Abstract
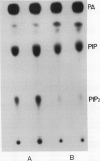
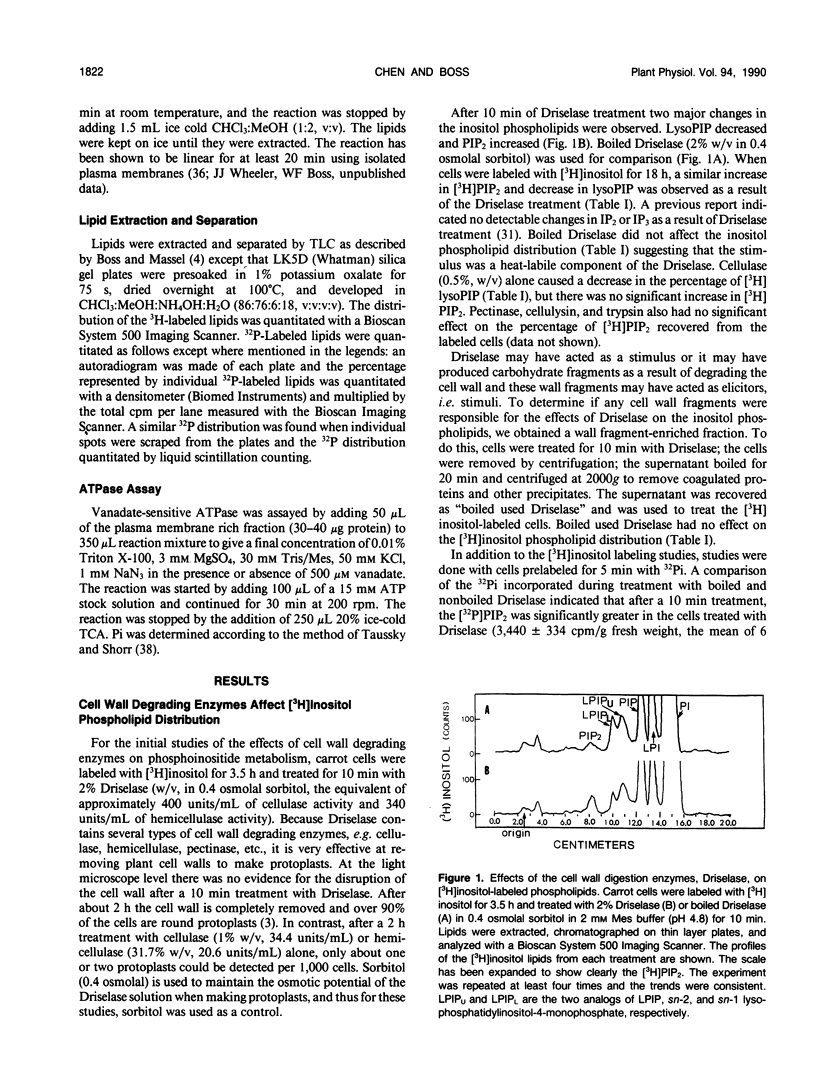
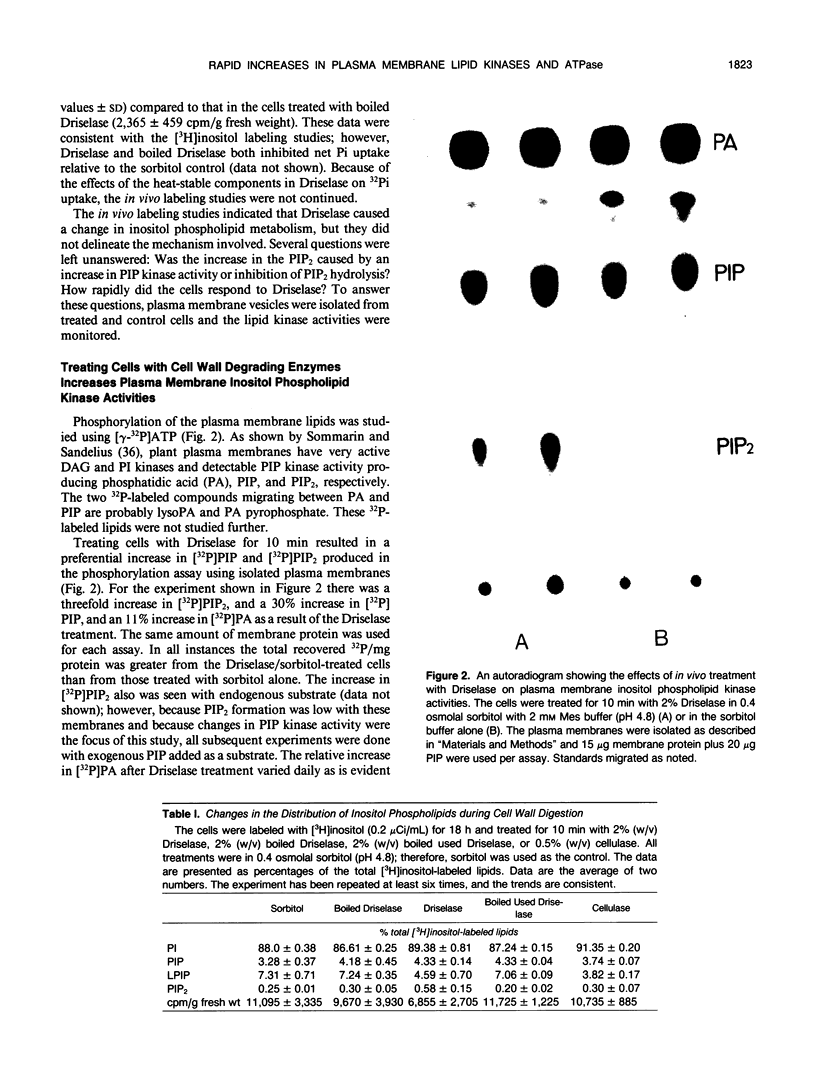
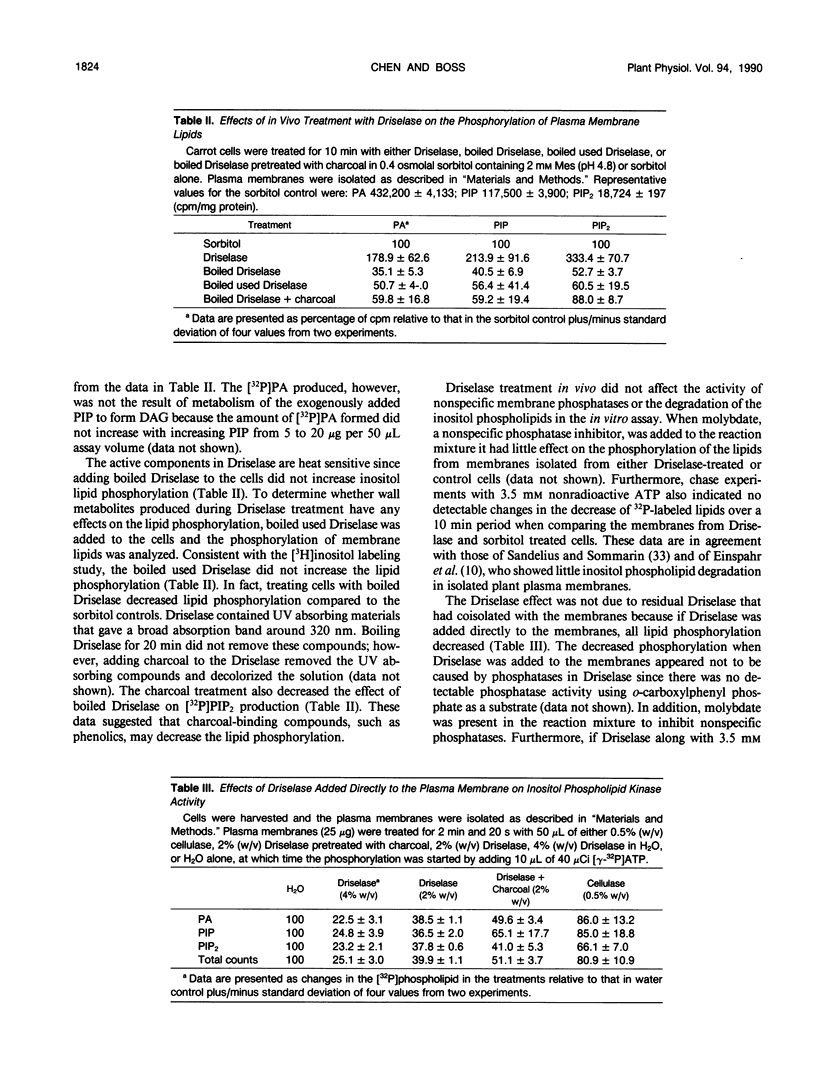
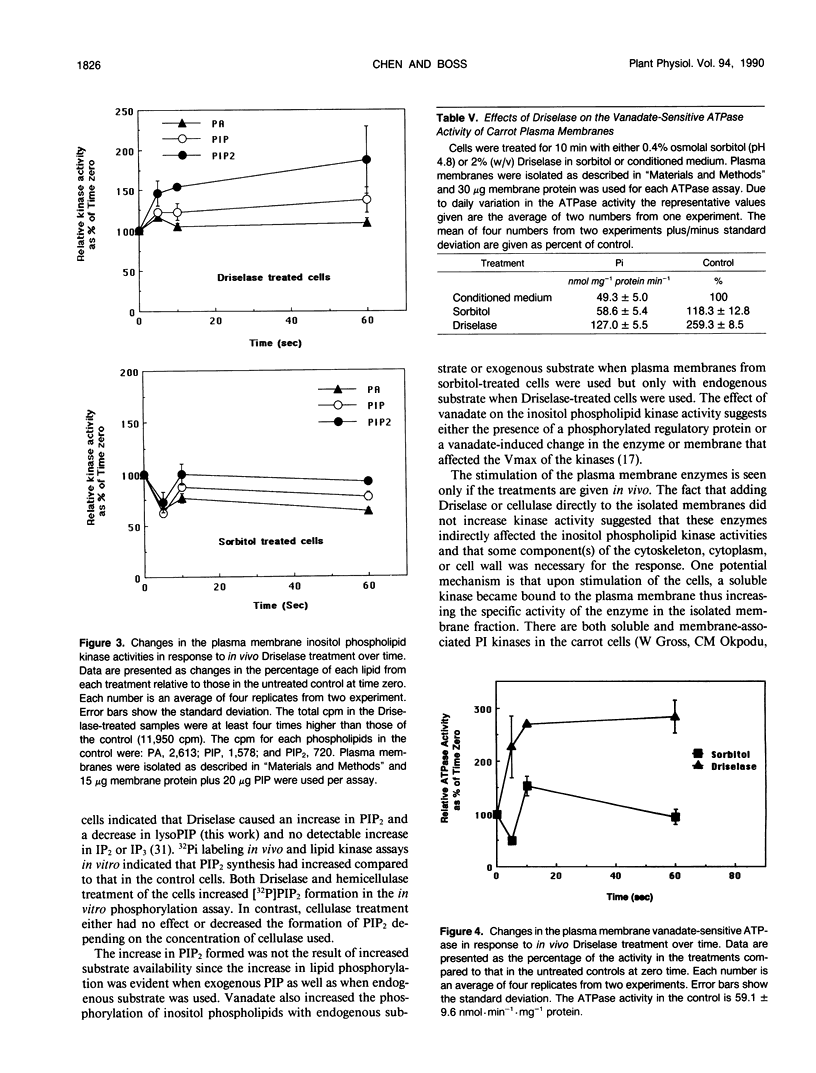
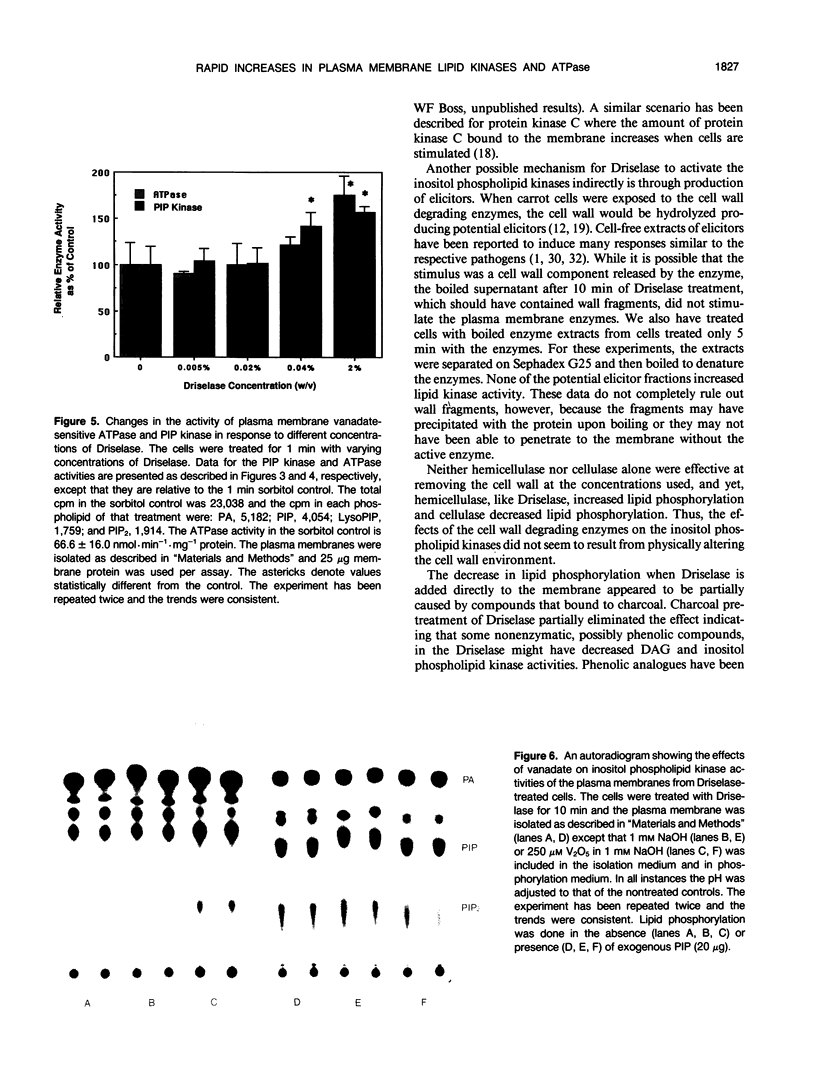
Treating carrot (Daucus carota L.) suspension culture cells with a mixture of cell wall degrading enzymes, Driselase, resulted in an increase in the percentage of [3H]phosphatidylinositol bisphosphate. Analysis of the lipid kinase activities in the isolated plasma membranes after whole cell treatment indicated that treatment with Driselase (2% weight/volume; the equivalent of 340 units per milliliter of hemicellulase and 400 units per milliliter of cellulase activity) or treatment with hemicellulase (31.7% weight/volume, 20.7 units per milliliter) resulted in an increase in the inositol phospholipid kinase activity. However, treatment with cellulase alone had no effect at 0.5% (weight/volume, 17.2 units per milliliter) or inhibited the kinase activity at 1% (weight/volume, 34.4 units per milliliter). The active stimulus in Driselase was heat sensitive. The plasma membrane vanadate-sensitive ATPase activity also increased when the cells were treated with Driselase. A time course study indicated that both the inositol phospholipid kinases and the plasma membrane vanadate-sensitive ATPase responded to as little as 5 seconds of treatment with 2% Driselase. However, at the lowest concentration of Driselase (0.04%, weight/volume) that resulted in an increase in inositol phospholipid kinase activity, the ATPase activity was not affected. Because inositol phospholipids have been shown to activate the vanadate-sensitive ATPase from plants (AR Memon, Q Chen, WF Boss [1989] Biochem Biophys Res Commun 162: 1295-1301), a stimulus-response pathway involving both the inositol phospholipid kinases and the plasma membrane vanadate-sensitive ATPase activity is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blowers D. P., Boss W. F., Trewavas A. J. Rapid Changes in Plasma Membrane Protein Phosphorylation during Initiation of Cell Wall Digestion. Plant Physiol. 1988 Feb;86(2):505–509. doi: 10.1104/pp.86.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss W. F., Massel M. O. Polyphosphoinositides are present in plant tissue culture cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1018–1023. doi: 10.1016/0006-291x(85)91908-4. [DOI] [PubMed] [Google Scholar]
- Chauhan V. P., Brockerhoff H. Phosphatidylinositol-4,5-bisphosphate may antecede diacylglycerol as activator of protein kinase C. Biochem Biophys Res Commun. 1988 Aug 30;155(1):18–23. doi: 10.1016/s0006-291x(88)81043-x. [DOI] [PubMed] [Google Scholar]
- Choquette D., Hakim G., Filoteo A. G., Plishker G. A., Bostwick J. R., Penniston J. T. Regulation of plasma membrane Ca2+ ATPases by lipids of the phosphatidylinositol cycle. Biochem Biophys Res Commun. 1984 Dec 28;125(3):908–915. doi: 10.1016/0006-291x(84)91369-x. [DOI] [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B., Dawson A. P., Irvine R. F. Inositol-containing lipids in suspension-cultured plant cells: an isotopic study. Plant Physiol. 1988 May;87(1):217–222. doi: 10.1104/pp.87.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Peeler T. C., Thompson G. A. Phosphatidylinositol 4,5-Bisphosphate Phospholipase C and Phosphomonoesterase in Dunaliella salina Membranes. Plant Physiol. 1989 Jul;90(3):1115–1120. doi: 10.1104/pp.90.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Esch T., Bonner W. D., Jr Hydrolysis of inositol phosphates by plant cell extracts. Biochem J. 1989 Dec 15;264(3):851–856. doi: 10.1042/bj2640851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl R., Varsányi M., Neumann E. Phosphorylation of phosphatidylinositol associated with the nicotinic acetylcholine receptor of Torpedo californica. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1251–1258. doi: 10.1016/s0006-291x(87)80205-x. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin S. H., Fain J. N. Ca2+-Mg2+-ATPase in rat hepatocyte plasma membranes: inhibition by vasopressin and purification of the enzyme. Prog Clin Biol Res. 1984;168:25–30. [PubMed] [Google Scholar]
- Lipsky J. J., Lietman P. S. Neomycin inhibition of adenosine triphosphatase: evidence for a neomycin-phospholipid interaction. Antimicrob Agents Chemother. 1980 Oct;18(4):532–535. doi: 10.1128/aac.18.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon A. R., Boss W. F. Rapid light-induced changes in phosphoinositide kinases and H(+)-ATPase in plasma membrane of sunflower hypocotyls. J Biol Chem. 1990 Sep 5;265(25):14817–14821. [PubMed] [Google Scholar]
- Memon A. R., Chen Q. Y., Boss W. F. Inositol phospholipids activate plasma membrane ATPase in plants. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1295–1301. doi: 10.1016/0006-291x(89)90814-0. [DOI] [PubMed] [Google Scholar]
- Memon A. R., Rincon M., Boss W. F. Inositol Trisphosphate Metabolism in Carrot (Daucus carota L.) Cells. Plant Physiol. 1989 Oct;91(2):477–480. doi: 10.1104/pp.91.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Pfaffmann H., Drobes B., Wilkinson F. E., Hartmann E. Diacylglycerol Levels Unchanged during Auxin-Stimulated Growth of Excised Hypocotyl Segments of Soybean. Plant Physiol. 1989 May;90(1):275–279. doi: 10.1104/pp.90.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka H., Imoto M., Sawa T., Hamada M., Naganawa H., Takeuchi T., Umezawa K. Screening of phosphatidylinositol kinase inhibitors from Streptomyces. J Antibiot (Tokyo) 1989 May;42(5):823–825. doi: 10.7164/antibiotics.42.823. [DOI] [PubMed] [Google Scholar]
- Rincón M., Chen Q., Boss W. F. Characterization of Inositol Phosphates in Carrot (Daucus carota L.) Cells. Plant Physiol. 1989 Jan;89(1):126–132. doi: 10.1104/pp.89.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K. R., Albert F., Anderson A. J. Lipid peroxidation is a consequence of elicitor activity. Plant Physiol. 1988 Feb;86(2):547–553. doi: 10.1104/pp.86.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Wheeler J. J., Boss W. F. Polyphosphoinositides are present in plasma membranes isolated from fusogenic carrot cells. Plant Physiol. 1987 Oct;85(2):389–392. doi: 10.1104/pp.85.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]