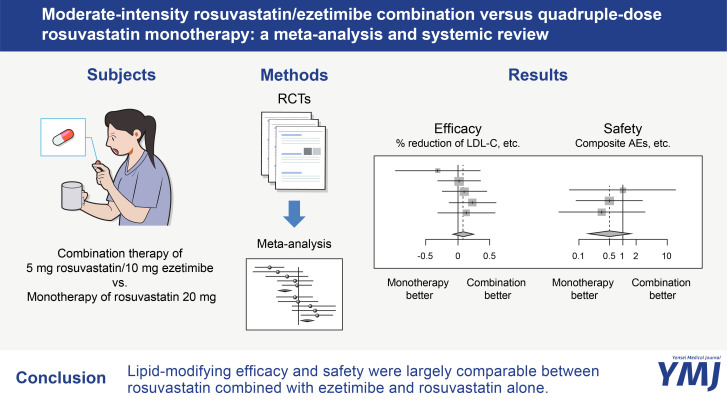
Abstract
Purpose
There are few studies in the literature on the dosage of statin that equivalently reduces low-density lipoprotein cholesterol (LDL-C) compared to an ezetimibe combination and whether such regimens have differences in safety. We compared the lipid-modifying efficacy and safety of 5 mg rosuvastatin/10 mg ezetimibe to those of 20 mg rosuvastatin.
Materials and Methods
A literature search was conducted using the PubMed, EMBASE, Cochrane, Web of Sciences, and SCOPUS databases up to December 2021. Human studies investigating the two aforementioned regimens with a randomized controlled design were selected. Outcome variables included the percentage reduction in LDL-C and other lipid parameters and rates of composite adverse events (AEs), including muscle-related symptoms. A random-effects meta-analysis was performed after heterogeneity testing between studies.
Results
Seven studies were included in this meta-analysis. The percentage LDL-C reduction did not differ between the combination and monotherapy groups [standardized mean difference (SMD) 0.08; 95% confidence interval (CI) -0.09 to 0.26; p=0.35]. The risk of composite AEs (odds ratio 0.50; 95% CI 0.15 to 1.72; p=0.27) of the combination was not different compared to the monotherapy group. The percentage of total cholesterol reduction was greater in the combination group (SMD 0.22; p=0.02), whereas that of triglyceride reduction and high-density lipoprotein cholesterol elevation did not differ between the two groups.
Conclusion
This meta-analysis showed that 5 mg rosuvastatin/10 mg ezetimibe had largely comparable lipid-modifying efficacy and tolerability as 20 mg rosuvastatin.
Keywords: Hypercholesterolemia, drug therapy, hydroxymethylglutary-CoA reductase inhibitors, preventive medicine
Graphical Abstract
INTRODUCTION
Low-density lipoprotein cholesterol (LDL-C) lowering using a statin-focused regimen is pivotal for primary and secondary cardiovascular prevention.1,2,3 This pharmacotherapy is recommended with high classes in diverse guidelines on lipid-lowering therapy.4,5 In the revised guidelines of the past decade, LDL-C treatment targets have been steadily lowered for high- and very high-risk groups.6 As a result, high-intensity statins are frequently prescribed and many more patients require combination drugs, such as ezetimibe. Ezetimibe is the most common option when the treatment goal is not achieved using statin monotherapy or when an individual experiences a drug intolerance.4,6
It has long been an issue of interest whether lower-intensity statin/ezetimibe combinations and higher-intensity statins have differences in efficacy and tolerability.7,8 The recent RACING (randomized comparison of the efficacy and safety of lipid-lowering therapy with statin monotherapy versus statin-ezetimibe combination for high-risk cardiovascular disease) trial compared statin/ezetimibe combination and double-dose statin therapy based on non-inferiority analysis. In the study, 10 mg rosuvastatin/10 mg ezetimibe was non-inferior to double-dose statin monotherapy, revealing potentially better results with regard to safety.9
According to previous studies, including the Treating to New Target (TNT) trial, high-dose statins caused more adverse events (AEs) than low-dose statins,10 while the ezetimibe combination has been reported to have minimal effect on safety.11 However, studies on the ezetimibe combination-equivalent statin dose, which equally reduces LDL-C, and studies evaluating whether the two regimens have differences in safety, have been extremely limited. Therefore, the present study compared the lipid-modifying efficacy and safety of a 5 mg rosuvastatin/10 mg ezetimibe combination therapy with 20 mg rosuvastatin, which are likely to reduce LDL-C equivalently. This procedure was conducted using a meta-analysis of randomized controlled trials. Furthermore, 5 mg rosuvastatin/10 mg ezetimibe and 20 mg rosuvastatin were appropriate for analysis as their effects have recently been commonly reported by clinical trials, particularly those conducted in Korea.
MATERIALS AND METHODS
This analysis was designed and conducted according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-analysis statement (PRISMA).12 As this was a meta-analysis of randomized controlled trials, approval of the protocol and patient informed consent were waived by the institutional review board of Severance Hospital, Seoul, Korea.
Search strategy
PubMed, EMBASE, Cochrane, Web of Sciences, and SCOPUS databases were searched using the following terms in the title or abstract: “rosuvastatin” and “ezetimibe” and “efficacy” or “effect” and “combination” and “patient.” The literature search was conducted from March 5, 2022, to April 4, 2022. Y.K. and J.M.P. examined each article to minimize the possibility of duplication, reviews, case studies, and experimental studies.
Study selection
Eligible studies were full-text peer-reviewed articles with the following conditions: 1) published by December 31, 2021, 2) human studies, 3) investigations on the effects of 5 mg rosuvastatin/10 mg ezetimibe and 20 mg rosuvastatin, 4) having a randomized controlled design, and 5) data regarding lipid modification and/or tolerability from the two regimens. The exclusion criteria were as follows: 1) non-clinical studies, 2) observational studies, 3) lack of data on lipid parameters, or 4) articles not published in English.
Data extraction
Y.K. and J.M.P. extracted the data, including the first author name, country, type of study, number and characteristics of participants, type and dose of prescribed drugs, treatment duration, lipid parameters, and AEs. Lipid parameters included LDL-C, total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C). AE was defined as any abnormal sign, symptom, laboratory test, combination of such abnormalities, or any unexpected deterioration in a concurrent illness. Drug-related AE was defined as an AE suspected of being drug-induced. Disagreements (selection of candidate studies and composite AEs) were resolved through discussions between authors. The publication bias was tested by funnel plots.
Statistical analysis
Statistical analyses were performed using R software (version 4.2.2; R foundation, Vienna, Austria). The primary outcome variable was the percentage reduction in LDL-C levels, and the secondary outcome variables were the rates of composite AEs. Composite AEs included muscle-related symptoms, elevation of creatine kinase (>5 to 10×upper limit of normal), and elevation of liver function test (>3×upper limit of normal). The tertiary outcome variables were the percentage reduction in TC and TG, the percentage elevation of HDL-C, and the rates of drug-related AEs and any AEs. The study reporting was conducted in accordance with the PRISMA statement. In general, the mean and standard deviation of percentage change reported in the articles were used. However, the graph length was measured when the outcomes we need were not measured. When only the median and interquartile range values were reported, the mean and standard deviation were estimated using the method described by Hozo, et al.13 A random effects meta-analysis was performed using the limited maximum likelihood method. Heterogeneity between studies was evaluated using the tau-square and I-square statistics.
RESULTS
Search results and included studies
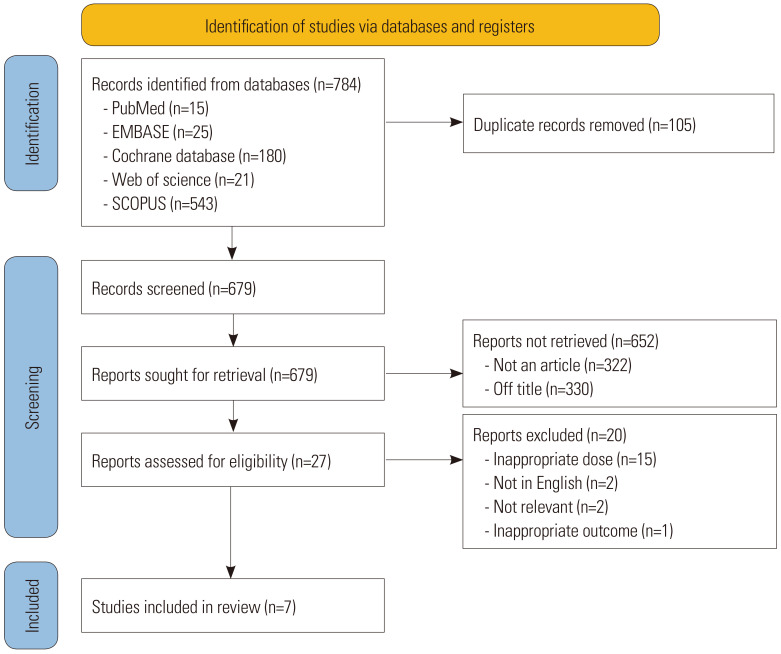
Of the 784 initially identified articles, 679 were screened after removing 105 duplicate records. Of the records not meeting the criteria of article (n=322) and title (n=330), 27 reports were found to be eligible for analysis. After excluding 20 articles for inappropriate doses, different languages, topics, or outcomes, seven articles were included in the current study (Fig. 1).14,15,16,17,18,19,20 The characteristics of the studies were systematically evaluated and are presented in Table 1. All seven included studies were randomized controlled trials (six from Korea and one from multiple countries). The enrolled participants had diabetes mellitus, hypercholesterolemia, or high/moderately high cardiovascular risk. One of these studies enrolled patients with acute coronary syndrome. Drug treatment was usually maintained for 6–8 weeks, except in one study where it was maintained for 6 months (Table 1). Data on lipid-lowering efficacy and tolerability were available from six and three studies, respectively. Funnel plots showed no publication bias for all outcome variables (Supplementary Fig. 1, only online).
Fig. 1. Flow chart of study inclusion.
Table 1. Characteristics of Included Studies.
| Study, first author or study name | Hwang, et al.14) | Rhee, et al.15) | I-ROSETTE16) | Kim, et al.17) | Yang, et al.18) | Oh, et al.19) | ACTE20) | |
|---|---|---|---|---|---|---|---|---|
| Type of study | RCT, multicenter | RCT, multicenter | RCT, multicenter | RCT, multicenter | RCT, multicenter | RCT, multicenter | RCT, multicenter | |
| Country | Korea | Korea | Korea | Korea | Korea | Korea | Multinational | |
| Population and number | Type 2 DM; 42 (ITT), 36 (PP) | Hypercholesterolemia; 407 (FAS) | Hypercholesterolemia; 389 (FAS), 353 (PP) | Hypercholesterolemia; 375 (FAS) | High/moderately high-risk group; 245 (FAS) | Acute coronary syndrome; 50 (randomized) | High/moderately high-risk group; 440 (randomized) | |
| Mean age, yr | 50–53 | 63–65 | 62–64 | 59–62 | 62–66 | 59–60 | 61–62 | |
| Males, % | 64 | 57 | 63 | 57 | 58 | 88 | 62 | |
| Comorbidities, % | ||||||||
| DM | 100 | 23 | 37 | 22 | 42 | 18 | N/A | |
| Hypertension | N/A | 70 | 71 | 21 | 67 | 36 | N/A | |
| Smoking | 33.3 | 15 | N/A | N/A | 19 | 36 | N/A | |
| CAD | N/A | 83 | 27 | 12 | 27 | 100 | N/A | |
| Mean baseline lipid levels, mg/dL | ||||||||
| TC | 235–237 | N/A | 225 | 228–229 | 219–236 | 183 | 178–188 | |
| TG | 136–173 | N/A | 156 | 153–158 | 136–161 | 121–128 | 116–143 | |
| HDL-C | 48–49 | N/A | 49 | 49–51 | 46–51 | 45–46 | 48–54 | |
| LDL-C | 154–157 | N/A | 152 | 160 | 148–157 | 124–128 | 98–107 | |
| Combination regimen | Rosuvastatin 5 mg/Ezetimibe 10 mg | Rosuvastatin 5–20 mg/Ezetimibe 10 mg | Rosuvastatin 5–20 mg/Ezetimibe 10 mg | Rosuvastatin 5–20 mg/Ezetimibe 10 mg | Rosuvastatin 5–20 mg/Ezetimibe 10 mg | Rosuvastatin 5 mg/Ezetimibe 10 mg | Rosuvastatin 5–10 mg/Ezetimibe 10 mg | |
| Monotherapy regimen | Rosuvastatin 20 mg | Rosuvastatin 5–20 mg | Rosuvastatin 5–20 mg | Rosuvastatin 5–20 mg | Rosuvastatin 5–20 mg | Rosuvastatin 20 mg | Rosuvastatin 10–20 mg | |
| Drug treatment duration | 6 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 6 months | 6 weeks | |
| Data used in meta-analysis | % changes in LDL-C | % changes in LDL-C, TC, TG; HDL-C | % changes in LDL-C, TC, TG, HDL-C, tolerability | % changes in LDL-C. TC, TG, HDL-C, tolerability | % changes in LDL-C, TC, TG, HDL-C | % changes in LDL-C, TC, TG, HDL-C | Tolerability | |
RCT, randomized controlled trial; DM, diabetes mellitus; ITT, intention-to-treat; PP, per protocol; DM, diabetes mellitus; CAD, coronary artery disease; N/A, not available; FAS, full analysis set; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Effect of two regimens on LDL-C and tolerability
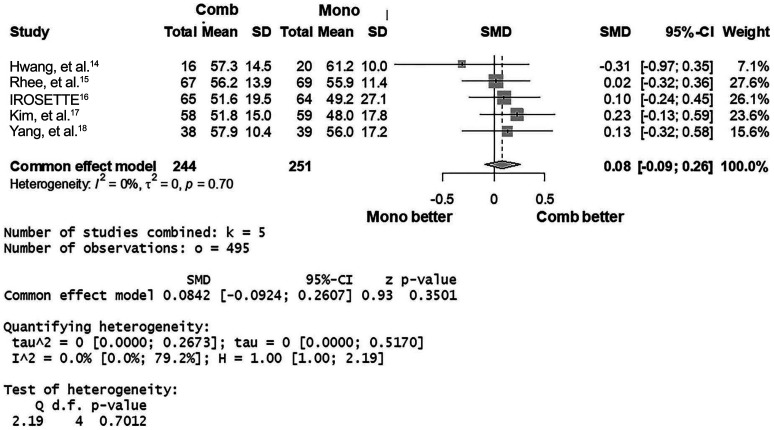
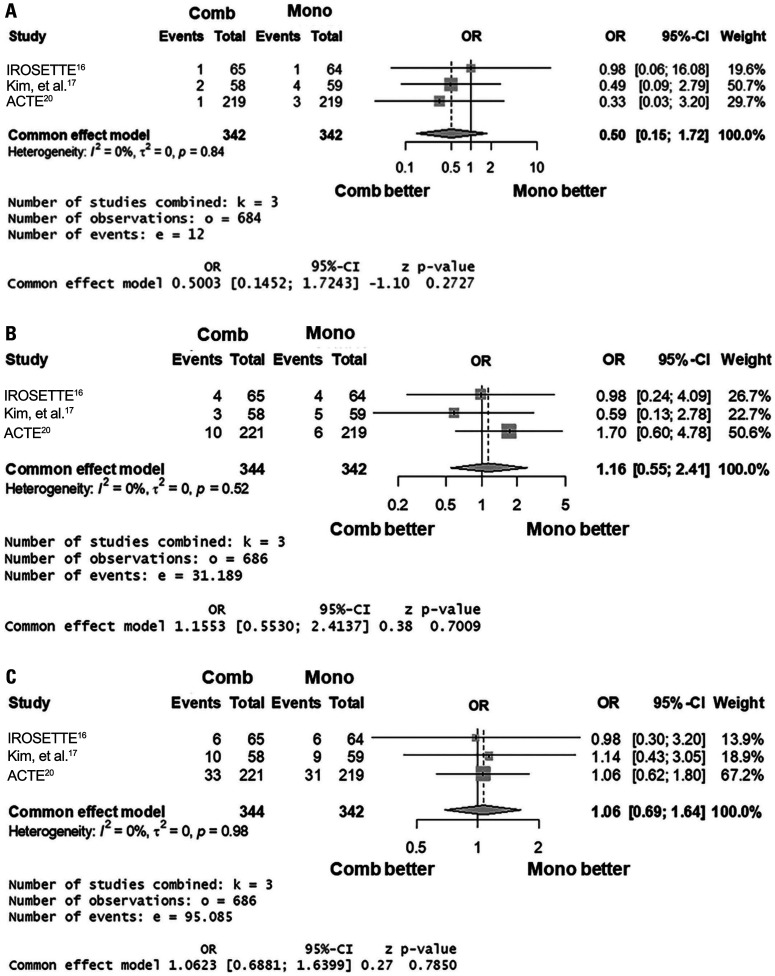
Based on five studies (I2=0%; p for heterogeneity=0.70), the percentage LDL-C reduction did not differ between 5 mg rosuvastatin/10 mg ezetimibe and 20 mg rosuvastatin [common effect model; standardized mean difference (SMD) 0.08; 95% CI -0.09 to 0.26; p=0.35] (Fig. 2). Based on three studies (I2=0%; p for heterogeneity=0.84), the risk of composite AEs (common effect model; odds ratio 0.50; 95% CI 0.15 to 1.72; p=0.27) of the combination was not different compared to the monotherapy group (Fig. 3A). The risks of drug-related AEs or any AEs of the two groups also did not differ (Fig. 3B and C).
Fig. 2. Forest plot showing the mean standardized difference and 95% confidence interval (CI) of percentage reduction of low-density lipoprotein cholesterol. Comb, combination regimen (5 mg rosuvastatin/10 mg ezetimibe); Mono, monotherapy regimen (20 mg rosuvastatin). SMD, standardized mean difference.
Fig. 3. Forest plots showing odds ratio (OR) and 95% confidence interval (CI) of composite adverse events (AEs) (A), drug-related AEs (B), and any AEs (C). Comb, combination regimen (5 mg rosuvastatin/10 mg ezetimibe); Mono, monotherapy regimen (20 mg rosuvastatin).
Effect of two regimens on other lipid parameters
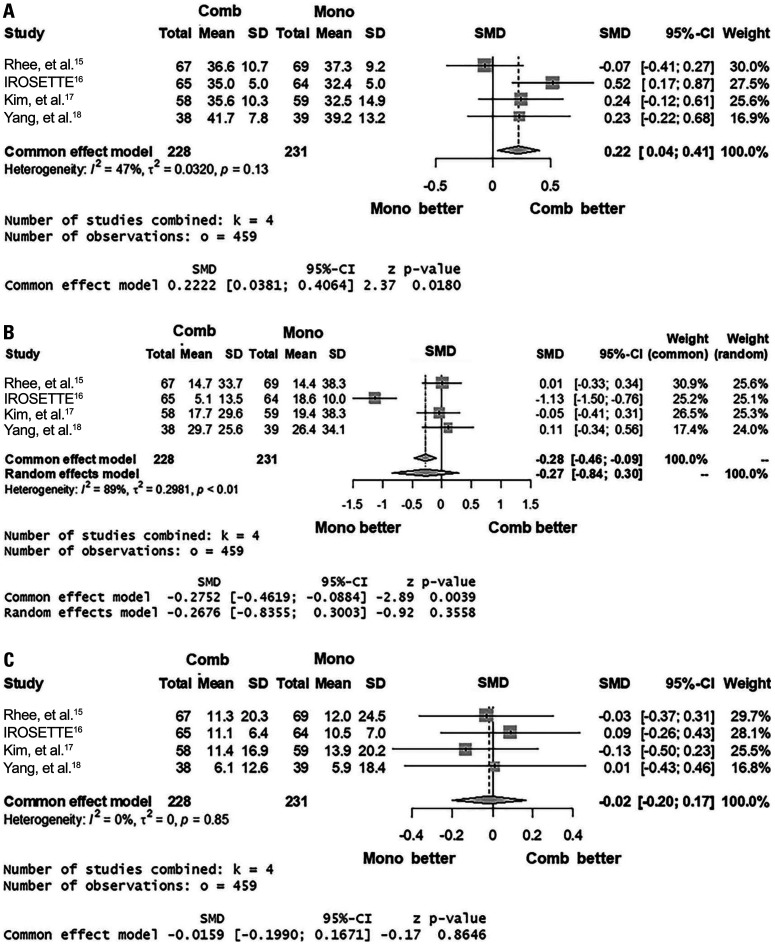
Based on four studies (I2=47%; p for heterogeneity=0.13), the percentage TC reduction was greater in the 5 mg rosuvastatin/10 mg ezetimibe group (common effect model; SMD 0.22; 95% CI 0.04 to 0.41; p=0.02) (Fig. 4A). The percentage TG reduction of the two regimens (I2=89%; p for heterogeneity<0.01) did not differ (random effect model; SMD -0.27; 95% CI -0.84 to 0.30; p=0.36) (Fig. 4B). Likewise, the percentages HDL-C elevation (I2=0%; p for heterogeneity=0.85) were not different between the two groups (common effect model; 95% CI -0.20 to 0.17; p=0.86) (Fig. 4C).
Fig. 4. Forest plots showing the mean standardized difference and 95% confidence interval (CI) of percentage reductions of total cholesterol (A) and triglyceride (B) and percentage elevation of high-density lipoprotein cholesterol (C). Comb, combination regimen (5 mg rosuvastatin/10 mg ezetimibe); Mono, monotherapy regimen (20 mg rosuvastatin). SMD, standardized mean difference.
DISCUSSION
There are no randomized controlled trials for cardiovascular events using 5 mg rosuvastatin/10 mg ezetimibe versus 20 mg rosuvastatin. Furthermore, as stated earlier, studies comparing “ezetimibe combination regimen and such combination-equivalent statin monotherapy (equally reducing LDL-C)” have been highly limited. Therefore, we conducted the current study to analyze and obtain data regarding these two regimens. The major findings of the current study were as follows: 1) the reduction in LDL-C levels did not differ between the two regimens; 2) the risk of composite AEs did not differ between the two regimens; and 3) the reduction in TC was higher with the combination regimen than with the monotherapy regimen, whereas TG reduction and HDL-C elevation were similar between the two regimens. These results, for the first time, exhibited largely similar efficacy and tolerability of 5 mg rosuvastatin/10 mg ezetimibe versus quadruple dose rosuvastatin by a meta-analysis.
A previous meta-analysis based on 11 clinical trials showed a greater LDL-C reduction in the statin/ezetimibe combination group than in the double-dose statin monotherapy group.21 No safety data were analyzed in this study. In the RACING trial comparing 10 mg rosuvastatin/10 mg ezetimibe and 20 mg rosuvastatin, LDL-C levels decreased from 80 mg/dL to 58 mg/dL and 66 mg/dL in each group, respectively.9 It is predicted that the incremental LDL-C reduction by ezetimibe will be higher than that achieved by doubling the statin dose. In this regard, the statin/ezetimibe combination and double-dose statins may not be comparable regimens targeting the same degree of lipid-lowering in clinical practice. Our study has clinical importance, as we analyzed the efficacy and safety of two regimens assumed to have an equivalent lipid-lowering effect.
In a previous study, Yamazaki, et al.22 compared 2.5 mg rosuvastatin/10 mg ezetimibe to 10 mg rosuvastatin, and the mean LDL-C change was very similar in each group (-21.9 mg/dL and -20.3 mg/dL), which was in line with our results. In a prior analysis, we found that atorvastatin 5 mg/ezetimibe 5 mg combination and quadruple dose atorvastatin (20 mg) comparably reduced LDL-C levels, whereas the combination regimen had better effects on hemoglobin A1c and apoB/A1 ratio.7 In another report, we identified that the same combination regimen lowered postprandial triglyceride more than quadruple-dose atorvastatin with similar reduction of LDL-C.23 Conversely, we demonstrated that atorvastatin 20 mg reduced the blood levels of lipoprotein-associated phospholipase A2, a marker of atherothrombosis, more than atorvastatin 5 mg/ezetimibe 5 mg combination.8 Based on these findings, it is very likely that lower-dose statin/ezetimibe and quadruple-dose statin have comparable LDL-C-lowering efficacy. However, there can be differences in other metabolic and biological effects between these two regimens, and one of the two regimens is not always better than the other on such effects. Further studies on this issue may help in the selection of lipid-modifying agents and personalizing cardiovascular prevention.
In the TNT study analyzing 80 mg and 10 mg of atorvastatin, the AE rates and drug discontinuation owing to AEs were lower in the latter group.6 Conversely, the addition of ezetimibe to simvastatin did not alter the risk of transaminase elevation, muscle AEs, or drug discontinuation.5 A recent meta-analysis using 14 studies on statin/ezetimibe combination versus double dose statins revealed similar safety profiles of the two regimens.23 The RACING trial, on the contrary, indicated better safety in the combination group.9 The rosuvastatin dose in the monotherapy group was 20 mg in the RACING trial and in our meta-analysis. The rosuvastatin dose administered to the combination group was lower in our study, and the AE rates did not differ between the combination and monotherapy groups. Although the reason for the difference between studies is not clear through our data, the small number of our study population and low power to differentiate safety of the regimens might be some of the probable reasons. As the odds ratio of composite AEs of the combination versus monotherapy group was numerically lower, it is difficult to rule out a greater sample size might have given statistical difference. The elementary safety index used in the current study was a composite of three major AEs. Although this variable is important to compare AEs typically associated with statin-based regimens, it may be difficult to include the overall clinical tolerability. As a result, it could have been difficult to obtain statistical significance by the index of our study. In addition to the RACING trial, a large cohort study compared cardiovascular outcomes and drug maintenance rates of moderate-intensity statin/ezetimibe versus high-intensity statin regimens.24 Further review or meta-analysis on these trials may provide more insights on the safety of combination with variable statin doses. Recently, a randomized controlled study comparing the side effects of rosuvastatin 20 mg versus rosuvastatin 5 mg/ezetimibe 10 mg in elderly patients with atherosclerotic cardiovascular disease has been ongoing. That study may be able to provide solid data regarding the tolerability of these two regimens.25
To summarize, AEs appear more frequent when higher-intensity statins compared to lower-intensity statins are used, whereas the effect of adding ezetimibe on AE risk appears minimal. Recently, as the research proving the clinical benefit of ezetimibe combination has been published, the net benefit of statin/ezetimibe combination is also being spotlighted. However, to date, reports have not provided sufficient evidence on the net clinical benefit of the statin/ezetimibe combination compared to high-intensity statins. This should be estimated based on the efficacy and safety of the two regimens with comparable LDL-C reduction. In this regard, it is worth mentioning that the current meta-analysis compared two regimens with very similar LDL-C reduction. By doing so, we could produce clinically relevant and helpful data.
Our study has several potential limitations. First, although we pooled the largest number of available studies, the total number of studies and participants were relatively small. Our meta-analysis did not use the primary comparisons from source trials. Most enrolled studies evaluated rosuvastatin/ezetimibe combination and rosuvastatin monotherapy with variable doses, whereas our study analyzed regimens with a specific dose. Calculated powers of the primary and secondary outcome variables were not sufficiently high, and this may be an important limitation of our study. Therefore, the current data should be interpreted with caution. Although most efficacy and safety variables did not differ between the two groups, we cannot entirely rule out the possibility of difference being observed in a larger study population. Second, the results on the efficacy of the combination regimen did not deviate from the original concept, and this may limit the value of the current study. Third, there may be a difference of cost-effectiveness between the two regimens analyzed in the current study. Although this is one of the major points when choosing drugs in clinical practice, it was beyond the scope of our study. In addition, the majority of studies included in our meta-analysis were from the Korean population, and this could limit the application of our results to other races.
In conclusion, our meta-analysis showed for the first time that 5 mg rosuvastatin/10 mg ezetimibe and 20 mg rosuvastatin had comparable lipid-lowering efficacy and tolerability, especially for LDL-C and drug-related AEs. These results provide useful information for physicians and may help in their clinical decision-making.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea and funded by the Korean Government (Grant No. 2022R1A2C1004946).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sang-Hak Lee.
- Data curation: Yura Kang and Jung Mi Park.
- Formal analysis: Yura Kang and Jung Mi Park.
- Funding acquisition: Sang-Hak Lee.
- Investigation: all authors.
- Methodology: Yura Kang and Jung Mi Park.
- Project administration: Sang-Hak Lee.
- Supervision: Sang-Hak Lee.
- Visualization: Yura Kang and Jung Mi Park.
- Writing—original draft: all authors.
- Writing—review & editing: Sang-Hak Lee.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
Funnel plots for outcome variables. The plots showed no publication bias for % LDL-C reduction (A), rates of AEs (B), and % changes of other lipid parameters (C). LDL-C, low-density lipoprotein cholesterol; SMD, standardized mean difference; AEs, adverse events; OR, odds ratio; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol.
References
- 1.Razavi AC, Mehta A, Sperling LS. Statin therapy for the primary prevention of cardiovascular disease: pros. Atherosclerosis. 2022;356:41–45. doi: 10.1016/j.atherosclerosis.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Kim GS, Seo J, Kim BG, Jin MN, Lee HY, Kim BO, et al. Impact of statin treatment intensity after endovascular revascularization on lower extremity peripheral artery disease. Yonsei Med J. 2022;63:333–341. doi: 10.3349/ymj.2022.63.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YS, Kim HL, Kim SH, Moon MK Committee of Clinical Practice Guideline; Korean Society of Lipid and Atherosclerosis; Korean Diabetes Association and Clinical Practice Guideline Committee. Lipid management in Korean people with type 2 diabetes mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement. J Lipid Atheroscler. 2023;12:12–22. doi: 10.12997/jla.2023.12.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CJ, Yoon M, Kang HJ, Kim BJ, Choi SH, Jeong IK, et al. 2022 consensus statement on the management of familial hypercholesterolemia in Korea. J Lipid Atheroscler. 2022;11:213–228. doi: 10.12997/jla.2022.11.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Her AY, Kim JY, Kang SM, Choi D, Jang Y, Chung N, et al. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. J Cardiovasc Pharmacol Ther. 2010;15:167–174. doi: 10.1177/1074248409357922. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Kang SM, Park S, Jang Y, Chung N, Choi D. The effects of statin monotherapy and low-dose statin/ezetimibe on lipoprotein-associated phospholipase A2. Clin Cardiol. 2011;34:108–112. doi: 10.1002/clc.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380–390. doi: 10.1016/S0140-6736(22)00916-3. [DOI] [PubMed] [Google Scholar]
- 10.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang YC, Jun JE, Jeong IK, Ahn KJ, Chung HY. Comparison of the efficacy of rosuvastatin monotherapy 20 mg with rosuvastatin 5 mg and ezetimibe 10 mg combination therapy on lipid parameters in patients with type 2 diabetes mellitus. Diabetes Metab J. 2019;43:582–589. doi: 10.4093/dmj.2018.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee MY, Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, et al. Ezetimibe and rosuvastatin combination treatment can reduce the dose of rosuvastatin without compromising its lipid-lowering efficacy. Clin Ther. 2019;41:2571–2592. doi: 10.1016/j.clinthera.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Hong SJ, Jeong HS, Ahn JC, Cha DH, Won KH, Kim W, et al. A phase III, multicenter, randomized, double-blind, active comparator clinical trial to compare the efficacy and safety of combination therapy with ezetimibe and rosuvastatin versus rosuvastatin monotherapy in patients with hypercholesterolemia: I-ROSETTE (Ildong rosuvastatin & ezetimibe for hypercholesterolemia) randomized controlled trial. Clin Ther. 2018;40:226–241.e4. doi: 10.1016/j.clinthera.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Kim W, Yoon YE, Shin SH, Bae JW, Hong BK, Hong SJ, et al. Efficacy and safety of ezetimibe and rosuvastatin combination therapy versus those of rosuvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2018;40:993–1013. doi: 10.1016/j.clinthera.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Yang YJ, Lee SH, Kim BS, Cho YK, Cho HJ, Cho KI, et al. Combination therapy of rosuvastatin and ezetimibe in patients with high cardiovascular risk. Clin Ther. 2017;39:107–117. doi: 10.1016/j.clinthera.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Oh M, Kim H, Shin EW, Sung C, Kim DH, Moon DH, et al. Comparison of high-dose rosuvastatin versus low-dose rosuvastatin plus ezetimibe on carotid atherosclerotic plaque inflammation in patients with acute coronary syndrome. J Cardiovasc Transl Res. 2020;13:900–907. doi: 10.1007/s12265-020-10009-4. [DOI] [PubMed] [Google Scholar]
- 20.Bays HE, Davidson MH, Massaad R, Flaim D, Lowe RS, Tershakovec AM, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study) Am J Cardiol. 2011;108:523–530. doi: 10.1016/j.amjcard.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 21.Yu M, Liang C, Kong Q, Wang Y, Li M. Efficacy of combination therapy with ezetimibe and statins versus a double dose of statin monotherapy in participants with hypercholesterolemia: a meta-analysis of literature. Lipids Health Dis. 2020;19:1. doi: 10.1186/s12944-019-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki D, Ishida M, Watanabe H, Nobori K, Oguma Y, Terata Y, et al. Comparison of anti-inflammatory effects and high-density lipoprotein cholesterol levels between therapy with quadruple-dose rosuvastatin and rosuvastatin combined with ezetimibe. Lipids Health Dis. 2013;12:9. doi: 10.1186/1476-511X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Y, Hu H, Yang J, Yao Q, Xu H, Yu Y, et al. The efficacy and safety of statin in combination with ezetimibe compared with double-dose statin in patients with high cardiovascular risk: a meta-analysis. Bosn J Basic Med Sci. 2020;20:169–182. doi: 10.17305/bjbms.2019.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Kang D, Park H, Kang M, Choi KH, Park TK, et al. Moderate-intensity statins plus ezetimibe vs. high-intensity statins after coronary revascularization: a cohort study. Cardiovasc Drugs Ther. 2023;37:141–150. doi: 10.1007/s10557-021-07256-1. [DOI] [PubMed] [Google Scholar]
- 25.Cha JJ, Hong SJ, Kim JH, Lim S, Joo HJ, Park JH, et al. Effect of rosuvastatin 20 mg versus rosuvastatin 5 mg plus ezetimibe on statin side-effects in elderly patients with atherosclerotic cardiovascular disease: rationale and design of a randomized, controlled SaveSAMS trial. Am Heart J. 2023;261:45–50. doi: 10.1016/j.ahj.2023.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plots for outcome variables. The plots showed no publication bias for % LDL-C reduction (A), rates of AEs (B), and % changes of other lipid parameters (C). LDL-C, low-density lipoprotein cholesterol; SMD, standardized mean difference; AEs, adverse events; OR, odds ratio; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol.