Abstract
Objective
The purpose of this scoping review was to explore the effects of neural mobilization (NM) on outcomes in adults with diabetic peripheral neuropathy (DPN).
Methods
Five databases were searched—PubMed, Web of Science (Web of Science Core Collection), Physiotherapy Evidence Database (PEDro), and Scopus—from inception to January 2022. The studies included were randomized controlled trials, pre-post single group design, multiple case studies, controlled case studies, quasi-experimental studies, and single case studies, which are published in full text in English.
Results
Six studies were included in this review, and most were of low-level evidence. The sample size of the studies ranges from 20 to 43, except for 1 case study, with a total of 158 participants in all the studies combined. In 4 out of 6 studies, only NM was given, whereas in 2 studies, NM was used along with other treatment strategies. The tibial nerve was the most studied nerve, whereas 1 study administered NM to nerves of the upper limbs, and only 1 trial examined the sciatic nerve. The outcomes included the Michigan Neuropathy Screening Instrument questionnaire, nerve conduction velocity, vibration perception threshold, heat/cold perception threshold, weight-bearing asymmetry and range of motion of lower limb, quality of life, and magnetic imaging changes.
Conclusion
At present, only a few low-level studies exist on the use of NM for the treatment of adults with DPN. The evidence for use of NM on DPN is still limited and insufficient.
Key Indexing Terms: Diabetic Complications, Diabetic Neuropathies, Tibial Nerve, Neural Conduction, Quality of Life
Introduction
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes mellitus (DM), affecting approximately 50% of patients.1 DPN affects nearly 30% of hospitalized patients with diabetes and nearly 20% to 30% of non-hospitalized patients with diabetes.2 In type 2 diabetes, DPN affects 59% of patients.3 Several studies have implicated poor glycemic control, duration of diabetes, hyperlipidemia (particularly hypertriglyceridemia), elevated albumin excretion rates, and obesity as risk factors for the development of DPN.4
In DPN, there is involvement of both small and large myelinated nerves fibers in limb-length dependent manner,5 though the involvement of small nerve fibers occurs first.6,7 Involvement of large nerve fibers results in impairment of proprioception, vibration, and pressure, whereas damage to small nerve fibers leads to impaired thermal sensation, pain, and autonomic function5,8 or both. In the early stages of DPN, sensory impairment is predominant, and no clinical impairment in motor function is detectable.9,10 Disruption of sensorimotor function leads to decreased ankle reflexes, strength, impairment in balance, coordination, and gait control.11,12 There is an increased incidence of foot ulcers,13,14 resulting in foot infection and even amputation in later stages of DPN.15 Patients with DPN exhibit slower nerve conduction velocities (NCV) of tibial, peroneal, and median nerves.16,17 Arezzo reported a decrease of ∼0.5 m/s in NCV of patients with DPN annually.18
Regular exercise leads to improvements in body composition, insulin sensitivity, glucose concentrations, lipid profile, vascular function,19 and even nerve functions.20 However, studies have reported weight-bearing exercise-induced injuries in people with DPN.19 Neural mobilization (NM) facilitates movement between neural structures and their surroundings. It reduces nerve mechano-sensitivity and increases the compliance of nerve tissues by improving neural flexibility.21 The NM techniques are useful in restoring normal physiological function through both the intraneural and extraneural effects.21,22 According to Schmid et al, NM techniques work by improving axonal transport and blood flow and reduces pressure within the nerves.21 In the diabetic population, NM of sciatic nerve improves flexibility of lower limb muscles.23 Long-term exercise training was used in patients with DPN to improve blood pressure, body mass index, free fat mass, fat mass, hemoglobin A1c, lipid profile,16 and alleviate conduction velocity of nerves.20 The addition of neurodynamics over manual therapy showed positive effects on nerve conduction (6%), pain, and functional status in carpal tunnel syndrome.24 Despite the availability of literature on the benefits of NM, few studies have focused on the effect of NM on nerve functions and muscle activity in DPN. Therefore, the purpose of this scoping review was to explore the effects of NM on outcomes in adults with DPN.
Materials and Methods
The current scoping review follows the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews.25
Eligibility Criteria
Studies were included in the review if they met the following criteria. Participants included people with a diagnosis of DPN, older than or equal to 18 years. Intervention included trials investigating the effects of NM given with or without other intervention techniques in patients with DPN. No restrictions were placed on the control or comparison group. No restrictions were placed on outcome measures. Types of study included randomized controlled trials, studies with pre-post single group design, multiple case studies, controlled case studies, quasi-experimental studies, and single case studies, published in the full text in English. The studies were excluded if the cause of neuropathy was due to other pathology other than diabetes and if published in languages other than English. Letters to the editor, conference papers, mini-reviews, and commentaries were excluded.
Information Sources
The following databases were searched: PubMed, Web of Science (Web of Science Core Collection), Physiotherapy Evidence Database (PEDro), Scopus, and general search in Google Scholar from inception to January 2022. Search terms used were a combination of keywords, “neurodynamic, neural/nerve mobilization, slider/tensioner techniques of mobilization, diabetic peripheral neuropathy, diabetic polyneuropathy.” The Boolean operators “AND,” “OR,” and/or “NOT” were used for further accuracy of the search. The details of the search strategy are given in Table 1.
Table 1.
Search Strategy
| Database | Search Strategy | Filter |
|---|---|---|
| Scopus | “nerve mobilization”" OR “neural mobilization” OR “manual therapy” AND “diabetic peripheral neuropathy” OR “diabetic complications” | Document type: Articles Language: English |
| Web of Science (Web of Science Core Collection) | https://www.webofscience.com/wos/woscc/summary/275923fb-e66a-4c0e-a150-9e3ed19ef608-227869fe/relevance/1 | Document type: Articles Language: English |
| PubMed | (((((“nerve mobilization” [Title/Abstract]) OR (“neural mobilization” [Title/Abstract])) OR (“manual therapy” [Title/Abstract])) AND (“Diabetic peripheral neuropathy” [Title/Abstract])) OR (“nerve conduction velocity” [Title/Abstract]) | Language: English Species: Human |
| Physiotherapy Evidence Database (PEDro) | “Diabetic peripheral neuropathy” | Therapy: stretching, mobilization, manipulation, massage Method: Clinical trial When searching: Match all search terms (AND) |
| Google Scholar | Neural mobilization in diabetic peripheral neuropathy; nerve mobilization in diabetes; neural mobilization in diabetic neuropathy | Type: Any type |
Selection of Studies
Two reviewers (MA and SP) independently selected the titles and abstracts identified during the literature search. Potentially eligible studies for inclusion in this review were thoroughly analyzed and subsequently assessed in terms of appropriateness according to the eligibility criteria. Whenever there was a disagreement in either the selection process, data extraction, or assessing the risk of bias, a third author (MN) reviewed the assessment, and the consensus was reached by discussion.
Data Extraction
Two reviewers (MA and SP) extracted the data independently, and the third author (MN) checked the extracted data. Any disagreements were resolved by discussion between the review authors. The following data were extracted from each study: author's name and year of publication, diabetic neuropathy definition, Questionnaire of the Michigan Neuropathy Screening Instrument score, ability to walk, type of DM, and duration, number of participants in both experimental and control groups, types of interventions in both the groups, mean follow-up time, mean age of the participants, treatment outcomes (baseline, follow-up, and end of treatment), primary outcome measures, study design, study results, and conclusions.
Quality Assessment of Included Trials
For assessing the methodological quality of all the retrieved evidence, we used the 11-point PEDro scale designed to rate the quality of randomized controlled trials.26 Trials were independently assessed for quality by 2 authors (MA and SP). If there was any disagreement on any criterion, it was re-assessed by each reviewer independently. Unresolved disagreements were identified and discussed in a consensus meeting. Any conflict that remained unresolved was then taken to a third reviewer (MN), who was independent of the initial deliberations, and a final consensus was reached. Each criterion was rated either yes (score = 1) or no (score = 0) to minimize the ambiguity in responses. The total score for the methodological quality of each included study was calculated by summing all the responses (maximum score = 10). Studies were then classified as poor (score of < 4), fair (score of 4 or 5), good (score of 6 to 8), and excellent (score of >8) quality based on the total score obtained.27
Results
Selection of Sources of Evidence
A total of 3080 articles were identified from the search performed. After removing the duplicates, 3038 records went for screening. Mendeley desktop version 1.19.8 was used for duplication removal. A total of 2676 records were removed after screening based on the title and abstract. Lastly, 6 articles were found to be relevant based on the predesigned eligibility criteria and were included in the review with consensus from the authors. The selection of the studies is illustrated in Figure 1.
Fig 1.
PRISMA flowchart of the study.
Characteristics of Included Trials
Two randomized controlled trials,28,29 1 case series,30 1 cross-sectional study,31 1 study with single group pre-post design,32 and 1 case study33 were included in the review. All of the studies included were done on participants with DPN, but the severity of the DPN was not indicated for inclusion criteria. A single case study was included, and the sample size of the remaining studies ranged from 20 to 43, with a total of 158 participants in all the studies combined. The inclusion and exclusion criteria of the studies included are summarized in Table 2.
Table 2.
Inclusion and Exclusion Criteria of the Studies
| Trials | Study Type | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|
| Kumar et al29 | Pilot RCT | • Known case of T2DM, with stable glycemic levels (on HbA1c) for a minimum of 6 months • Complaint of bilateral neuropathic pain in the legs and feet (screened using neuropathic pain scale) for a minimum of 6 months • VPT greater than 25 volts in both feet when assessed using a biothesiometer • Tested positive on structural differentiation during lower extremity neurodynamic testing on both sides of lower limbs. • Mechanical allodynia to manual palpation of nerve trunks in bilateral legs and feet |
• Any comorbid musculoskeletal disorders • History of fractures, trauma and surgery to lower limbs • Inability to understand therapist's instructions |
| Ali et al30 | Case series | • Men and women with age above 40 years • Diagnosed T2DM more than 2 years with peripheral neuropathy in upper limb |
• Participants with frozen shoulder • History of altered mental health status • History of particular shoulder injury surgery |
| Manu et al33 | Case study | • Diagnosed case of diabetic symmetrical sensorimotor diabetic peripheral neuropathy | • Not mentioned |
| Gehlot et al28 | RCT | • Not mentioned | • Not mentioned |
| Boyd et al31 | Cross-sectional study | • Diagnosed T2DM • Participants have to meet flexibility requirements of isolated hip flexion ≥90°, full knee extension, ankle dorsiflexion ≥0° and plantar flexion ≥30° |
• Low back or leg pain lasting >3 consecutive days in the past 6 months • Complex regional pain syndrome, lumbar spine surgeries • Chemical dependence or alcohol abuse • A history of sciatica or trauma to the nerves of the lower extremity • Chemotherapy in the past year |
| Doshi et al32 | Pre-post study | • Both male and female participants • Age group of 50-60 years • Patients diagnosed with diabetic neuropathy with bilateral pinprick sensation over the sole of foot • Have the ability to understand and cooperate for instructions of the test |
• Diabetic ulcer • Comorbid disorders |
HbA1c, glycosylated hemoglobin; RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus; VPT, vibration perception threshold.
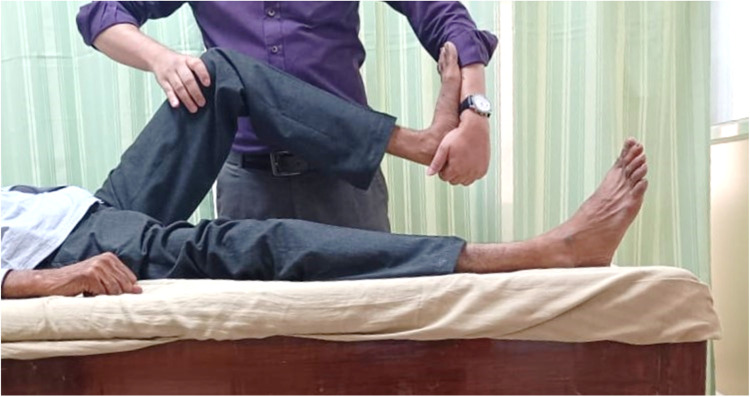
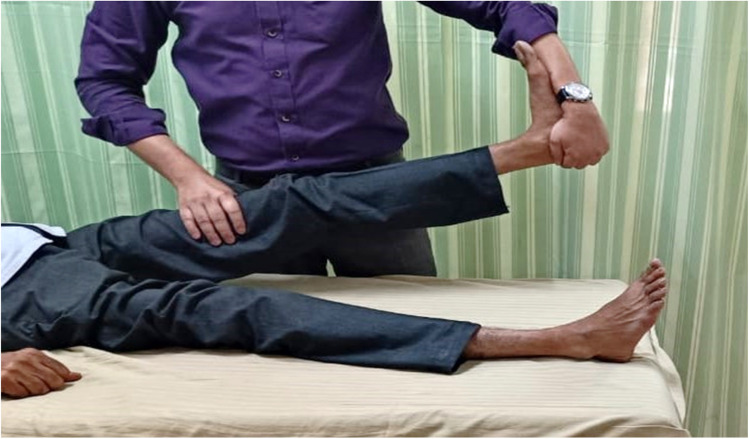
In 4 out of 6 studies, NM was given alone, whereas 2 studies combined NM with other treatment strategies. One study used NM along with conventional therapy,28 whereas in another trial NM was combined with nerve massage.29 Two studies targeted tibial nerve,32,33 1 study administered NM to nerves of upper limb,30 and only 1 trial examined the sciatic nerve.28 Two studies used the slider technique, and only 1 study used a combination of slider and tensioner technique. The slider and tensioner techniques of tibial nerve are generally employed in lower limb NM. The techniques are shown in Fig 2, Fig 3, respectively. In 1 study, sciatic NM plus conventional therapy was given, and only conventional therapy in the control group, for 5 sessions per week for 4 weeks.28 Ali et al delivered NM to each nerve of upper limb for 10 seconds with a 2-second rest between each repetition, and a total of 10 repetitions were performed.30 In 3 studies,30, 31, 32 there were no control groups as they were case series, cross-sectional, and pre-post studies. The characteristics of the studies are given in Table 3.
Fig 2.
Slider technique of the tibial nerve.
Fig 3.
Tensioner technique of the tibial nerve.
Table 3.
Characteristics of the Included Studies
| Trials | Type of Study | Sample Size, n | Age, y | Sex, M/F | Intervention in Experimental Group | Intervention in Control Group | Outcome Measures | Major Findings |
|---|---|---|---|---|---|---|---|---|
| Gehlot et al28 | RCT | 20 | Not reported | Not reported | Group A: Sciatic nerve mobilization+ Conventional PT Duration: 5 d/wk, 4 wk |
Group B: Conventional PT Duration: 5 d/wk, 4 wk |
Weight bearing asymmetry ROM (flexion and extension) |
↑ in ROM and improvement in weight bearing asymmetry |
| Kumar et al29 | Pilot RCT | 34 | 53.86 ± 9.85 | 26/18 | Experimental limb: Nerve slider technique + nerve massage | Control limb: Sham intervention consisted of midrange rhythmic passive joint movements performed by a physiotherapist at .5Hz, each movement for 5 repetitions, at ankle, subtalar, midfoot, forefoot and toes | VPT, HPT, and CPT | Greater ↓ in VPT, HPT, and CPT on the experimental limb |
| Ali et al30 | Case series | 40 | 51.22 ± 6.71 | 8/31 | Nerve sliders for radial, ulnar and median nerves Duration: 10 s with 2 s rest and 10 repetitions |
No control | DN4 | ↓ in DN4 |
| Boyd et al31 | Cross-sectional study | 43 | 56.3 ± 11.1 | 22/21 | Straight leg raise neurodynamic tests were performed with ankle plantar flexion (PF/SLR) and dorsiflexion (DF/SLR) | No control | MDNS, MNSIq, VPT, EMG, SLR | Strong correlations between MDNS and MNSIc (0.82, P < .0005); MDNS and VPT-AVG (0.76, P < .0005). ↑Hip ROM from P1 to P2 in SLR for both DF/SLR and PF/SLR (P < .0005). In start position, 2 times ↑MVC (5.3 ± 4.1% MVC to 9.7 ± 8.9% MVC). |
| Doshi et al32 | Pre-post study | 20 | 50-60 | 9/11 | Tibial nerve mobilization | No control | SNCV | ↑ in SNCV |
| Manu et al33 | Case study | 1 | 63 | 1/0 | Tibial nerve mobilization | No control | FA, ADC, Pain, QOL, ROM | ↑ FA, ↓ADC, ↑QOL, ↑ ROM, ↓Pain |
ADC, apparent diffusion coefficient; AVG, average; CPT, cooling perception thresholds; DF, dorsiflexion; DN4, douleur neuropathic; EMG, electromyography; FA, fractional anisotropy; HPT, heat perception thresholds; MDNS, Michigan Diabetic Neuropathy Score; MNSIc, Michigan Neuropathy Screening Instrument clinical examination; MNSIq, Michigan Neuropathy Screening Instrument questionnaire; M/F, male/female; MVC, maximum voluntary contraction; PF, platarflexion; PT, physical therapy; QOL, quality of life; RCT, randomized controlled trials; ROM, range of motion; SLR, straight leg raise; SNCV, sensory nerve conduction velocity; VPT, vibration perception threshold.
Methodological Quality of Studies
Quality scoring was performed for all the included trials in the study. The average PEDro score was 4.1 (fair quality). Three trials scored 3 out of 10,30, 31, 32 1 scored 2 out of 10,33 1 scored 6 out of 10,28 and 1 scored 8 out of 10.29 Only 1 study reported assessor blinding.29 None of the studies mentioned blinding of the participants. All studies reported dropout and intention to treat analysis. The summary is given in Table 4.
Table 4.
Quality Assessment of Included Studies
| Trials | Random Allocation | Concealed Allocation | Group Similarity at Baseline | Participant Blinding | Therapist Blinding | Assessor Blinding | <15% Dropouts | Intention to Treat Analysis | Between-Group Differences Reported | Point Estimates and Variability Reported | Total Score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gehlot et al28 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | Good |
| Kumar et al29 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | Good |
| Ali et al30 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | Poor |
| Manu et al33 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | Poor |
| Boyd et al31 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 | Poor |
| Doshi et al32 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 | Poor |
Effect of NM on Sensorimotor Variables
All of the included trials showed a positive effect of NM in patients with DPN. Effects of NM alone or in combination with other intervention has been shown in NCV, range of motion (ROM), vibration perception threshold (VPT), and other symptoms of DPN. The study done on the sensory part of the tibial nerve showed an increase in conduction velocity after mobilization.32 Improvement in ROM was found in 1 trial done by Gehlot et al.28 Two studies reported a positive improvement in VPT.29,31
Discussion
This scoping review summarized the effects of NM on sensory and motor symptoms in adults with DPN. The present review included 6 studies that met the eligibility criteria. However, these studies were low level on the evidence hierarchy. The included studies found that NM produced positive results in people with DPN. The severity levels of neuropathy were not defined in the studies. The Michigan Diabetic Neuropathy score, which includes nerve study and clinical examination, can be used to categorize the severity or class of DPN.12 Starting with sensory impairments to balance issues, the moderate to severe type DPN can affect balance, gait, posture, and other body functions.
Conduction Velocity
In this review, in a study with 20 DPN patients, when tibial NM was given for 3 weeks, there was an increase in the sensory nerve velocity. NM causes the edema to disperse, which in turn eases the hypoxia and diminishes the related symptoms and changes in the nerve conduction speed.32 NCV is considered the most accurate, sensitive, and reliable measure for the study of peripheral nerve functions.31 During the course of the pathology, there is considerable decrease in the conduction velocity.17,34 The changes in conduction speed are due to hyperglycemia, which prompts osmotic swelling of the nerves with damage to the axons and myelin sheath. The nerve conduction velocity mainly reflects the myelin changes, while the action potential amplitude indicates the axonal changes.35 Studies have revealed that peroneal motor NCV can predict foot problems and is a reliable measure to predict new ulcerations and deaths in patients with type 2 diabetes.36,37
Sensorimotor Changes
A study done by Gehlot et al showed increased hip and knee flexion and extension ROM after combined sciatic NM and conventional therapy given for 4 weeks in patients with DPN.28 This suggests that improved elasticity of nerves and musculoskeletal tissues may be due to an increase in the intraneural blood flow and improvement of the axoplasmatic flow.38 Literature provides evidence regarding the neuromuscular effects of NM. These findings are in coherence with the other study, which showed changes in ROM and flexibility of the hamstring and calf muscles were significant when sciatic NM was given in patients with diabetes.23
Pain Perception and Quality of Life
In 1 trial, NM given on an experimental limb along with massage showed decreased perception threshold of vibration, cold, and hot.29 In DPN, due to damage in small and large nerve fibers, there might be a reduction or absence of sensation of pain, temperature, VPT, light touch, and proprioception. After NM, the alleviation of the perception might be due to the movement components, which might have induced afferent kinesthetic impulses from ankle and foot muscles, which in turn could possibly influence the cutaneous receptor afferents, thus altering the perception of sensory thresholds.39 Effects of NM on pain and related outcomes are being studied in conditions like diabetes29 and low back pain.14,40,41 One case study has examined the role of NM on pain in patients with DPN.33 When tibial nerve mobilization was given, there was reduction in the visual analog scale score. The attributed reasons overlap the explanation given by Bialosky et al, who assumed that nerve mobilization could affect the intraneural circulation, axoplasmic flow, viscoelasticity behavior of neural connective tissue, and desensitizes abnormal impulse-generating sites.42 The increase in fractional anisotrophy and decrease in apparent diffusion coefficient further supports the finding that NM causes increased functions of the nerves, thus reducing the edema around the nerves. Fractional anisotrophy depicts the preferred direction, whereas apparent diffusion coefficient quantifies the speed of diffusion of water molecules.43 Quality of life is decreased in DPN, as described by studies due to direct or indirect effects. Pain, dietary routine, and/or presence of comorbidities are prominent factors that affect the quality of life.44
Limitations
This study has some limitations. We only searched a limited number of databases, which means that we may have missed some relevant studies. Our search terms were limited and may not have included all key words that would have identified relevant studies, so this may have also resulted in missed studies in this review.
Although, this review explored studies on the effects of NM in patients with DPN, there are limitations and lack of strong conclusive studies. Though nerve damage is evident in DPN which affects the conduction velocity, the mobilization techniques, which may be safe in that specific severity level can be given as treatment with limited adverse effects. To our knowledge, this is the first review of NM in DPN. More controlled trials are required to further enhance the use of NM as an intervention strategy and the generalizability of the technique in other conditions. Future studies can be done on the effects of NM on different severity levels of neuropathy patients.
Conclusion
At present, only a few low-level studies exist on the use of NM for the treatment of adults with DPN. The evidence for use of NM on DPN is still limited and insufficient.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): M.A., M.N.
Design (planned the methods to generate the results): M.A.
Supervision (oversight, organization and implementation): S.P., M.N.
Data collection/processing (experiments, organization, or reporting data): M.A., S.P.
Analysis/interpretation (analysis, evaluation, presentation of results): M.A., S.P., M.N.
Literature search (performed the literature search): M.A., S.P.
Writing (responsible for writing a substantive part of the manuscript): M.A., S.P.
Critical review (revised manuscript for intellectual content): M.N.
Practical Applications.
-
•
This is a first scoping review on neural mobilization (NM) in patients with diabetic peripheral neuropathy (DPN).
-
•
At present, only a few low-level studies exist on the use of NM for the treatment of adults with DPN.
-
•
The evidence for use of NM on DPN is still limited and insufficient.
Alt-text: Unlabelled box
References
- 1.Kisozi T, Mutebi E, Kisekka M, et al. Prevalence, severity and factors associated with peripheral neuropathy among newly diagnosed diabetic patients attending Mulago hospital: a cross-sectional study. Afr Health Sci. 2017;17(2):463–473. doi: 10.4314/ahs.v17i2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewanjee S, Das S, Das AK, et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 2018;833:472–523. doi: 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Res. 2016;5 doi: 10.12688/f1000research.7898.1. F1000 Faculty Rev-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diab Metab Res Rev. 2012;28(Suppl 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI. In: Controversies in Treating Diabetes: Clinical and Research Aspects. LeRoith D, Vinik AI, editors. Humana Press; Totowa, NJ: 2008. Diabetic neuropathies; pp. 135–156. [Google Scholar]
- 6.Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3–22. doi: 10.1016/B978-0-444-53480-4.00001-1. [DOI] [PubMed] [Google Scholar]
- 7.Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab (Seoul) 2016;31(2):230–238. doi: 10.3803/EnM.2016.31.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am. 2004;88(4):947–999. doi: 10.1016/j.mcna.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Boulton AJM, Ward JD. Diabetic neuropathies and pain. Clin Endocrinol Metab. 1986;15(4):917–931. doi: 10.1016/s0300-595x(86)80080-9. [DOI] [PubMed] [Google Scholar]
- 10.Said G. Diabetic neuropathy–a review. Nat Clin Pract Neurol. 2007;3(6):331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160(2):174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 12.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 13.Janghorbani M, Rezvanian H, Kachooei A, et al. Peripheral neuropathy in type 2 diabetes mellitus in Isfahan, Iran: prevalence and risk factors. Acta Neurologica Scandinavica. 2006;114(6):384–391. doi: 10.1111/j.1600-0404.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim TH. The treatment effect of hamstring stretching and nerve mobilization for patients with radicular lower back pain. J Phys Ther Sci. 2017;29(9):1578–1582. doi: 10.1589/jpts.29.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Sloan G, Ye Y, et al. New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol. 2020;10:929. doi: 10.3389/fendo.2019.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Almeida S, Riddell MC, Cafarelli E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve. 2008;37(2):231–240. doi: 10.1002/mus.20919. [DOI] [PubMed] [Google Scholar]
- 18.Arezzo JC. The use of electrophysiology for the assessment of diabetic neuropathy. Neurosci Res Commun. 1997;21(1):13–23. [Google Scholar]
- 19.Gholami F, Nikookheslat S, Salekzamani Y, Boule N, Jafari A. Effect of aerobic training on nerve conduction in men with type 2 diabetes and peripheral neuropathy: a randomized controlled trial. Neurophysiol Clin. 2018;48(4):195–202. doi: 10.1016/j.neucli.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Dixit S, Maiya AG, Shastry BA. Effect of aerobic exercise on peripheral nerve functions of population with diabetic peripheral neuropathy in type 2 diabetes: A single blind, parallel group randomized controlled trial. J Diabetes Complications. 2014;28(3):332–339. doi: 10.1016/j.jdiacomp.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Schmid AB, Brunner F, Luomajoki H, et al. Reliability of clinical tests to evaluate nerve function and mechanosensitivity of the upper limb peripheral nervous system. BMC Musculoskelet Disord. 2009;10:11. doi: 10.1186/1471-2474-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal M, Mehta S, Rana N, et al. Motor nerve conduction velocity and function in carpal tunnel syndrome following neural mobilization: a randomized clinical trial. Int J Health Allied Sci. 2016;5(2):104–110. [Google Scholar]
- 23.Siddiqui H, Khan SA, Saher T, Siddiqui ZA. Effect of sciatic nerve mobilisation on muscle flexibility among diabetic and non-diabetic sedentary individuals: a comparative study. Comp Exerc Physiol. 2021;17(3):229–233. [Google Scholar]
- 24.Wolny T, Saulicz E, Linek P, Shacklock M, Myśliwiec A. Efficacy of manual therapy including neurodynamic techniques for the treatment of carpal tunnel syndrome: a randomized controlled trial. J Manipulative Physiol Ther. 2017;40(4):263–272. doi: 10.1016/j.jmpt.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 26.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 27.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 28.Gehlot A, Misra A, Garg D. Effect of neurodynamic techniques in diabetic neuropathy patients on weight bearing asymmetry of lower extremity. Indian J Appl Res. 2021;11(10):52–53. [Google Scholar]
- 29.Kumar PS. Immediate effects of longitudinal vs. transverse tibial nerve massage on vibration perception thresholds and thermal perception thresholds in asymptomatic subjects: a pilot randomized clinical trial. Physiother Occup Ther J. 2010;3(1):13–23. [Google Scholar]
- 30.Ali M, Anwar S, Perveen W, Akhtar M, Hashmi R, Jabeen Z. Effects of neurodynamic exercises on the management of diabetic peripheral neuropathy of the upper limb: a case series. Physiother Quart. 2023;31(3):53–56. [Google Scholar]
- 31.Boyd BS, Wanek L, Gray AT, Topp KS. Mechanosensitivity during lower extremity neurodynamic testing is diminished in individuals with Type 2 Diabetes Mellitus and peripheral neuropathy: a cross sectional study. BMC Neurol. 2010;10:75. doi: 10.1186/1471-2377-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doshi M, Singarvelan DRM. Effect of tibial nerve mobilization on nerve conduction velocity in diabetic neuropathy patient. Int J Heal Sci Res. 2019;9:218–224. [Google Scholar]
- 33.Manu G, Amit M, Asir John S. Effect of massage, passive neural mobilization and transcutaneous electrical nerve stimulation on magnetic resonance diffusion tensor imaging (MR-DTI) of the tibial nerve in a patient with type 2 diabetes mellitus induced neuropathy: a case report. Physiother Theory Pract. 2022;38(13):3273–3282. doi: 10.1080/09593985.2021.1994070. [DOI] [PubMed] [Google Scholar]
- 34.Hyllienmark L, Golster H, Samuelsson U, Ludvigsson J. Nerve conduction defects are retarded by tight metabolic control in type I diabetes. Muscle Nerve. 2001;24(2):240–246. doi: 10.1002/1097-4598(200102)24:2<240::aid-mus90>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Tehrani KHN. A study of nerve conduction velocity in diabetic patients and its relationship with tendon reflexes (T-Reflex) Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.7288. e2020066-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrington AL, Shaw JE, Van Schie CHM, Abbott CA, Vileikyte L, Boulton AJM. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care. 2002;25(11):2010–2015. doi: 10.2337/diacare.25.11.2010. [DOI] [PubMed] [Google Scholar]
- 37.Charles M, Soedamah-Muthu SS, Tesfaye S, et al. Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes: the EURODIAB prospective complications study. Diabetes Care. 2010;33(12):2648–2653. doi: 10.2337/dc10-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shacklock M. Clinical neurodynamics and sports medicine: origins and development. Aspetar Sports Med J. 2014;3:50–56. [Google Scholar]
- 39.Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol. 2007;580(2):649–658. doi: 10.1113/jphysiol.2006.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos M, Cruz CAH, Laurentino MF, Ashmawi HA, Santos FM, Chacur M. Effects of neural mobilization on individuals with chronic low back pain. Braz J Pain. 2020;3(2):205–212. [Google Scholar]
- 41.Čolaković H, Avdić D. Effects of neural mobilization on pain, straight leg raise test and disability in patients with radicular low back pain. J Health Sci. 2013;3(2):109–112. [Google Scholar]
- 42.Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J Orthop Sports Phys Ther. 2009;39(10):709–723. doi: 10.2519/jospt.2009.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh K, Gupta K, Kaur S. High resolution ultrasonography of the tibial nerve in diabetic peripheral neuropathy. J Ultrason. 2017;17(71):246–252. doi: 10.15557/JoU.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, Zis P. Quality of life in painful peripheral neuropathies: a systematic review. Pain Res Manag. 2019;2019 doi: 10.1155/2019/2091960. [DOI] [PMC free article] [PubMed] [Google Scholar]