Abstract
Objective
The purpose of this study was to compare the effect of adding transfer energy capacitive and resistive (TECAR) therapy to conventional therapy on patients’ symptoms of myofascial pain syndrome (MPS) in the upper trapezius.
Methods
Fifty patients with MPS in the upper trapezius were randomized into 2 groups. The intervention group received TECAR therapy (capacitive mode, 2 sessions weekly), and conventional treatment comprised of stretching exercise (9 times a day) and medication (acetaminophen and tizanidine) for 3 weeks, and the comparison group received conventional treatment (same as the intervention group) alone. The patients completed the Numeric Pain Scale, Neck Disability Index, and Shoulder Pain and Disability Index before, after, and 1 month after the treatment.
Results
All variables were significantly reduced within both groups by time and treatment separately (P < .001), while a general comparison among the groups showed a significant reduction for all variables in the intervention group compared with the comparison group (P < .05) except for shoulder disability (P = .114). Moreover, the intervention group had better results compared with the comparison group based on the minimal clinically important difference.
Conclusion
A combination of TECAR therapy, exercise, and medication substantially reduced symptom severity for patients with MPS in the upper trapezius when compared to only exercise and medication.
Key Indexing Terms: Myofascial Pain Syndromes, Physical Therapy Modalities, Superficial Back Muscles, Disability Evaluation
Introduction
Myofascial pain syndrome (MPS) is a painful disorder related to muscle and surrounding fascia, which may leave a patient in excruciating pain for years. The attenuated forms of MPS are often neglected by the patients and are difficult to diagnose.1 It affects 30% to 93% of those who experience musculoskeletal discomfort.2 It often begins in the neck and shoulder, particularly when the upper trapezius (UT) muscle is involved.3 Patients with MPS are characterized by the presence of myofascial trigger points (MTrPs), which are defined as hyper-reactive taut bands or nodules in skeletal muscles. These discrete sensitive spots elicit a characteristic twitch and referred pain in response to palpation or pressure. The induced pain prevents full lengthening of the muscle and causes weakness, which is the hallmark of MPS.4,5 The main probable pathophysiology of MTrPs is sustained contractile activity that reduces blood flow and increases metabolic stress.6 These trigger points are thought to occur as a result of muscle overuse, direct trauma to the muscle, psychological stress, and systemic pathologies.7
Although there is no set strategy for managing MPS, several pharmaceutical and non-pharmacological treatments are recommended, including nonsteroidal anti-inflammatory injectable medications, opioids, topical creams, physiotherapy, manipulative therapy, vibration therapy, and exercise.8 In this regard, diathermy was shown to relieve pain in muscles and joints.9
Transfer energy capacitive and resistive (TECAR) therapy is a nonablative and noninvasive form of the combined contact diathermy and electrotherapy that improves therapeutic effects in the body, using long radiofrequency waves with high frequency (300 KHz to 1 MHz) without radiant energy from the exterior.10,11 Its distinguishing characteristic in diathermy equipment is raising the local temperature and stimulating the body's healing response through vasodilation and improved microcirculation, especially in deeper tissues like muscles, while healing properties of other diathermy modalities like ultrasound and short-wave diathermy are limited to superficial soft tissues.10,12 This therapy type is preferred as it induces adjustable tissue response through 2 modes of electrodes (capacitive and resistive) based on the tissue's features and its pathology nature.12 Adipose tissue, muscle, cartilage, and lymph are examples of superficial layers with greater electrolytic and water content where the insulating ceramic layer in capacitive mode merely produces heat. An absence of an insulating ceramic layer in resistive mode results in the passage of the energy into the deeper body tissues with more resistance and less water, such as bone, fascia, and tendons.10,12,13 These technical features enable TECAR therapy to improve symptoms of osteoarticular and muscular disorders (arthrosis, lumbago, and sciatica) and muscle injuries in different areas (including the knee, shoulder, hip, ankle, spinal column, and hands) in athletes.11 TECAR therapy has had better and more sustainable effects in the treatment of patients with shoulder pain and patients with low back pain compared to other modalities.14,15
Few studies have looked at the efficacy of TECAR therapy on MPS specifically because the pathophysiology and therapeutic results of this illness are distinct from those of ordinary pain.16 The purpose of this study was to compare the effect of adding TECAR therapy to conventional therapy on clinical symptoms of patients with UT MPS. Based on the pathophysiology of MPS and MTrPs and the mechanism of TECAR therapy, we hypothesized that TECAR therapy would be effective on symptoms of patients with MPS of UT.
Methods
Study Characteristics
In this randomized clinical trial, patients referred to physical medicine and rehabilitation clinics affiliated with Isfahan University of Medical Sciences, Isfahan, Iran (including Amin's Hospital and Al-Zahra's Hospital), from July 23, 2021, to March 20, 2022, were enrolled and gave consent for participation. The inclusion criteria were patients with a diagnosis of myofascial pain in UT, aged 18 to 60 years, pain score >3 for more than 30 days (based on the Numeric Pain Scale [NPS] score), no therapeutic intervention received in the last 30 days, no history of cervical surgery or fracture in the past 2 years, no cervical myelopathy or radiculopathy, no fibromyalgia (based on the latest version of the diagnostic criteria determined by the American College of Rheumatology in 201617), no history of psychological disorder or dissociative disorders, no pregnancy or breastfeeding, no acute infection or coagulative disorder, and no history of oral or injectable corticosteroids or narcotics use. Patients who refused to participate or were reluctant to participate, did not complete the entire study, missed the final follow-up appointments, or did not submit the questionnaire in the allotted time were excluded from the study. Furthermore, the patients who had deteriorated or showed symptom exacerbation, required surgical intervention, or had other treatments requiring referral for additional therapies were excluded from this study.
To diagnose MPS, the following criteria defined by Simons et al were used by a physical medicine and rehabilitation specialist18: (1) palpable taut, rope-like band of muscle fibers in UT, (2) point tenderness of a nodule in a taut band, (3) manual pressure of MTrPs eliciting pain at that area that mirrors the patient's experience and elicits pain at a distant site (identifies an active TrP), and (4) range of motion limitation in terms of pain.
The specialist explained the study's design and objectives to eligible participants and asked them to read and sign the written informed consent for participation. Those who gave consent were enrolled as the study participants. The specialist recorded the participants’ demographics (sex and age) in the study's checklist and asked them to complete 3 self-report questionnaires to evaluate their pain and disability in the neck and shoulder.
Based on the formula for comparing means in clinical trial studies (N = [2(Z1-a/2 + Z1-b) 2 S2 /d2]), the sample size was estimated, considering the type I error (a) = 0.05, study power (1-b) = 0.8, Z1-a/2 = 1.96, Z1-b = 0.84, S = 1.7 (SD of the average NPS scores in previous studies19), and d = 1.35 for the interpretation of the results for the difference of TECAR therapy combined with conventional treatment compared with conventional treatment. Two-tailed hypothesis and 2-sided significance level were used for sample size estimation. Because there were 2 groups of patients in this research, 25 patients were estimated for each therapy group, and 50 individuals were then computed as the final sample size. We estimated a 24% dropout rate in each group during the study. Therefore, 31 patients were recruited in each group.
After completing the questionnaires, the patients were randomized into 2 groups of 31, including TECAR therapy combined with the conventional treatment (intervention group) and conventional treatment alone (comparison group), using Random Allocation Software (Graph-Pad software, Inc, CA). The allocation ratio in this research was 1:1 and used a parallel group trial design. Each patient received a unique number from a statistician who was not involved in the other steps and was assigned to 1 of the groups. The patients were referred to another physician in the physiotherapy clinic of Amin's Hospital with the particular number in a closed envelope.
The physician taught exercises and explained medication use to the patients, and during the study, evaluated patients’ compliance about exercise and medication use via phone call. Moreover, he provided TECAR therapy for patients in the intervention group in the physiotherapy clinic of Amin's Hospital.
The first endpoint time was immediately after the end of interventions, and the second endpoint stage was 1 month after the completion of study interventions. Therefore, the patients were asked to refer to the physical medicine and rehabilitation clinics (including Amin's Hospital and Al-Zahra's Hospital) immediately after the end of the interventions and 1 month after that for a follow-up examination and to complete the questionnaires again. The physical medicine and rehabilitation specialist was blinded to the patients’ grouping allocations when examining the patients at each follow-up and collecting the outcome measures.
Furthermore, the statistician who performed the statistical analysis was blinded to the allocations and analyzed the data by patients’ assigned codes.
Blinding
The patients were not blinded due to the nature of the study.
Data Collection Tools
Numeric Pain Scale
The first questionnaire was an 11-point NPS used to evaluate the pain severity in UT muscle and neck regions. The patients were asked to mark their pain on an 11-point scale, from 0 (indicating no pain) to 10 (indicating the worst pain possible). The pain intensity was considered mild at given scores between 1 to 3, moderate at scores between 4 to 7, and severe at scores between 8 to 10.
Neck Disability Index
The second questionnaire was the Neck Disability Index (NDI), a valid index for evaluating neck disability, designed in 1991 by Vernon and Mior20 and validated in patients with chronic non-traumatic neck pain.21 It has 10 questions: 7 questions about daily activities, 2 questions about pain, and 1 about concentration. This questionnaire focuses on the patient's ability to perform personal care, lifting objects, reading, concentration, driving, sleeping, and hobbies. Each item scored from 0 to 5, and the sum of the scores forms the total score. Each patient scores within a range of 0 to 50; higher scores show greater pain and disability. We used a Persian-translated version of the questionnaire that was previously validated.22
Shoulder Pain and Disability Index
The third and last questionnaire was the Shoulder Pain and Disability Index (SPADI), which was used to evaluate the patient's pain and disability in the shoulders. This questionnaire was developed by Heald et al23 in 1997 with 2 domains: a 5-item pain domain and an 8-item functionality domain. A total score of 0 to 130 is the sum of each question score of 0 to 10. Higher scores show greater pain and disability. A Persian-translated version of this questionnaire was previously validated.24
Study Interventions
The comparison group received conventional treatment consisting of exercise and medication for 3 weeks. The exercise therapy was composed of 30-second UT stretching exercises 9 times a day (3 times in the morning, 3 times in the afternoon, and 3 times at night). The pharmacological therapy was composed of an analgesic (daily oral intake of 1 tablet of acetaminophen, 325 mg) and a muscle relaxant (tizanidine, 4 mg, a one-fourth tablet for the first 3 nights, continued by a one-half tablet each night).
In the intervention group, in addition to 3-week conventional therapy, the patients underwent TECAR therapy by Back 3SE manual (capacitive electrode transfer [CET] model, 500 kHz, 400 A, WINBACK, Biarritz, France). For 3 weeks straight, they received TECAR therapy in 6 sessions (2 sessions weekly, on Saturdays and Tuesdays), each lasting 20 minutes and using medium electrodes (60 mm) with a power range of 20% to 50%. After impregnating the neutral surface of the device and the CET electrode with conductive cream, the neutral plate was placed in front of the patient's chest, and the CET electrode was moved in the UT area by rubbing while the patient was in the prone position.
Ethical Considerations
The protocol of this study was approved by Ethics Committee of Isfahan University of Medical Sciences (code: IR.MUI.MED.REC.1400.219) and registered in the Iranian Registry of Clinical Trials (code: IRCT20210623051688N1). Before inclusion, informed consent was given by all the participants. The patients’ privacy was maintained via the study, and all intervention costs and expenses were supported by the authors.
Statistical Analysis
The collected information was input into the statistical software IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY), used for all of the statistical analysis. The descriptive data were presented in mean, SD, percentages, and absolute numbers. The χ2 test, independent t test, and generalized linear models were used for analytics. P values <.05 were considered significant. We used a Bonferroni test to adjust P values in independent t tests and to reduce type 1 error; therefore, P values <.05/3 (.017) were statistically significant. Moreover, we calculated Cohen's d effect size for each of the t tests, which was considered as follows: small ≥0.20, medium ≥0.50, and large ≥0.80. For the minimal clinically important difference, we considered 2 points for an 11-point NPS,25 6 for NDI,26 and 8 for each subscale of SPADI.27
Results
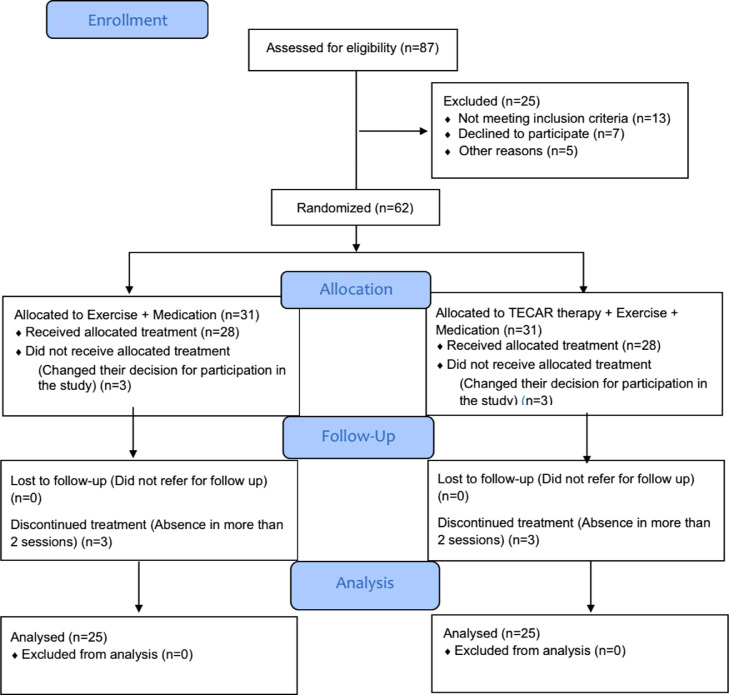
Eighty-seven patients were assessed for eligibility criteria by the researcher, who is an experienced physiatrist. Sixty-two patients were eligible. After completing the questionnaires, the patients were randomized into 2 groups of 31. Three patients in each group did not receive allocated treatment because they changed their decision to participate in the study. Furthermore, 3 patients in each group discontinued treatment, which means they were absent in more than 2 sessions. Therefore, a total of 50 patients with UT MPS completed the study, 25 in each group (Fig 1).
Fig 1.
Flow diagram for participant screening and enrollment for this study.
The demographic characteristics of participants are presented in Table 1; there was no significant difference among the groups in terms of mean age and sex distribution (P = .867 and .758, respectively).
Table 1.
Demographic Characteristics of Participants
| Variables | Comparison Group (n = 25) | Intervention Group (n = 25) | P Value | Cohen's d Effect Size |
|---|---|---|---|---|
| Age (y) | ||||
| Mean ± SD | 43.68 ± 9.32 | 43.2 ± 10.82 | .867a | −0.048 |
| Min/Max | 20/56 | 23/58 | ||
| Sex—n (%) | ||||
| Female | 7 (28.0) | 8 (32.0) | .758b | |
| Male | 18 (72.0) | 17 (68.0) |
Independent t test.
χ2 test.
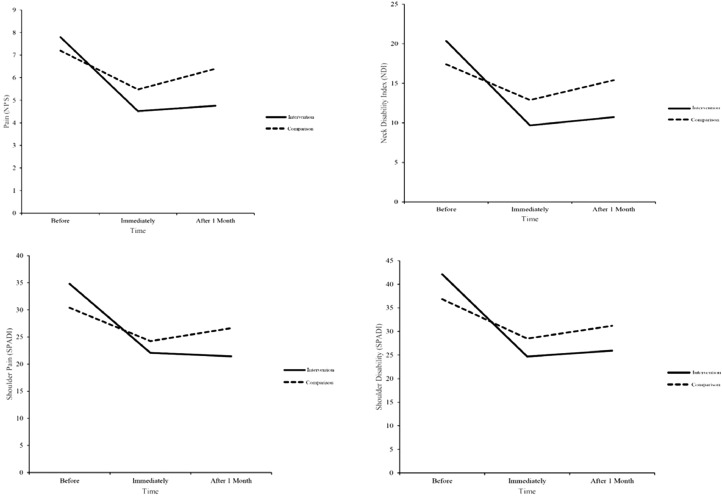
The trend of changes in the variables in each group showed that conventional treatment alone or in combination with TECAR therapy led to statistically significant improvement for all variables (P < .001). The outcomes improved for all variables by time within both groups (P < .001). The general comparison between the 2 groups revealed that intervention (using TECAR therapy in addition to conventional treatment) was statistically superior to the comparison (using conventional treatment alone) for all variables (P < .05), with the exception of shoulder disability (P = .114).
Moreover, the mean of all variables in the intervention group significantly differed immediately after the treatment completion and 1 month after the treatment completion compared with before the treatment (more than 2 for NPS, more than 6 for NDI, and more than 8 for each subscale of SPADI). In contrast, the mean of NPS, NDI, and shoulder pain scores in the comparison group across the study stages did not show a significant difference, except for shoulder disability, the difference of mean between before the treatment and only immediately after the treatment was significant (>8).
The mean values of pain scores, NDI, and SPADI and the trend of their changes trend during the study are shown for the 2 study groups in Table 2 and Figure 2.
Table 2.
Comparison of Pain and Disability of the Neck and Shoulder Between 2 Groups
| Groups (Mean ± SD) |
P1 | 95% CI for Cohen's d Effect Size |
Cohen's d Effect Size e | P2 (Treatment) | P3 (Time) | P4 (Treatment and Time) | |||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Comparison (n = 25) | Intervention (n = 25) | Lower | Upper | |||||
| Numeric Pain Scale (NPS) | |||||||||
| Before | 7.2 ± 1.89 | 7.8 ± 1.78 | .254 | −0.462 | 1.116 | 0.327 | |||
| Immediately after | 5.48 ± 1.5 | 4.52 ± 1.08 | .013 | −1.545 | 0.075 | −0.735 | <.001 | <.001 | <.001 |
| After 1 month | 6.4 ± 1.89 | 4.76 ± 1.92 | .004 | −1.68 | −0.041 | −0.861 | |||
| Neck disability (NDI) | |||||||||
| Before | 17.4 ± 4.83 | 20.36 ± 3.82 | .021 | −0.127 | 1.486 | 0.68 | |||
| Immediately after | 12.88 ± 4.19 | 9.68 ± 1.4 | .001 | −1.858 | −0.191 | −1.024 | <.001 | <.001 | <.001 |
| After 1 month | 15.4 ± 5.08 | 10.72 ± 1.51 | <.001 | −2.106 | −0.392 | −1.249 | |||
| Shoulder pain (SPADI) | |||||||||
| Before | 30.4 ± 8.78 | 34.8 ± 8.81 | .083 | −0.296 | 1.296 | 0.5 | |||
| Immediately after | 24.24 ± 7.11 | 22.08 ± 6.92 | .282 | −1.097 | 0.481 | −0.308 | <.001 | <.001 | .043 |
| After 1 month | 26.64 ± 8.26 | 21.44 ± 4.51 | .009 | −1.595 | 0.032 | −0.781 | |||
| Shoulder disability (SPADI) | |||||||||
| Before | 36.84 ± 10.89 | 42.12 ± 14.3 | .149 | −0.377 | 1.208 | 0.415 | |||
| Immediately after | 28.48 ± 8.69 | 24.68 ± 8.33 | .121 | −1.24 | 0.347 | −0.446 | <.001 | <.001 | .114 |
| After 1 month | 31.2 ± 9.97 | 25.92 ± 8.67 | .052 | −1.365 | 0.234 | −0.565 | |||
P1 indicates independent t test (P < .017 is significant). P2-P3-P4 indicates GLM (P < .05 is significant).
GLM, Generalized Linear Model; NPS, Numeric Pain Scale; NDI, Neck Disability Index; SPADI, Shoulder Pain and Disability Index.
Fig 2.
Treatment process in the study groups. NDI, Neck Disability Index; NPS, Numeric Pain Scale; SPADI, Shoulder Pain and Disability Index.
Discussion
We evaluated the effect of adding TECAR therapy to the conventional treatment of UT MPS. The comparison and intervention group patients did not show significant differences in demographic features from one another. Hence, the probable confounding role of demographics on the outcomes was minimized.
The results showed that TECAR therapy combined with conventional therapy resulted in greater improvement of pain severity originating from the UT, neck, and shoulder, as well as neck disability, compared with the conventional treatment alone. Adding TECAR to conventional management does not seem to have any additional effect on shoulder disability.
TECAR therapy is a novel physical therapeutic modality with approved effectiveness in several musculoskeletal disorders, such as adhesive capsulitis.11,28 The mechanism of action for this diathermy method is providing direct (moderate) heat to pathological lesions, which increases microcirculation and oxygen supply to musculoskeletal tissues.12 However, the pathogenesis of MPS is different from ordinary pain, and the role of MTrPs in MPS should be considered. Thus, new therapeutic strategies have focused on MTrPs.29 Therefore, prospective studies need to study the independent effectiveness of TECAR therapy on MPS until their results can extend the TECAR therapy efficacy.
Additional TECAR therapy showed significant positive effects on patient's pain in the UT, neck, and shoulder regions, and the intervention group had significantly lower pain scores compared to the comparison group, which highlights the superiority of combined TECAR-conventional therapies over the conventional treatment alone, statistically and clinically. Other studies confirmed the analgesic effect of TECAR therapy in different muscles, including the shoulder; however, as they have not considered MPS, they cannot be discussed.30 There are few studies on the effectiveness of TECAR therapy on MPS that could be comparable with the present study. In a double-blind placebo-controlled trial, the researchers studied patients with active MTrP in the UT muscle (myofascial chronic neck pain). Eight sessions of 448 kHz capacitive resistive monopolar radiofrequency treatment were given to their patients. In contrast to the placebo group, the intervention group's visual analog scale ratings exhibited a tendency toward decline both after the first session and at the conclusion of the eighth session, according to their findings.16 These outcomes match those of the current research. It was suggested that the mechanism of TECAR therapy on MPS may be due to sodium ion transport by K-ATPase and hydrogen bonding of water molecules with ions/ligands, created by the interaction of the electromagnetic field with transient oscillating-induced electrons and protons, which affects the bonds of hydrogen in hydrated proteins.31 Nonetheless, this mechanism is still a hypothesis which was not established yet; therefore, more studies are required to investigate the molecular mechanism behind this effect.
In addition to pain, we evaluated the effect of intervention on disability using NDI and SPADI. For neck disability, the results showed the superiority of combined TECAR therapy in neck functionality improvement compared with conventional treatment alone, statistically and clinically. Therefore, the combination of TECAR therapy with conventional treatment gave patients better improvement in all regions with pain sensations and neck functionality. This is in contrast with Diego et al who found neither difference between the groups in post-intervention NDI scores nor in the right cervical rotation.16 This difference could be due to the difference in the therapeutic methods used for each group, as we studied the effect of adding TECAR therapy to exercise and medication. In another study, the effect of TECAR therapy (frequency of 500 kHz, and intensity of 40%) on MPS was investigated on professional basketball players compared with the control group (device off). The intervention group saw a higher rise in absolute temperature, but there was no change in their range of motion or VAS scores.32 These results are inconsistent with that of the present study. This discrepancy could be due to the fact that this study has measured the short-term outcomes (up to 30 minutes after 1 session), while we considered long-term results after 6 sessions.
Another disability score evaluated in the present study was shoulder disability. The conventional treatment, with or without TECAR therapy, had the same effect on the shoulder functionality. Furthermore, the patients in the intervention group showed significant reduction in shoulder disability score at both follow-ups based on the minimal clinically important difference; the difference in the control group, however, was only appreciable right after the treatment. Further research is required to clarify this variable because it has not yet been examined in any other study.
Most of the treatment strategies suggested for MPS have short-term effectiveness, and there are few strategies with sustained efficacy for more than 3 months.8,33 Regarding the therapies that were effective until 1 month, especially in the intervention group, it is suggested to evaluate this effect in a longer follow-up period to determine whether this effect sustains over a more extended period of time.
Limitations
The short duration of follow-up is a limitation of this study; thus, the effects of long-term intervention were not examined. Another limitation was that our sample size may not have been sufficient to identify minute variations between the research groups. Additionally, the samples were chosen from referrals to a single city; thus, care should be given when extrapolating the findings to the whole community. Considering the limitations of the present study, and few studies in this regard, further studies are required for inclusive results. We encourage the researchers to conduct multicenter trials with large study populations and follow their patients for a more prolonged period of time. Furthermore, molecular studies are appreciated to discover the TECAR mechanism of action.
Conclusion
A combination of TECAR therapy, exercise, and medication substantially reduced symptom severity for patients with MPS in the UT when compared with only exercise and medication.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): P.T., S.S., R.M.
Design (planned the methods to generate the results): P.T., S.S., R.M.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): P.T., S.S., R.M.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): P.T., S.S., R.M.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): P.T., S.S., R.M.
Literature search (performed the literature search): P.T., S.S., R.M.
Writing (responsible for writing a substantive part of the manuscript): P.T., S.S., R.M.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): P.T., S.S., R.M.
Practical Applications.
-
•
A comparison among the groups showed a significant reduction for all variables in the intervention group compared with the comparison group except for shoulder disability.
-
•
The intervention group had better results compared with the comparison group based on the minimal clinically important difference.
-
•
Our results showed that adding transfer energy capacitive and resistive therapy to the conventional treatment improved patients’ symptoms.
Alt-text: Unlabelled box
References
- 1.Plaut S. Scoping review and interpretation of myofascial pain/fibromyalgia syndrome: an attempt to assemble a medical puzzle. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0263087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons DG. Clinical and etiological update of myofascial pain from trigger points. J Musculoskelet Pain. 1996;4(1-2):93–122. [Google Scholar]
- 3.Ribeiro DC, Belgrave A, Naden A, Fang H, Matthews P, Parshottam S. The prevalence of myofascial trigger points in neck and shoulder-related disorders: a systematic review of the literature. BMC Musculoskelet Disord. 2018;19(1):252. doi: 10.1186/s12891-018-2157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg-Stein J, Simons DG. Focused review: myofascial pain. Arch Phys Med Rehabil. 2002;83(3 Suppl 1):S40–S47. doi: 10.1053/apmr.2002.32155. S48-49. [DOI] [PubMed] [Google Scholar]
- 5.Cao Q-W, Peng B-G, Wang L, et al. Expert consensus on the diagnosis and treatment of myofascial pain syndrome. World J Clin Cases. 2021;9(9):2077–2089. doi: 10.12998/wjcc.v9.i9.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jafri MS. Mechanisms of myofascial pain. Int Sch Res Notices. 2014;2014 doi: 10.1155/2014/523924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep. 2012;16(5):439–444. doi: 10.1007/s11916-012-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urits I, Charipova K, Gress K, et al. Treatment and management of myofascial pain syndrome. Best Pract Res Clin Anaesthesiol. 2020;34(3):427–448. doi: 10.1016/j.bpa.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Tranquilli C, Ganzit G, Ciufetti A, Bergamo P, Combi F. Multicentre study on Tecar® therapy in sports pathologies. 2009 [Google Scholar]
- 10.ResearchGate. Tecar® therapy in the treatment of acute and chronic pathologies in sports. Available at:https://www.researchgate.net/profile/Gian-Ganzit/publication/267853490_TECARR_THERAPY_IN_THE_TREATMENT_OF_ACUTE_AND_CHRONIC_PATHOLOGIES_IN_SPORTS/links/558bea9f08aee43bf6ad2853/TECARR-THERAPY-IN-THE-TREATMENT-OF-ACUTE-AND-CHRONIC-PATHOLOGIES-IN-SPORTS.pdf. Accessed December 20, 2022.
- 11.Hawamdeh M. The effectiveness of capacitive resistive diathermy (Tecartherapy®) in acute and chronic musculoskeletal lesions and pathologies. Eur J Sci Res. 2014;118(3):336–340. [Google Scholar]
- 12.Clijsen R, Leoni D, Schneebeli A, et al. Does the application of tecar therapy affect temperature and perfusion of skin and muscle microcirculation? A pilot feasibility study on healthy subjects. J Altern Complement Med. 2020;26(2):147–153. doi: 10.1089/acm.2019.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parolo E, Combi F. Hyperthermia through resistive and capacitive energy transfer in the treatment of acute and chronic musculoskeletal lesions. Medicine (Baltimore) 2009 ID: 78397094. [Google Scholar]
- 14.Notarnicola A, Maccagnano G, Gallone MF, Covelli I, Tafuri S, Moretti B. Short term efficacy of capacitive-resistive diathermy therapy in patients with low back pain: a prospective randomized controlled trial. J Biol Regul Homeost Agents. 2017;31(2):509–515. [PubMed] [Google Scholar]
- 15.Paolucci T, Pezzi L, Centra MA, et al. Effects of capacitive and resistive electric transfer therapy in patients with painful shoulder impingement syndrome: a comparative study. J Int Med Res. 2020;48(2) doi: 10.1177/0300060519883090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diego IMA, Fernández-Carnero J, Val SL, et al. Analgesic effects of a capacitive-resistive monopolar radiofrequency in patients with myofascial chronic neck pain: a pilot randomized controlled trial. Rev Assoc Med Bras (1992) 2019;65(2):156–164. doi: 10.1590/1806-9282.65.2.156. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Simons DG, Travell JG, Simons LS. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. Travell & Simons' myofascial pain and dysfunction: upper half of body. [Google Scholar]
- 19.Hakgüder A, Birtane M, Gürcan S, Kokino S, Turan FN. Efficacy of low level laser therapy in myofascial pain syndrome: an algometric and thermographic evaluation. Lasers Surg Med. 2003;33(5):339–343. doi: 10.1002/lsm.10241. [DOI] [PubMed] [Google Scholar]
- 20.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415. [PubMed] [Google Scholar]
- 21.En MC, Clair DA, Edmondston SJ. Validity of the Neck Disability Index and Neck Pain and Disability Scale for measuring disability associated with chronic, non-traumatic neck pain. Man Ther. 2009;14(4):433–438. doi: 10.1016/j.math.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Mousavi SJ, Parnianpour M, Montazeri A, et al. Translation and validation study of the Iranian versions of the Neck Disability Index and the Neck Pain and Disability Scale. Spine (Phila Pa 1976) 2007;32(26):E825–E831. doi: 10.1097/BRS.0b013e31815ce6dd. [DOI] [PubMed] [Google Scholar]
- 23.Heald SL, Riddle DL, Lamb RL. The shoulder pain and disability index: the construct validity and responsiveness of a region-specific disability measure. Phys Ther. 1997;77(10):1079–1089. doi: 10.1093/ptj/77.10.1079. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimzadeh MH, Birjandinejad A, Golhasani F, Moradi A, Vahedi E, Kachooei AR. Cross-cultural adaptation, validation, and reliability testing of the Shoulder Pain and Disability Index in the Persian population with shoulder problems. Int J Rehabil Res. 2015;38(1):84–87. doi: 10.1097/MRR.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 25.Farrar JT, Young JP, Jr., LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 26.Hung M, Saltzman CL, Kendall R, et al. What are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res. 2018;476(10):2027–2036. doi: 10.1097/CORR.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul A, Lewis M, Shadforth MF, Croft PR, Van Der Windt DAWM, Hay EM. A comparison of four shoulder-specific questionnaires in primary care. Ann Rheum Dis. 2004;63(10):1293–1299. doi: 10.1136/ard.2003.012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahdatpour B, Haghighat S, Sadri L, Taghian M, Sadri S. Effects of transfer energy capacitive and resistive on musculoskeletal pain: a systematic review and meta-analysis. Galen Medical Journal. 2022;11:e2407. doi: 10.31661/gmj.v11i.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai MJ, Saini V, Saini S. Myofascial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21–36. doi: 10.1007/s40122-013-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro S, Henriques B, Cardoso R. The effectiveness of tecar therapy in musculoskeletal disorders. International Journal of Public Health and Health Systems. 2018;3(5):77–83. [Google Scholar]
- 31.Lastra JCR, Méndez EP. Contemporary Advances in Sports Science. IntechOpen; London, United Kingdom: 2021. Use of an Evolution in tecartherapy for muscle improvement and treatment of sports injuries. [Google Scholar]
- 32.Yeste-Fabregat M, Baraja-Vegas L, Vicente-Mampel J, Perez-Bermejo M, Gonzalez Bautista, IJ Barrios C. Acute effects of tecar therapy on skin temperature, ankle mobility and hyperalgesia in myofascial pain syndrome in professional basketball players: a pilot study. Int J Environ Res Public Health. 2021;18(16):8756. doi: 10.3390/ijerph18168756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin HJ, Shin JC, Kim WS, Chang WH, Lee SC. Application of ultrasound-guided trigger point injection for myofascial trigger points in the subscapularis and pectoralis muscles to post-mastectomy patients: a pilot study. Yonsei Med J. 2014;55(3):792–799. doi: 10.3349/ymj.2014.55.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]