Key Teaching Points.
-
•
Atrial appendage pathways are rare causes of atrioventricular reciprocating tachycardia that should be suspected when conventional valve annular mapping and ablation fail to effectively eliminate pathway conduction.
-
•
An appendage-to-ventricle pathway should be suspected when there is broad activation along the valve annulus adjacent to the ipsilateral atrial appendage and if mapping electrodes proximal to the valve annulus appear to demonstrate earlier activation than those on the annulus directly.
-
•
Mapping of the entire length of the appendage overlying the ventricular epicardial surface should be performed to localize the insertion site of the pathway.
-
•
Irrigated ablation within the appendage with reduced power and limited inferiorly directed contact force over the ventricular epicardium can limit the risk of appendage perforation and adjacent coronary artery injury.
Introduction
Accessory atrioventricular (AV) pathwaysarising from atrial appendages are rarely encountered during catheter ablation for supraventricular tachycardia (SVT). Failure to recognize or properly ablate at these unusual pathway locations can result in treatment failure or potential procedural complications. We present a case of successful ablation of a left atrial appendage pathway in a pediatric patient with Wolff-Parkinson-White (WPW) syndrome and provide recommendations and tips learned from the case to aid in early recognition and planning of ablation of appendage pathways.
Case report
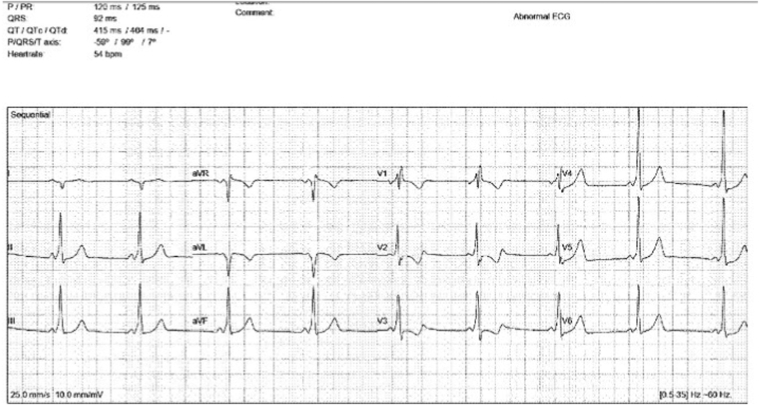
A 9-year-old, 32-kg male patient was referred to our electrophysiology group for symptomatic events suggestive of re-entrant SVT. Surface 12-lead electrocardiogram demonstrated sinus rhythm with ventricular pre-excitation suggestive of a left-sided accessory pathway (Figure 1). Given the frequency of our patient’s symptoms in the setting of WPW and the curative potential of catheter ablation, the family elected to proceed with procedural intervention.
Figure 1.
A 12-lead electrocardiogram demonstrating sinus rhythm with ventricular pre-excitation. The pattern of pre-excitation suggests a left-sided pathway.
The procedure was performed under general anesthesia with endotracheal intubation. Three-dimensional mapping was performed using Biosense Webster’s CARTO3 mapping system (Biosense Webster, Irvine, CA). Femoral venous access was obtained, and sheaths were placed in both groins. A 5F fixed-curve, quadripolar catheter was positioned at the right ventricular apical septum, a 5F steerable decapolar catheter was positioned across the tricuspid valve along the proximal ventricular septum for His recording, and a 7F steerable decapolar catheter was cannulated in the coronary sinus (CS) with the distal electrodes reaching the area of the lateral mitral valve annulus. Finally, a 7F steerable multielectrode high-density mapping catheter (PentaRay, D curve, Biosense Webster) was used for mapping and atrial pacing.
Intracardiac electrograms and baseline pacing maneuvers confirmed the presence of a manifest, bidirectional conducting accessory pathway on the left side of the heart. Atrial pacing repeatedly induced orthodromic reciprocating tachycardia (ORT) using the same left-sided connection. Antegrade pathway conduction properties were considered low risk (antegrade pathway block cycle length 310 ms). Atrial fibrillation was not induced during atrial pacing sequences to permit assessment of the shortest pre-excited R-R interval. As ORT was easily and repeatedly induced, ablation was pursued and a transseptal puncture was performed for left atrial access. The PentaRay mapping catheter and subsequently an 8F contact force–sensing, open-irrigated radiofrequency (RF) ablation catheter (ThermoCool SmartTouch SF; Biosense Webster) were used for mapping during pre-excited sinus rhythm, ventricular pacing, and ORT.
Based on initial findings from electroanatomic activation mapping, the pathway appeared to be at the anterolateral mitral valve annulus, but distal to the coronary sinus catheter tip. Unfortunately, the decapolar catheter in the CS could not be advanced further into the great cardiac vein in order to fully bracket the area of interest. An initial RF lesion was delivered at the anterolateral annulus but failed to affect pathway conduction. This prompted further mapping, with subsequent RF lesion deliveries being placed progressively more anterior along the annulus. Additional RF deliveries along the atrial and ventricular aspects of the anterior and anterolateral annulus were attempted during sinus rhythm, with favorable local bipolar and unipolar signals. These resulted in prompt loss of pathway conduction (2.7 seconds and 4.4 seconds, respectively) but recurrence either during RF delivery or shortly after completion of a 60-second lesion. Ablation during ORT with backup ventricular pacing terminated tachycardia in 0.8 seconds but pre-excitation returned 2 minutes after this lesion.
Given repeated instances of transient pathway disruption during ablation with subsequent recurrence, together with the observation of a broad area over which early activation and reasonable local signals were identified, the entire mitral valve annulus was remapped. Annular mapping again localized competitive signals across the anterior and anterolateral annulus. While mapping near the area of anterior and anterolateral annular intersection, earlier signals were identified on the proximal electrode pair of the ablation catheter compared to the distal pair (Figure 2A and 2B). This finding of earlier proximal activation was present during pre-excited sinus rhythm, ORT, and ventricular pacing. The proximal electrode pair was oriented near the entrance into the left atrial appendage. With insertion of the ablation catheter into the appendage, bipolar signals demonstrated dominant atrial signals with tightly coupled ventricular signals during pre-excited conduction and unipolar signals showed a favorable and early qS ventricular component (Figure 2C).
Figure 2.
A: Bipolar and unipolar signals from the tip of the ablation catheter at one of the sites of ablation failure on the mitral valve annulus. B: Left anterior oblique view showing the ablation catheter at the anterolateral mitral valve (MV) annulus. The distal electrode pair is at the MV annulus while the proximal pair contacts the left atrial tissue more proximally near the base of the left atrial appendage. B, C: Ventricular activation timing was earlier by 14 msec at the proximal pair compared to the distal tip pair, suggesting earlier activation in the appendage. D: Bipolar and Unipolar signals from the tip of the ablation catheter (Abl-unipol) just before ablation at the site of success in the left atrial appendage. Abl dist = distal ablation catheter electrode pair (ABL 1-2); Abl prox = proximal ablation catheter electrode pair (ABL 3-4); Abl unipol = ablation unipolar; CS- Prox = proximal coronary sinus; CS-dist = distal coronary sinus; LLPV = left lower pulmonary vein; LUPV = left upper pulmonary vein; RV = right ventricle.
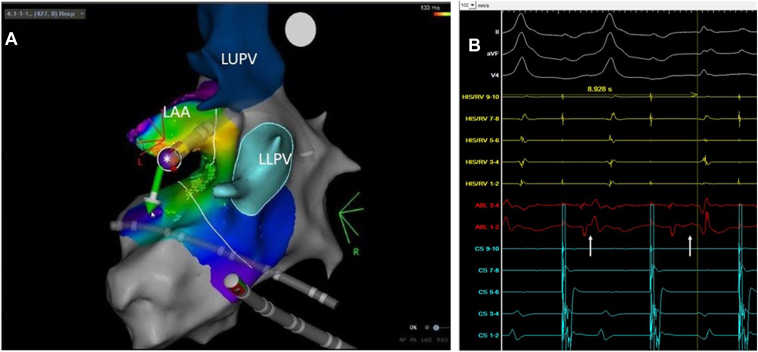
The floor of the appendage from base to tip was carefully mapped with the site of earliest ventricular activation during sinus rhythm localized to the base of the appendage overlying the anterolateral/anterior mitral annular border. At this location, though a distinct pathway potential was never identified, continuous AV signals were present. Given the location of the ablation catheter inside the appendage with high impedance values (190 ohms), irrigated ablation at 20 watts and 8 grams of applied contact force were delivered, with abrupt loss of pathway conduction at 8.9 seconds and without recurrence thereafter (Figure 3A and 3B). A consolidation lesion was placed at the site of success, followed by several consolidation lesions at the ventricular aspect of the mitral valve annulus immediately adjacent to the site of success in the appendage. There were no ST-segment changes or provocation of ventricular arrhythmias during ablation delivery to suggest thermal injury or compression involving the left circumflex artery. Fluoroscopy was used to assess the cardiac silhouette, which was found to be normal, without evidence of pericardial effusion. Postablation electrophysiologic testing, including atrial and ventricular burst pacing, extrastimulus pacing, and adenosine administration, were performed, without evidence of accessory pathway recurrence or inducible arrhythmia. Nearly 1 year postablation, the patient is symptom free. Follow-up electrocardiogram demonstrated no ventricular pre-excitation and transthoracic echo showed normal findings.
Figure 3.
A: The ablation catheter is positioned at the floor of the left atrial appendage (LAA) with inferiorly directed contact force during radiofrequency (RF) delivery. Asterisk (∗) indicates site of success. LLPV = left lower pulmonary vein, LUPV = left upper pulmonary vein. B: RF delivery at the floor of the appendage resulted in abrupt and durable loss of pathway conduction (white arrows highlight change in local atrioventricular signal).
Discussion
Atrial appendage pathways have rarely been reported as the target site of successful ablation of accessory AV connections in individuals with ORT. The published experience in treating appendage pathways is primarily limited to individual case reports and small case series. To our knowledge, there have been fewer than 15 published pediatric cases of atrial appendage pathways. Of the documented cases, right atrial appendage pathways appear to be more common, with the larger surface area of the right compared to the left atrial appendage potentially serving as a contributor to this observed laterality.
Although the anatomic location of appendage pathways involves the epicardial ventricular surface, they can be localized and treated successfully during endocardial mapping and ablation. Both radiofrequency ablation and cryoablation have been used in left and right appendage pathways in pediatric patients as young as 4 months.1,2 Reports of accessory pathways with associated SVT have also been reported in conjunction with appendage structural anomalies such as appendage aneurysms and giant appendages, as well as postsurgical states with acquired electrical connections between the right atrial appendage and ventricle, as can occur in older iterations of Fontan palliation.3, 4, 5 Mah and colleagues6 reported on a small series of 3 patients with high-risk WPW syndrome involving appendage pathways with unsuccessful endocardial ablation. These patients underwent open surgical dissection along with epicardial surface ablation, resulting in pathway elimination. The open surgical approach to treatment permitted direct visualization of structures, which lends insight into how these connections may develop. In all 3 cases involving left and right appendage-to-ventricle connections, fibrofatty and muscular connections, sometimes over a broad area between the ventricular epicardial surface and underside of the appendage, were identified. Pre-excitation and inducible ORT were eliminated following complete dissection and appendage mobilization, resulting in the disruption of the appendage-ventricle connections.
This case offers several important considerations for early recognition, mapping, and ablation of appendage pathways. First, the recognition of a broad area of early activation around the annulus overlying the appendage should raise suspicion for the possibility of appendage involvement. In this case, early and competitive signals were found in a region spanning the anterior and anterolateral annulus, with some sites even demonstrating transient pathway elimination during ablation. Comprehensive annular mapping should always be performed, especially if reference catheter signals fail to fully bracket the site of earliest activation. An appendage location should be considered if multiple adjacent sites along the annulus appear to be suitable targets for ablation.
Second, the local signals of the proximal electrode pair of the ablation catheter should not be ignored during annular mapping. In the case of a bidirectional accessory pathway, typical wavefront activation should result in primarily atrial-dominant signals on the proximal electrode pair, with earliest ventricular activation at the tip electrode pair during antegrade pathway conduction and earliest atrial activation at the tip followed by later atrial activation at the proximal pair during retrograde conduction when the ablation catheter is positioned in a prograde orientation at the valve annulus. The opposite pattern of early activation was seen in our case, with the proximal electrode pair demonstrating earlier ventricular activation during pre-excited sinus rhythm compared to the distal pair with the tip on the annulus near the base of the left atrial appendage. As such, the proximal pair was anatomically closer to the location of the pathway in the appendage compared to the distal pair at the mitral valve annulus.
Finally, mapping and ablation within the atrial appendage should be carefully approached. Cardiac perforation involving the atrial appendage has been described involving various tools, including guide wires and even the splines of the PentaRay mapping catheter.7 The left circumflex artery is also known to course near the base of the left atrial appendage along the left AV groove and may be externally compressed or injured during catheter ablation.8
In the presented case, the local electrogram signal at the site of success lacked a clearly identifiable pathway potential, but a continuous local AV signal was present and was earlier than sites along the mitral valve annulus. Given some descriptions of the anatomic basis for these appendage-to-ventricular connections, the very short distance between the thin-walled appendage and ventricular epicardium, and the orthogonal orientation of the tip electrode to the connection when inserted in the appendage, a clear pathway potential may not be present or identifiable. Care should be taken to map the entire length of the appendage overlying the ventricular epicardial surface in order to localize and deliver targeted ablation at the insertion site of the connection within the appendage. Attention should be paid to limit excessive contact force and high-power delivery during RF ablation in order to minimize the risk of thermal injury to or compression of the circumflex artery. In their case of ablation of a left appendage pathway, Benhayon and colleagues2 described their incorporation of intracardiac echocardiography for direct imaging of the left ventricle during ablation to identify wall motion abnormalities that would suggest coronary compromise. Close monitoring for ST-segment changes or development of acute ventricular arrhythmias is mandatory during ablation delivery. Although ablation of a ventricular insertion may be possible, full transmural lesions from the ventricular endocardium may be difficult to achieve given that the pathway location involves the ventricular epicardial surface. Similar to ablation inside the CS or coronary veins, given the limited and slow flow of blood within the appendage, together with its thin walls, care must be taken to avoid excessive tip pressure or high-power RF lesions, given a risk of perforation or steam pop. Temperature-limited, nonirrigated RF delivery may also result in prompt maximum temperature achievement and resultant low power, with no local tissue effect and minimal tissue penetration. Low-power, irrigated ablation with careful attention to impedance trends and contact force can be safely and successfully performed, as was demonstrated in the present case. Alternatively, cryoablation can be employed for ablative treatment within the appendage.
The use of advanced or adjunct imaging, such as computed tomography, magnetic resonance imaging, and transesophageal or intracardiac echocardiography, as well as coronary and atrial chamber angiography, may provide helpful information to define the relationship of the appendage and coronary artery branches to each other. However, data are lacking to guide what tools should be routinely used, and their incorporation would be at the discretion of the operator performing the procedure.
Conclusion
Atrial appendage–to-ventricle accessory pathways are a rare substrate associated with ORT. Such pathways should be suspected if there is broad activation along the adjacent valve annulus and earlier activation away from the annulus. Safe ablation, even in pediatric patients, can be performed by carefully targeting the insertion site of the pathway along the floor of the appendage and carefully delivering ablative therapy to minimize perforation or adjacent coronary injury.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors declare that they have no conflict of interest.
References
- 1.Dean P.N., Skeet A., Moak J.P., Berul C.I. Cryoablation and angiographic evidence of a concealed right atrial appendage to right ventricle accessory pathway in an infant. Congenit Heart Dis. 2013;8:E183–E187. doi: 10.1111/chd.12037. [DOI] [PubMed] [Google Scholar]
- 2.Benhayon D., Sinisterra S., Young M.-L. Wolff-Parkinson-White syndrome due to a left atrial appendage-to-left ventricular connection: a case of a successful pathway elimination from inside of the left atrial appendage. HeartRhythm Case Rep. 2018;4:519–522. doi: 10.1016/j.hrcr.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emi M., Aoki H., Nakamura Y., Hirano Y., Takahashi K., Kayatani F. Rare accessory pathway between giant left atrial appendage and the left ventricle. HeartRhythm Case Rep. 2020;6:131–134. doi: 10.1016/j.hrcr.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ergül Y., Öztürk E., Özgür S. Successful radiofrequency ablation of accessory pathway associated with left atrial appendage aneurysm in a low birthweight premature patient. Turk J Pediatr. 2019;61:142–146. doi: 10.24953/turkjped.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Peinado R., Gnoatto, Merino J.L., Oliver J.M. Catheter ablation of multiple, surgically created atrioventricular connections following Fontan-Björk procedure. EP Europace. 2007;9:848–850. doi: 10.1093/europace/eum077. [DOI] [PubMed] [Google Scholar]
- 6.Mah D., Miyake C., Clegg R., et al. Epicardial left atrial appendage and biatrial appendage accessory pathways. Heart Rhythm. 2010;7:1740–1745. doi: 10.1016/j.hrthm.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Guan F., Stähli B.E., Jakob P., Wolber T. Perforation of multipolar electroanatomic mapping catheter in the left atrial appendage during left atrial mapping. HeartRhythm Case Rep. 2022;8:P615–P617. doi: 10.1016/j.hrcr.2022.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh A., Makkar A., Ho S.Y., et al. Manifestations of coronary arterial injury during catheter ablation of atrial fibrillation and related arrhythmias. Heart Rhythm. 2013;10:1638–1645. doi: 10.1016/j.hrthm.2013.09.001. [DOI] [PubMed] [Google Scholar]