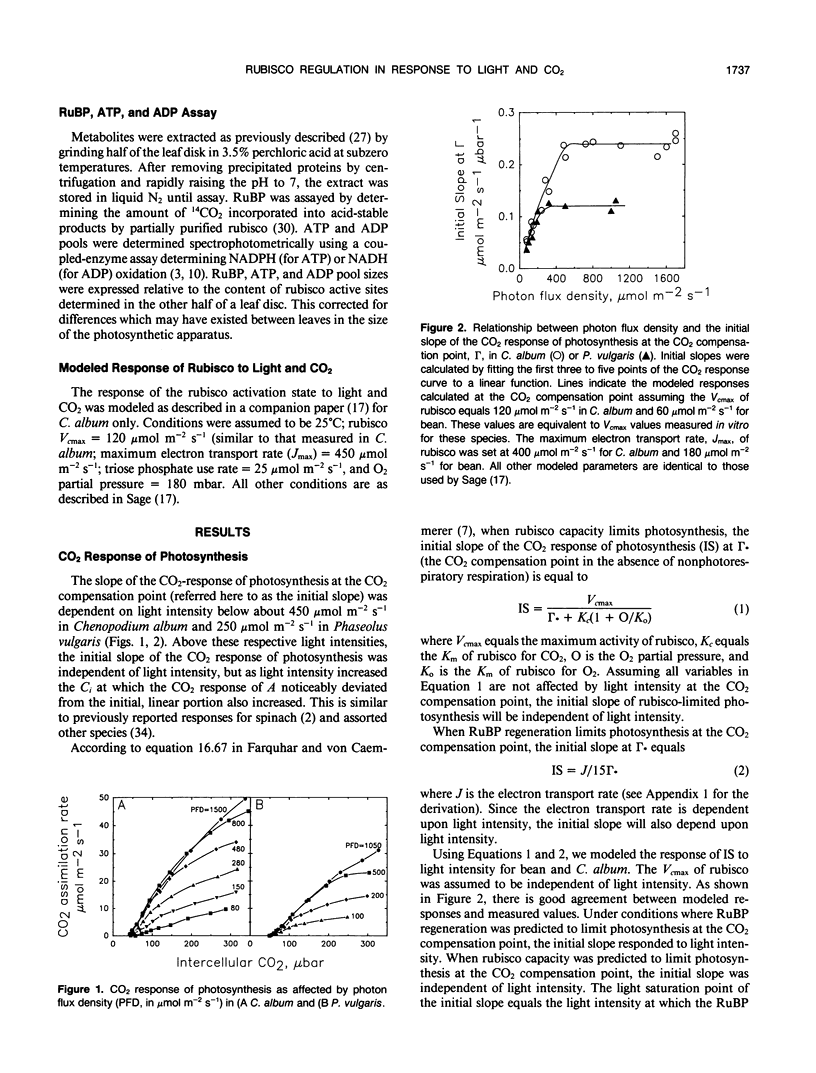
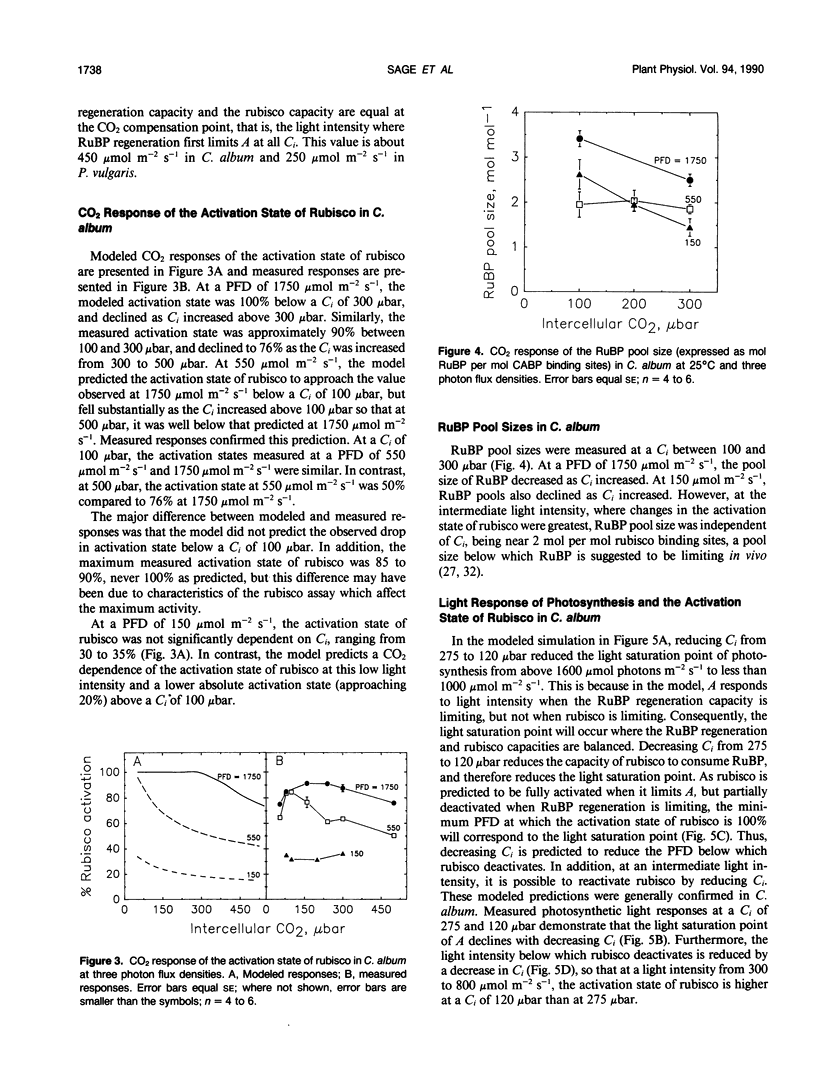
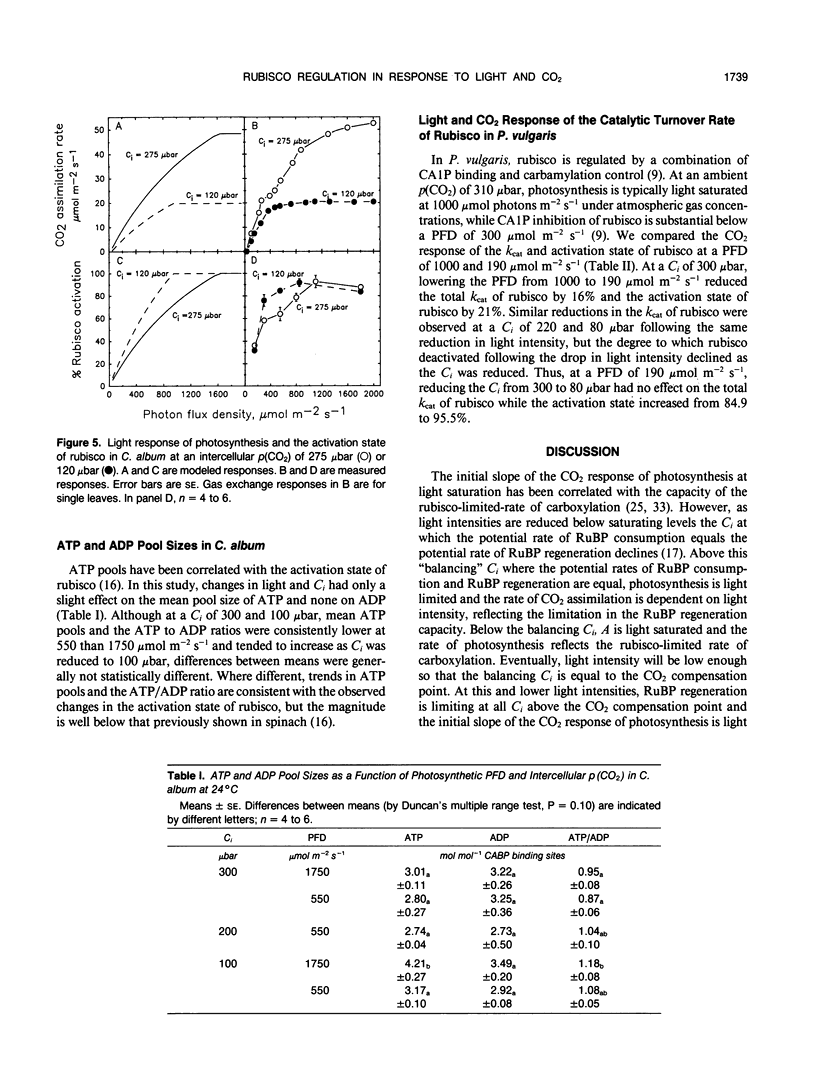
The light and CO2 response of (a) photosynthesis, (b) the activation state and total catalytic efficiency (kcat) of ribulose-1,5-bisphosphate carboxylase (rubisco), and (c) the pool sizes of ribulose 1,5-bisphosphate, (RuBP), ATP, and ADP were studied in the C3 annuals Chenopodium album and Phaseolus vulgaris at 25°C. The initial slope of the photosynthetic CO2 response curve was dependent on light intensity at reduced light levels only (less than 450 micromoles per square meter per second in C. album and below 200 micromoles per square meter per second in P. vulgaris). Modeled simulations indicated that the initial slope of the CO2 response of photosynthesis exhibited light dependency when the rate of RuBP regeneration limited photosynthesis, but not when rubisco capacity limited photosynthesis. Measured observations closely matched modeled simulations. The activation state of rubisco was measured at three light intensities in C. album (1750, 550, and 150 micromoles per square meter per second) and at intercellular CO2 partial pressures (C1) between the CO2 compensation point and 500 microbars. Above a C1 of 120 microbars, the activation state of rubisco was light dependent. At light intensities of 550 and 1750 micromoles per square meter per second, it was also dependent on C1, decreasing as the C1 was elevated above 120 microbars at 550 micromoles per square meter per second and above 300 microbars at 1750 micromoles per square meter per second. The pool size of RuBP was independent of C1 only under conditions when the activation state of rubisco was dependent on C1. Otherwise, RuBP pool sizes increased as C1 was reduced. ATP pools in C. album tended to increase as C1 was reduced. In P. vulgaris, decreasing C1 at a subsaturating light intensity of 190 micromoles per square meter per second increased the activation state of rubisco but had little effect on the kcat. These results support modelled simulations of the rubisco response to light and CO2, where rubisco is assumed to be down-regulated when photosynthesis is limited by the rate of RuBP regeneration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks A., Portis A. R., Sharkey T. D. Effects of Irradiance and Methyl Viologen Treatment on ATP, ADP, and Activation of Ribulose Bisphosphate Carboxylase in Spinach Leaves. Plant Physiol. 1988 Nov;88(3):850–853. doi: 10.1104/pp.88.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz N. D., Sharkey T. D. Activity ratios of ribulose-1,5-bisphosphate carboxylase accurately reflect carbamylation ratios. Plant Physiol. 1989 Mar;89(3):735–739. doi: 10.1104/pp.89.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobza J., Seemann J. R. Mechanisms for light-dependent regulation of ribulose-1,5-bisphosphate carboxylase activity and photosynthesis in intact leaves. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3815–3819. doi: 10.1073/pnas.85.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K. A., Jensen R. G., O'leary J. W., Berry J. A. Photosynthesis and Ribulose 1,5-Bisphosphate Concentrations in Intact Leaves of Xanthium strumarium L. Plant Physiol. 1984 Dec;76(4):968–971. doi: 10.1104/pp.76.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F. A Model Describing the Regulation of Ribulose-1,5-Bisphosphate Carboxylase, Electron Transport, and Triose Phosphate Use in Response to Light Intensity and CO(2) in C(3) Plants. Plant Physiol. 1990 Dec;94(4):1728–1734. doi: 10.1104/pp.94.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F., Sharkey T. D., Seemann J. R. Acclimation of Photosynthesis to Elevated CO(2) in Five C(3) Species. Plant Physiol. 1989 Feb;89(2):590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Anderson J. C. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987 Sep;85(1):66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D. Salinity and Nitrogen Effects on Photosynthesis, Ribulose-1,5-Bisphosphate Carboxylase and Metabolite Pool Sizes in Phaseolus vulgaris L. Plant Physiol. 1986 Oct;82(2):555–560. doi: 10.1104/pp.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R., Berry J. A. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Response to Changing Partial Pressure of O(2) and Light in Phaseolus vulgaris. Plant Physiol. 1986 Jul;81(3):788–791. doi: 10.1104/pp.81.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiol. 1989 Apr;89(4):1060–1065. doi: 10.1104/pp.89.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. A., Tenhunen J. D., Gates D. M., Lange O. L. Effect of photosynthetic photon flux density on carboxylation efficiency. Plant Physiol. 1987 Sep;85(1):109–114. doi: 10.1104/pp.85.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


