Abstract
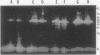
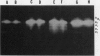
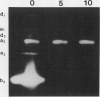
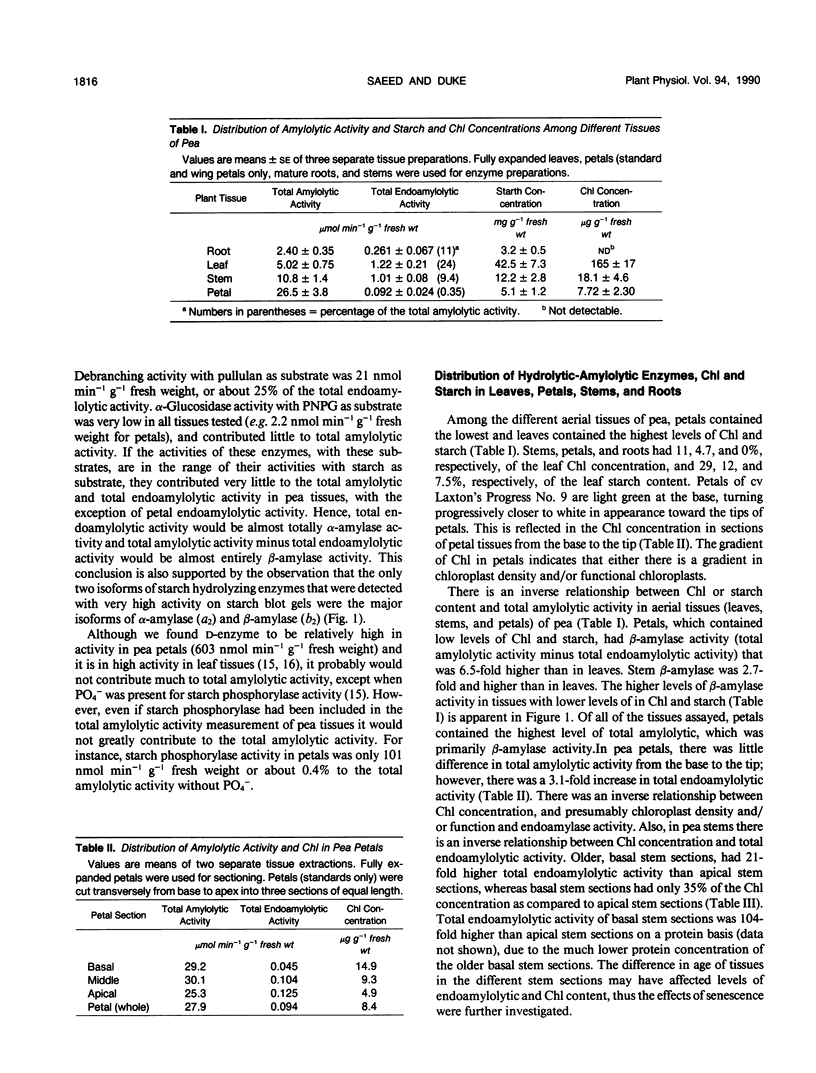
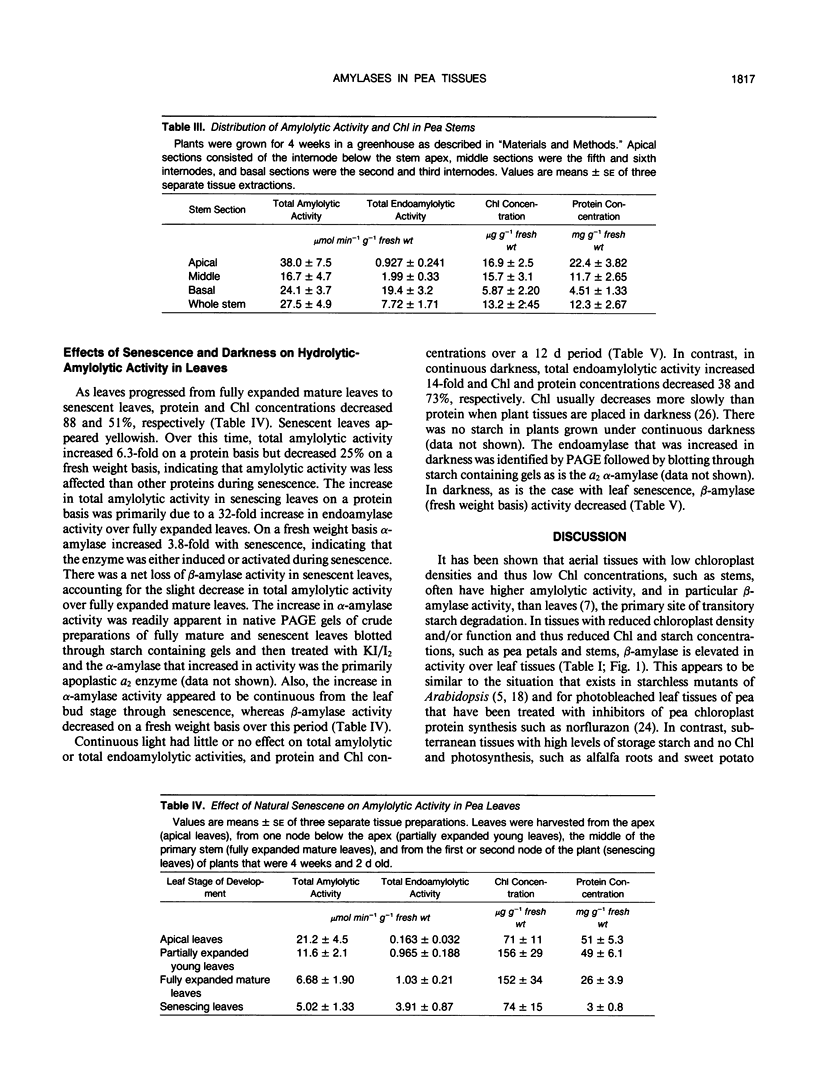
Pea (Pisum sativum L.) tissues with reduced chloroplast density (e.g. petals and stems) or function (i.e. senescent leaves and leaves darkened for prolonged periods) were surveyed to determine whether tissues with genetically or environmentally reduced chloroplast density and/or function also have significantly different amylolytic enzyme activities and/or isoform patterns than leaf tissues with totally competent chloroplasts. Native PAGE followed by electrophoretically blotting through a starch or β-limit dextrin containing gel and KI/I2 staining revealed that the primary amylases in leaves, stems, petals, and roots were the primarily vacuolar β-amylase (EC 3.2.1.2) and the primarily apoplastic α-amylase (EC 3.2.1.1). Among tissues of light grown pea plants, petals contained the highest levels of total amylolytic (primarily β-amylase) activity and considerably higher ratios of β- to α-amylase. In aerial tissues there was an inverse relationship between chlorophyll and starch concentration, and β-amylase activity. In sections of petals and stems there was a pronounced inverse relationship between chlorophyll concentration and the activity of α-amylase. Senescing leaves of pea, as determined by age, and protein and chlorophyll content, contained 3.8-fold (fresh weight basis) and 32-fold (protein basis) higher α-amylase activity than fully mature leaves. Leaves maintained in darkness for 12 days displayed a 14-fold (fresh weight basis) increase in α-amylase activity over those grown under continuous light. In senescence and prolonged darkness studies, the α-amylase that was greatly increased in activity was the primarily apoplastic α-amylase. These studies indicate that there is a pronounced inverse relationship between chloroplast function and levels of apoplastic α-amylase activity and in some cases an inverse relationship between chloroplast density and/or function and vacuolar β-amylase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beers E. P., Duke S. H. Characterization of alpha-Amylase from Shoots and Cotyledons of Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Apr;92(4):1154–1163. doi: 10.1104/pp.92.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H., Henson C. A. Partial Characterization and Subcellular Localization of Three alpha-Glucosidase Isoforms in Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Oct;94(2):738–744. doi: 10.1104/pp.94.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H. Localization of alpha-Amylase in the Apoplast of Pea (Pisum sativum L.) Stems. Plant Physiol. 1988 Aug;87(4):799–802. doi: 10.1104/pp.87.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caspar T., Lin T. P., Monroe J., Bernhard W., Spilatro S., Preiss J., Somerville C. Altered regulation of beta-amylase activity in mutants of Arabidopsis with lesions in starch metabolism. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5830–5833. doi: 10.1073/pnas.86.15.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G. W., Jr, Pallas J. E., Jr, Mendicino J. The hydrolysis of maltodextrins by a -amylase isolated from leaves of Vicia faba. Biochim Biophys Acta. 1972 Aug 28;276(2):491–507. doi: 10.1016/0005-2744(72)91010-8. [DOI] [PubMed] [Google Scholar]
- Doehlert D. C., Duke S. H., Anderson L. Beta-Amylases from Alfalfa (Medicago sativa L.) Roots. Plant Physiol. 1982 May;69(5):1096–1102. doi: 10.1104/pp.69.5.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C., Duke S. H. Specific Determination of alpha-Amylase Activity in Crude Plant Extracts Containing beta-Amylase. Plant Physiol. 1983 Feb;71(2):229–234. doi: 10.1104/pp.71.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Henson C. A., Servaites J. C., Vogelzang R. D., Pendleton J. W. Low root temperature effects on soybean nitrogen metabolism and photosynthesis. Plant Physiol. 1979 May;63(5):956–962. doi: 10.1104/pp.63.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Hanson A. D., Chandler P. C. Water stress enhances expression of an alpha-amylase gene in barley leaves. Plant Physiol. 1986 Feb;80(2):350–359. doi: 10.1104/pp.80.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Characterization of Pea Chloroplast D-Enzyme (4-alpha-d-Glucanotransferase). Plant Physiol. 1989 Sep;91(1):136–143. doi: 10.1104/pp.91.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984 May;75(1):278–280. doi: 10.1104/pp.75.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Spilatro S. R., Preiss J. Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol. 1988 Jan;86(1):251–259. doi: 10.1104/pp.86.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte P. A., Henson C. A., Duke S. H. Purification and Characterization of Pea Epicotyl beta-Amylase. Plant Physiol. 1990 Mar;92(3):615–621. doi: 10.1104/pp.92.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. L., Preiss J. Localization of Carbohydrate Metabolizing Enzymes in Guard Cells of Commelina communis. Plant Physiol. 1987 Oct;85(2):360–364. doi: 10.1104/pp.85.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Duke S. H. Chloroplastic regulation of apoplastic alpha-amylase activity in pea seedlings. Plant Physiol. 1990 May;93(1):131–140. doi: 10.1104/pp.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Ziegler P., Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 1986 Dec;82(4):1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler P. Partial purification and characterization of the major endoamylase of mature pea leaves. Plant Physiol. 1988 Mar;86(3):659–666. doi: 10.1104/pp.86.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]