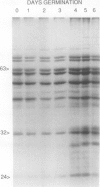
Abstract
Redox activities, NADH:ferricyanide reductase, NAD(P)H:cytochrome reductases, and NADH:ascorbate free-radical reductase, are present in endoplasmic reticulum (ER) and glyoxysomal membranes from the endosperm of germinating castor bean (Ricinus comminus L. var Hale). The development of these functions was followed in glyoxysomes and ER isolated on sucrose gradients from castor bean endosperm daily from 0 through 6 days of germination. On a per seed basis, glyoxysomal and ER protein, glyoxysomal and ER membrane redox enzyme activities, and glyoxylate cycle activities peaked at day 4 as did the ER membrane content of cytochrome P-450. NADH:ferricyanide reductase was present in glyoxysomes and ER isolated from dry seed. This activity increased only about twofold in glyoxysomes and threefold in ER during germination relative to the amount of protein in the respective fractions. The other reductases, NADH:cytochrome reductase and NADH:ascorbate free-radical reductase, increased about 10-fold in the ER relative to protein up to 4 to 5 days, then declined. NADPH:cytochrome reductase reached maximum activity relative to protein at day 2 in both organelles. The increases in redox activities during germination indicate that the membranes of the ER and glyoxysome are being enriched with redox proteins during their development. The development of redox functions in glyoxysomes was found to be coordinated with development of the glyoxylate cycle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden L., Lord J. M. Purification and comparative properties of microsomal and glyoxysomal malate synthase from castor bean endosperm. Plant Physiol. 1978 Feb;61(2):259–265. doi: 10.1104/pp.61.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch M. L., Donaldson R. P. Ascorbate free-radical reduction by glyoxysomal membranes. Plant Physiol. 1990 Oct;94(2):531–537. doi: 10.1104/pp.94.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Dietrich R. A., Maslyar D. J., Baden C. S., Harada J. J. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989 Mar;1(3):293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Donaldson R. P., Fang T. K. beta-Oxidation and Glyoxylate Cycle Coupled to NADH: Cytochrome c and Ferricyanide Reductases in Glyoxysomes. Plant Physiol. 1987 Nov;85(3):792–795. doi: 10.1104/pp.85.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P. Membrane lipid metabolism in germinating castor bean endosperm. Plant Physiol. 1976 Apr;57(4):510–515. doi: 10.1104/pp.57.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P. Nicotinamide cofactors (NAD and NADP) in glyoxysomes, mitochondria, and plastids isolated from castor bean endosperm. Arch Biochem Biophys. 1982 Apr 15;215(1):274–279. doi: 10.1016/0003-9861(82)90305-8. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P. Organelle Membranes from Germinating Castor Bean Endosperm: II. ENZYMES, CYTOCHROMES, AND PERMEABILITY OF THE GLYOXYSOME MEMBRANE. Plant Physiol. 1981 Jan;67(1):21–25. doi: 10.1104/pp.67.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. A., Houghton J. D., Jones O. T. The cytochromes in microsomal fractions of germinating mung beans. Biochem J. 1981 Mar 15;194(3):743–751. doi: 10.1042/bj1940743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D. B., Donaldson R. P. Electron transport in glyoxysomal membranes. Arch Biochem Biophys. 1982 Apr 15;215(1):280–288. doi: 10.1016/0003-9861(82)90306-x. [DOI] [PubMed] [Google Scholar]
- Ito A., Hayashi S., Yoshida T. Participation of a cytochrome b5-like hemoprotein of outer mitochondrial membrane (OM cytochrome b) in NADH-semidehydroascorbic acid reductase activity of rat liver. Biochem Biophys Res Commun. 1981 Jul 30;101(2):591–598. doi: 10.1016/0006-291x(81)91300-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster D. G., Bowditch M. I., Eldridge K. M., Donaldson R. P. Characterization of membrane-bound electron transport enzymes from castor bean glyoxysomes and endoplasmic reticulum. Arch Biochem Biophys. 1988 Aug 15;265(1):50–61. doi: 10.1016/0003-9861(88)90370-0. [DOI] [PubMed] [Google Scholar]
- Luster D. G., Donaldson R. P. Orientation of electron transport activities in the membrane of intact glyoxysomes isolated from castor bean endosperm. Plant Physiol. 1987 Nov;85(3):796–800. doi: 10.1104/pp.85.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Oxidation of NADH in Glyoxysomes by a Malate-Aspartate Shuttle. Plant Physiol. 1980 Oct;66(4):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Trelease R. N., Choinski J. S. Malate synthase activity in cotton and other ungerminated oilseeds: a survey. Plant Physiol. 1979 Jun;63(6):1068–1071. doi: 10.1104/pp.63.6.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. Developmental changes in the activity of messenger RNA isolated from germinating castor bean endosperm. Plant Physiol. 1979 Oct;64(4):630–634. doi: 10.1104/pp.64.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. Synthesis and posttranslational segregation of glyoxysomal isocitrate lyase from castor bean endosperm. Eur J Biochem. 1981 Sep;119(1):43–49. doi: 10.1111/j.1432-1033.1981.tb05574.x. [DOI] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E. M., Riezman H., Grienenberger J. M., Becker W. M., Leaver C. J. Regulation of glyoxysomal enzymes during germination of cucumber. Temporal changes in translatable mRNAs for isocitrate lyase and malate synthase. Eur J Biochem. 1980 Dec;112(3):469–477. [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Protein Bodies from the Endosperm of Castor Bean: Subfractionation, Protein Components, Lectins, and Changes during Germination. Plant Physiol. 1976 Dec;58(6):703–709. doi: 10.1104/pp.58.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]