Abstract
The Anterior Inferior Cerebellar Artery-Posterior Inferior Cerebellar Artery (AICA-PICA) common trunk is a rare variant of cerebral posterior circulation in which a single vessel originating from either the basilar or vertebral arteries supplies both cerebellum and brainstem territories. We present the first case of an unruptured right AICA-PICA aneurysm treated with flow diversion using a Shield-enhanced pipeline endovascular device (PED, VANTAGE Embolization Device with Shield Technology, Medtronic, Canada). We expand on this anatomic variant and review the relevant literature.
A 39-year-old man presented to our treatment center with vertigo and right hypoacusis. The initial head CT/CTA was negative, but a 4-month follow-up MRI revealed a 9 mm fusiform dissecting aneurysm of the right AICA. The patient underwent a repeat head CTA and cerebral angiogram, which demonstrated the presence of an aneurysm on the proximal portion of an AICA-PICA anatomical variant. This was treated with an endovascular approach that included flow diversion via a PED equipped with Shield Technology. The patient’s post-procedure period was uneventful, and he was discharged home after two days with an intact neurological status. The patient is still asymptomatic after a 7-month follow-up, with MR angiogram evidence of stable aneurysm obliteration and no ischemic lesions.
Aneurysms of the AICA-PICA common trunk variants have a high morbidity risk due to the importance and extent of the territory vascularized by a single vessel. Endovascular treatment with flow diversion proved to be both safe and effective in obliterating unruptured cases.
Keywords: Aneurysm, AICA-PICA variant, Coiling, Endovascular treatment, Posterior fossa, Stenting
INTRODUCTION
Aneurysms of the posterior circulation account for 10-15% of all intracranial aneurysms [29]. The common trunk of the anterior inferior cerebellar artery and the posterior inferior cerebellar artery (AICA-PICA) is a rare variant of the cerebral posterior circulation, first described in 1968 [40] and with only 11 cases currently reported in the literature, treated with surgical or endovascular techniques [2,7,12,18,32,39]. This entity is distinguished by a single vessel that originates from either the basilar artery (BA) or the vertebral artery (VA) and supplies blood to both the AICA and PICA vascular territories of the cerebellum and brainstem.
We present a unique case of a rapidly enlarging unruptured right AICA-PICA aneurysm successfully treated with flow diversion using a pipeline embolization device with Shield Technology (PED-Shield, VANTAGE Embolization Device with Shield Technology, Medtronic, Canada). The purpose of this report is to describe this rare clinical entity, with a focus on the endovascular techniques used, as well as a review of the relevant literature.
DESCRIPTION OF CASE
A 39-year-old Southeast Asian man was referred to our hospital with sudden positional vertigo and 2-months of right hypoacusis. The medical history was significant for high blood pressure on amlodipine treatment. Other comorbidities or a history of trauma were not revealed by clinical or familiar history.
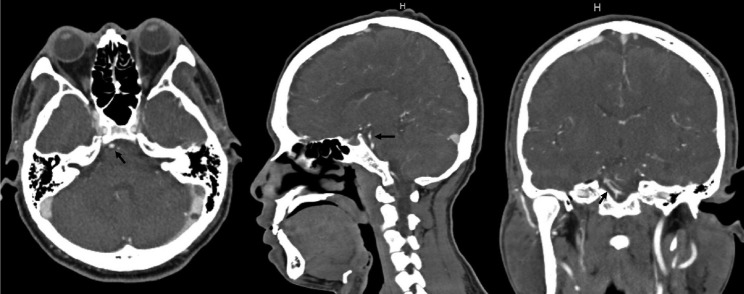
Blood pressure was 176/87 mmHg at the time of admission. Except for decreased hearing on the right side and an impaired right vestibulo-ocular reflex, the neurologic examination was unremarkable. Audiometry revealed a right moderate to severe sensorineural hearing loss. At the time, a head CT/CTA scan revealed no signs of vascular dilatations (Fig. 1).
Fig. 1.
Head CT/CTA scan done after emergency admission for vertigo and right hypoacusis. No signs of intracranial bleeding, vascular malformations, neoplasm or other relevant conditions. Specifically, no aneurysms or dissections can be seen arising from the basilar artery and right AICA (black arrow). CTA, computed tomography angiogram; AICA, anterior inferior cerebellar artery
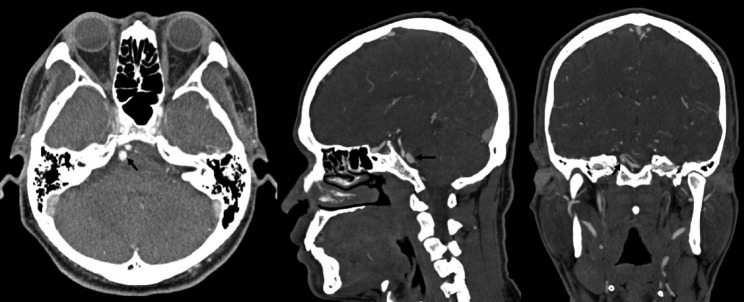
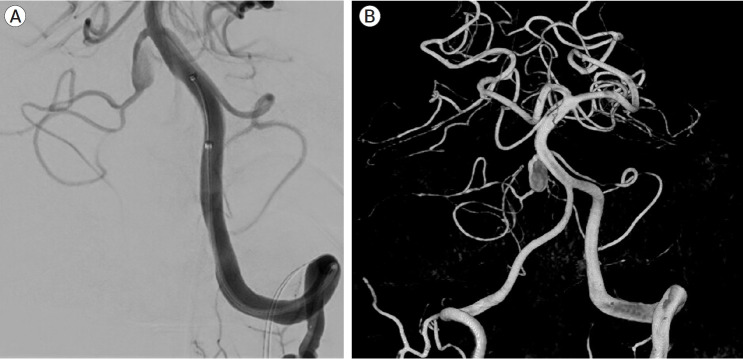
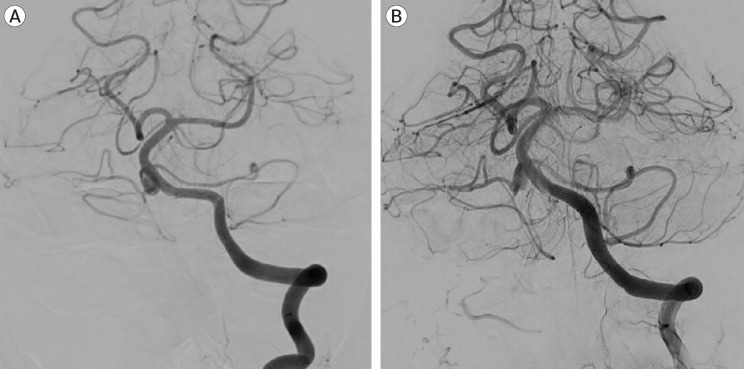
A follow-up MRI scan three months later revealed an unruptured aneurysm at the right cerebellopontine angle. A repeat head CT/CTA scan revealed the presence of an unruptured 9 mm aneurysm near the right proximal AICA, which had developed rapidly following the previous negative CTA (Fig. 2). A diagnostic cerebral angiogram was performed on the patient, which confirmed the aneurysm (Fig. 3). The scan also revealed a common AICA-PICA trunk that extended past the brainstem segment before bifurcating to supply both distal AICA and PICA territories. The left vertebral artery was dominant. The rapid growth of the aneurysm following the sudden onset of symptoms was thought to be a dissecting aneurysm with embolic phenomenon of the labyrinthine artery. The fusiform nature of the aneurysm excludes endovascular coiling in this case, but it was deemed suitable for flow diversion.
Fig. 2.
Repeat head CT/CTA now showing a 9 mm fusiform aneurysm arising from the presumed AICA vessel (black arrow). CTA, computed tomography angiogram; AICA, anterior inferior cerebellar artery
Fig. 3.
Diagnostic cerebral angiogram of the left vertebral artery, oblique view (A) and 3D reconstruction (B) showing the lack of PICA vessels bilaterally, with the AICA bifurcating and supplying both AICA and PICA territories bilaterally. There is a 9 mm fusiform aneurysm involving the proximal part of the AICA-PICA at its origin from the basilar artery. PICA, posterior inferior cerebellar artery; AICA, anterior inferior cerebellar artery
Surgical technique
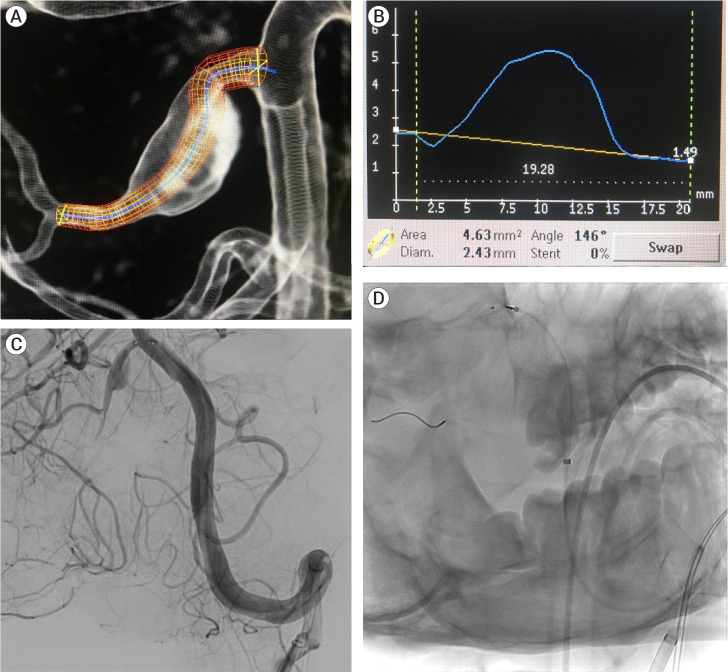
The patient was given aspirin 81 mg and clopidogrel 75 mg daily for three days before the procedure. A right common femoral artery puncture was performed and an 8F sheath was inserted after general anesthesia, neuromuscular paralysis, and endotracheal intubation. Using a roadmap and guidewire technique, a Neuron Max 088 support catheter (Penumbra, USA) was advanced to the left vertebral artery. The right AICA-PICA common trunk arose at a sharp angle from the midportion of the basilar artery. The Asahi Intecc micro-guidewire (USA), which provided adequate microcatheter support strength, was used to enter the trunk. The micro-guidewire and microcatheter system (Phenom plus catheter co-axial with Phenom 27 catheter) were advanced past the aneurysm into the AICA-PICA trunk’s inferior bifurcation, which supplied the distal PICA territory. This 1.25 mm-diameter segment was chosen as the flow diverting stent’s distal landing zone. The proximal landing zone, which measured 1.5 mm in diameter, was chosen as the segment between the beginning of the aneurysm and the origin of the AICA-PICA trunk at the basilar artery. The smallest PED-Shield available is 2.5 mm in diameter. As a result, a 2.5 mm×14 mm PED-Shield was successfully deployed without incident (Fig. 4). Final angiograms revealed that the stent was well-positioned, covering the entire neck of the aneurysm, with parent vessel patency (Fig. 5). The patient awoke neurologically intact from endovascular intervention and had an uneventful post-operative period. On post-operative day 2, he was discharged home.
Fig. 4.
Planning of stent deployment (A and B). Selective cannulation of the right AICA-PICA variant (C) and stent deployment covering the fusiform aneurysm on its entire length (D). AICA, the anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery
Fig. 5.
Cerebral angiogram pre (A) and post (B) stent deployment showing the stent covering the entire neck of the aneurysm, with parent vessel patency.
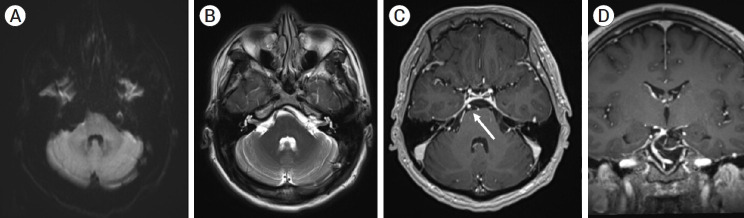
A normal neurological examination was performed at 7 months. The MRI scan revealed no ischemia in the AICA or PICA territories, a patent AICA-PICA vessel distal to the stent, and no aneurysm remnant (Fig. 6). Clopidogrel was stopped, and the patient is now on 81 mg aspirin indefinitely.
Fig. 6.
7-month follow-up MRI showing no evidence of cerebellar or brainstem parenchymal ischemia on T1 (A) and T2 weighted images of the AICA or PICA territory (B), with follow-up T1 post contrast MR angiography axial image (C) and coronal image (D) showing opacification of the AICA-PICA vessel distal to the stent (white arrow) and no residual aneurysm filling. MRI, magnetic resonance imaging; AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery
DISCUSSION
General considerations
A number of anatomical variants of the posterior circulation have been described [14,24,28,36]. Hou et al. [21] recently reviewed the angiography of 500 patients undergoing cerebral angiogram and discovered an AICA-PICA common trunk in 22.1% of cases, further subclassifying these anomalies. Common trunks of the AICA and PICA may arise from the basilar artery (BA type) or the vertebral artery (VA type), and then divide into two branches that supply the territory of the AICA or PICA, respectively [21]. This branching can take place either proximally, at the anterior brainstem segment (BP type), or distally, after the anterior brainstem segment (BD type) [21]. Our case fits the description of a BP-type AICA-PICA common trunk, with the aneurysm originating in the proximal (anterior brainstem) segment.
Injury to this variant at its source, whether during surgical or endovascular treatments, could result in a major stroke involving both the anterior and posterior surface of the cerebellum, as well as a portion of the brainstem, with potentially fatal consequences.
Literature review
Although AICA-PICA variations are common, AICA-PICA common trunk aneurysms are a rare clinical entity. Only 11 cases have previously been reported, according to our review of the literature [2,7,12,18,32,39]. 5 studies reported surgical treatment, while the remaining 6 cases were managed with coiling, 3 with stent assisted coiling, and 1 with coiling and surgical thrombectomy. A flow diverter was not used to treat this condition in any of the previous studies.
The majority of the studies, as shown in Table 1, were case reports, with the exception of Suh et al. [39] reporting a series of 5 cases and Gopalakrishnan et al. [18] describing 2 cases. Overall, 10 patients were female [2,7,12,18], with ages ranging from 26 to 71 years. The most common type of presentation was subarachnoid hemorrhage, which was described in 7 patients [7,12,18,39], with only 2 incidental findings [39] and 2 cases presenting with mass effect [2,32]. The AICA-PICA variant aneurysm location was proximal in 5 cases [2,39] and distal in 6 [7,12,18,32]. A small aneurysm (10 mm) was found in eight cases [7,12,18,39], a large aneurysm (11-24 mm) was discovered in two cases [2,39], and one giant lesion (25 mm) was described [32].
Table 1.
Overview of studies included in the literature review
| Authors | Age | Sex | Presentation | Size | Location | Origin of the trunk | in of Type of treatment | Occlusion |
|---|---|---|---|---|---|---|---|---|
| Ebara et al. [12] | 62 | F | SAH | Small | Distal | BA | Surgical – Midline suboccipital craniotomy and clipping | Complete |
| Baskaya et al. [7] | 44 | F | SAH | Small | Distal | BA | Surgical – Midline suboccipital craniotomy and clipping | Complete |
| Gopalakrishnan et al. [18] | 68 | F | SAH | Small | Distal | BA | Surgical – Midline suboccipital craniotomy and clipping | Complete |
| 63 | F | SAH | Small | Distal | BA | Surgical – Retrosigmoidal craniotomy and clipping | Complete | |
| Suh et al. [39] | 67 | F | SAH | Small | Proximal | NA | Endovascular – Coiling | Partial |
| 71 | F | SAH | Small | Proximal | NA | Endovascular – Coiling | Complete | |
| 66 | F | Incidental finding | Small | Proximal | NA | Endovascular – Stent assisted coiling | Complete | |
| 72 | F | Incidental finding | Small | Proximal | NA | Endovascular – Stent assisted coiling | Partial | |
| 46 | F | SAH | Large | Proximal | NA | Endovascular – Stent assisted coiling | Complete | |
| Ooigawa et al. [32] | 42 | M | Mass effect leading to cranial nerve deficit | Giant | Distal | BA | Endovascular – Coiling + Surgical – Thrombectomy | Complete |
| Ansari et al. [2] | 26 | F | Mass effect leading to cranial nerve deficit | Large | Proximal | VA | Surgical – Retrosigmoidal craniotomy and clipping | Complete |
| Current case report | 39 | M | Cranial nerve deficit | Small | Proximal | BA | Endovascular – Flow diversion with PED with Shield Technology | Partial |
BA, basilar artery; VA, vertebral artery
In our case, a proximal 9 mm aneurysm was discovered on a right AICA-PICA common trunk variant. Interestingly, when the patient first presented with right-sided balance and hearing problems, indicating cranial nerve VIII dysfunction, an initial CTA revealed no lesion. We believe this was due to a spontaneous dissection that included the origin of the labyrinthine artery, which was not visible on initial imaging due to spatial resolution limitations [37]. A fusiform dissecting aneurysm developed quickly as the dissection progressed.
Dissections of the vertebrobasilar system have previously been reported as an uncommon cause of acute sudden onset sensorineural hearing loss, either directly involving the labyrinthine artery or as an embolic phenomenon [13,31]. Gross et al. [20] described a case of a ruptured dissecting labyrinthine artery aneurysm treated with surgical clipping, where the labyrinthine artery originated directly from the basilar artery.
Pathogenesis and risk factors
The specific etiology of AICA-PICA variant aneurysms is unknown. Few hypotheses have been proposed, with the majority of them focusing on increased blood flow as a result of the anatomical variant, a more tortuous course leading to hemodynamic stresses at the vascular turns, and vascular injury such as dissection due to trauma or inflammatory diseases [12]. In our case, no specific cause was found; the patient was only receiving treatment for high blood pressure, with no other known comorbidities or trauma history. Interestingly, the aneurysm grew rapidly, expanding from 0 to 9 mm in less than 4 months, lending support to the theory of a dissection-related fusiform aneurysm.
Several risk factors for aneurysm growth have been reported in the literature (Table 2) [5,6,10]. The only risk factors identified in this case were hypertension, posterior circulation, and non-saccular shape. Furthermore, a meta-analysis of clinical and radiographic risk factors for aneurysm growth and rupture revealed that the majority of risk factors for aneurysm growth were also risk factors for rupture [10]. This consideration influenced our recommendations in favor of prompt treatment.
Table 2.
Risk factors associated with aneurysmal growth
| Risk factors |
|---|
| Demographics |
| Age ≥50 years |
| Female gender |
| Clinical history |
| Hypertension |
| Polycystic kidney disease |
| Smoking |
| Sympathomimetic drugs abuse |
| Anatomical-vascular characteristics |
| Aneurysm size ≥5 mm |
| Non-saccular shape |
| Posterior circulation |
| Presence of multiple aneurysms |
Management
Surgery for the treatment of posterior fossa aneurysms can be challenging due to the surrounding neurovascular complexity, the narrow surgical field, the rarity and deep locations of these aneurysms [39]. Endovascular techniques, on the other hand, which frequently involve stent placement in the posterior fossa, necessitate dual antiplatelet treatment, which increases morbidity and mortality risks, particularly in ruptured aneurysms [42].
According to the literature, most AICA aneurysms arise from the proximal segment and can be treated with neck clipping or coiling, particularly saccular ones [15,17,23]. Fusiform aneurysms are more difficult to treat, and surgery is considered the first option for large or giant lesions due to the relatively higher morbidity when treated with endovascular techniques [3,22,35]. Distal aneurysms, on the other hand, can be surgically or endovascularly trapped or occluded [17,34,41,44]. The fusiform nature of the aneurysm in our case precluded simple clipping or coiling. Indeed, either surgical or endovascular sacrifice of the parent artery would have increased the risk of ischemic stroke in both the proximal AICA and PICA territories.
In terms of safety and success rate, an endovascular approach with flow diversion was deemed optimal. Flow diversion has recently emerged as a safe and effective treatment option for patients with complex unruptured cerebral aneurysms that would otherwise be difficult to coil or clip microsurgically [8,25]. Flow diversion is also being used to treat ruptured aneurysms [26,33]. Flow diversion has been used successfully in posterior circulation fusiform aneurysms, including those involving the AICA or PICA [19,30]. Wu et al. [43] reported that despite being covered by the flow diverter, all AICA or PICA remained patentable in their cohort. This is in contrast to a previous meta-analysis that found an increased risk of ischemic strokes and perforator infarctions with posterior versus anterior circulation aneurysms [9].
In our case, we used the PED-Shield, a third-generation pipeline flow diverter device. The phosphorylcholine stent surface modification has been shown to reduce the thrombogenicity of the pipeline flow diverter [16], which is important in small caliber vessels that supply eloquent structures, as in this case. Most patients are kept on dual antiplatelet therapy after deployment [4], though single antiplatelet therapy may also be safe [1,11,27,38].
CONCLUSIONS
Aneurysms of the AICA-PICA common trunk variants carry a high morbidity risk given the importance and extension of the territory vascularized by a single vessel. In our opinion, endovascular treatment with flow diversion is safe and effective for these dissecting aneurysms of an AICA-PICA variant vessel.
Footnotes
Ethical approval and consent to participate
This study was approved by Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada. The patient provided consent for the publication of this paper.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Ansari A, Rahman A, Ahmed N, Hossain ABMM, Samadder S, Arifin MS, et al. Co-existence of arterio-venous malformation and saccular bifurcation aneurysm of a 48 years old patient, presented with massive intracranial hemorrhage: case report. J Neurol Stroke. 2019;9(4):189–94. [Google Scholar]
- 2.Ansari KA, Menon G, Nair S, Abraham M. Aneurysm of anterior inferior cerebellar artery-posterior inferior cerebellar artery variant. Asian J Neurosurg. 2018 Jan-Mar;13(1):53–5. doi: 10.4103/1793-5482.180935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anxionnat R, Tonnelet R, Derelle AL, Liao L, Barbier C, Bracard S. Endovascular treatment of ruptured intracranial aneurysms: indications, techniques and results. Diagn Interv Imaging. 2015 Jul-Aug;96(7-8):667–75. doi: 10.1016/j.diii.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Atasoy D, Kandasamy N, Hart J, Lynch J, Yang SH, Walsh D, et al. Outcome study of the pipeline embolization device with shield technology in unruptured aneurysms (PEDSU) AJNR Am J Neuroradiol. 2019 Dec;40(12):2094–101. doi: 10.3174/ajnr.A6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backes D, Rinkel GJE, Laban KG, Algra A, Vergouwen MDI. Patient- and aneurysm-specific risk factors for intracranial aneurysm growth. Stroke. 2016 Apr;47(4):951–7. doi: 10.1161/STROKEAHA.115.012162. [DOI] [PubMed] [Google Scholar]
- 6.Backes D, Vergouwen MDI, Tiel Groenestege AT, Bor ASE, Velthuis BK, Greving JP, et al. PHASES score for prediction of intracranial aneurysm growth. Stroke. 2015 May;46(5):1221–6. doi: 10.1161/STROKEAHA.114.008198. [DOI] [PubMed] [Google Scholar]
- 7.Başkaya MK, Coscarella E, Jea A, Morcos JJ. Aneurysm of the anterior inferior cerebellar artery-posterior inferior cerebellar artery variant: case report with anatomical description in the cadaver. Neurosurgery. 2006 Feb;58(2):e388. doi: 10.1227/01.NEU.0000199344.63306.DD. [DOI] [PubMed] [Google Scholar]
- 8.Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J. 2015 Aug;28(4):365–75. doi: 10.1177/1971400915602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013 Feb;44(2):442–7. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ, Murad MH, Wang Z, et al. Risk factors for growth of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016 Apr;37(4):615–20. doi: 10.3174/ajnr.A4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury D, Ahmed N, Chaurasia B, Barua KK. Azygos anterior cerebral artery aneurysm with subarachnoid hemorrhage. Neuroimmunology and Neuroinflammation. 2018 Sep;5(9):39. [Google Scholar]
- 12.Ebara M, Tanaka T, Sawauchi S, Morooka S, Yuhki K, Abe T. A ruptured aneurysm of the anterior and posterior inferior cerebellar artery: a case report. No Shinkei Geka. 1999 Nov;27(11):1013–7. [PubMed] [Google Scholar]
- 13.Eliezer M, Verillaud B, Guichard JP, Kania R, Toupet M, Herman P, et al. Labyrinthine infarction caused by vertebral artery dissection: consideration based on MRI. J Neurol. 2019 Oct;266(10):2575–7. doi: 10.1007/s00415-019-09456-0. [DOI] [PubMed] [Google Scholar]
- 14.Fujii K, Lenkey C, Rhoton AL. Microsurgical anatomy of the choroidal arteries. Fourth ventricle and cerebellopontine angles. J Neurosurg. 1980 Apr;52(4):504–24. doi: 10.3171/jns.1980.52.4.0504. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima S, Hirohata M, Okamoto Y, Yamashita S, Ishida S, Shigemori M. Anterior inferior cerebellar artery dissecting aneurysm in a juvenile: case report. Neurol Med Chir (Tokyo) 2009 Feb;49(2):81–4. doi: 10.2176/nmc.49.81. [DOI] [PubMed] [Google Scholar]
- 16.Girdhar G, Andersen A, Pangerl E, Jahanbekam R, Ubl S, Nguyen K, et al. Thrombogenicity assessment of Pipeline Flex, Pipeline Shield, and FRED flow diverters in an in vitro human blood physiological flow loop model. J Biomed Mater Res A. 2018 Dec;106(12):3195–202. doi: 10.1002/jbm.a.36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez LF, Alexander MJ, McDougall CG, Spetzler RF. Anteroinferior cerebellar artery aneurysms: surgical approaches and outcomes—a review of 34 cases. Neurosurgery. 2004 Nov;55(5):1025–35. doi: 10.1227/01.neu.0000141083.00866.82. [DOI] [PubMed] [Google Scholar]
- 18.Gopalakrishnan CV, Menon G, Nair S. Aneurysm of the anterior inferior cerebellar artery-posterior inferior cerebellar artery variant: two case reports and review of literature. Br J Neurosurg. 2009;23(5):554–6. doi: 10.1080/02688690902987778. [DOI] [PubMed] [Google Scholar]
- 19.Griessenauer CJ, Ogilvy CS, Adeeb N, Dmytriw AA, Foreman PM, Shallwani H, et al. Pipeline embolization of posterior circulation aneurysms: a multicenter study of 131 aneurysms. J Neurosurg. 2018 May;130(3):923–35. doi: 10.3171/2017.9.JNS171376. [DOI] [PubMed] [Google Scholar]
- 20.Gross BA, Frerichs KU, Du R. Microsurgical treatment of a ruptured dissecting labyrinthine artery aneurysm. Clin Neurol Neurosurg. 2013 Oct;115(10):2277–9. doi: 10.1016/j.clineuro.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Hou K, Li G, Luan T, Xu K, Xu B, Yu J. Anatomical study of anterior inferior cerebellar artery and its reciprocal relationship with posterior inferior cerebellar artery based on angiographic data. World Neurosurg. 2020 Jan;133:e459–72. doi: 10.1016/j.wneu.2019.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Kalani MYS, Yashar S, Ramey W, Albuquerque FC, McDougall CG, Nakaji P, et al. Revascularization and aneurysm surgery: techniques, indications, and outcomes in the endovascular era. Neurosurgery. 2014;74(5):482–98. doi: 10.1227/NEU.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 23.Kusaka N, Maruo T, Nishiguchi M, Takayama K, Maeda Y, Ogihara K, et al. Embolization for aneurysmal dilatation associated with ruptured dissecting anterior inferior cerebellar artery aneurysm with preservation of the parent artery: case report. No Shinkei Geka. 2006 Jul;34(7):729–34. [PubMed] [Google Scholar]
- 24.Lister RJ, Rhoton AL, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982 Feb;10(2):170–99. [PubMed] [Google Scholar]
- 25.Lv X, Yang H, Liu P, Li Y. Flow-diverter devices in the treatment of intracranial aneurysms: a meta-analysis and systematic review. Neuroradiol J. 2016 Feb;29(1):66–71. doi: 10.1177/1971400915621321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madaelil TP, Moran CJ, Cross DT 3rd, Kansagra AP. Flow diversion in ruptured intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol. 2017 Mar;38(3):590–5. doi: 10.3174/ajnr.A5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning NW, Cheung A, Phillips TJ, Wenderoth JD. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg. 2019 Jul;11(7):694–8. doi: 10.1136/neurintsurg-2018-014363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin RG, Grant JL, Peace D, Theiss C, Rhoton AL. Microsurgical relationships of the anterior inferior cerebellar artery and the facial-vestibulocochlear nerve complex. Neurosurgery. 1980 May;6(5):483–507. doi: 10.1227/00006123-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Menovsky T, André Grotenhuis J, Bartels RHMA. Aneurysm of the anterior inferior cerebellar artery (AICA) associated with high-flow lesion: report of two cases and review of literature. J Clin Neurosci. 2002 Mar;9(2):207–11. doi: 10.1054/jocn.2001.0945. [DOI] [PubMed] [Google Scholar]
- 30.Munich SA, Tan LA, Keigher KM, Chen M, Moftakhar R, Lopes DK. The pipeline embolization device for the treatment of posterior circulation fusiform aneurysms: lessons learned at a single institution. J Neurosurg. 2014 Nov;121(5):1077–84. doi: 10.3171/2014.7.JNS132595. [DOI] [PubMed] [Google Scholar]
- 31.Nagahata M, Hosoya T, Fuse T, Aoyagi M, Yamaguchi K. Arterial dissection of the vertebrobasilar systems: a possible cause of acute sensorineural hearing loss. Am J Otol. 1997 Jan;18(1):32–8. [PubMed] [Google Scholar]
- 32.Ooigawa H, Morikawa E, Ishihara S, Ogura T, Takeda R, Kurita H. Partially thrombosed giant aneurysm arising from a distal anterior inferior cerebellar artery–posterior inferior cerebellar artery variant: a case report. Interdisciplinary Neurosurgery. 2015;2(3):123–5. [Google Scholar]
- 33.Pasarikovski CR, Waggass G, Cardinell J, Howard P, da Costa L, Yang VX. Pipeline embolisation device with shield technology for the treatment of ruptured intracranial aneurysm. Neuroradiol J. 2019 Jun;32(3):189–92. doi: 10.1177/1971400919834692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peluso JPP, van Rooij WJ, Sluzewski M, Beute GN. Distal aneurysms of cerebellar arteries: incidence, clinical presentation, and outcome of endovascular parent vessel occlusion. AJNR Am J Neuroradiol. 2007 Sep;28(8):1573–8. doi: 10.3174/ajnr.A0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petr O, Brinjikji W, Thomé C, Lanzino G. Safety and efficacy of microsurgical treatment of previously coiled aneurysms: a systematic review and meta-analysis. Acta Neurochir (Wien) 2015 Oct;157(10):1623–32. doi: 10.1007/s00701-015-2500-y. [DOI] [PubMed] [Google Scholar]
- 36.Rhoton AL. The cerebellar arteries. Neurosurgery. 2000;47:S29–68. doi: 10.1097/00006123-200009001-00010. [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Kawagishi K. Labyrinthine artery detection in patients with idiopathic sudden sensorineural hearing loss by 7-T MRI. Otolaryngol Neck Surg. 2014 Mar;150(3):455–9. doi: 10.1177/0194599813517990. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan VM, Shlobin NA, Karahalios K, Scherschinski L, Rahmani R, Graffeo CS, et al. Adoption of advanced microneurosurgical technologies: an international survey. World Neurosurg. 2022 Jan;157:e473–83. doi: 10.1016/j.wneu.2021.10.128. [DOI] [PubMed] [Google Scholar]
- 39.Suh SH, Kim DJ, Kim DI, Kim BM, Chung TS, Hong CK, et al. Management of anterior inferior cerebellar artery aneurysms: endovascular treatment and clinical outcome. AJNR Am J Neuroradiol. 2011 Jan;32(1):159–64. doi: 10.3174/ajnr.A2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi M, Wilson G, Hanafee W. The anterior inferior cerebellar artery: its radiographic anatomy and significance in the diagnosis of extra-axial tumors of the posterior fossa. Radiology. 1968;90(2):281–7. [Google Scholar]
- 41.Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, et al. Trapping of ruptured dissecting aneurysm of distal anterior inferior cerebellar artery--case report. Brain Nerve. 2009 Feb;61(2):203–7. [PubMed] [Google Scholar]
- 42.Tumialán LM, Zhang YJ, Cawley CM, Dion JE, Tong FC, Barrow DL. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms: a cautionary report. J Neurosurg. 2008 Jun;108(6):1122–9. doi: 10.3171/JNS/2008/108/6/1122. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Tian Z, Liu J, Zhang Y, Li W, Zhang Y, et al. Patency of posterior circulation branches covered by flow diverter device: a hemodynamic study. Front Neurol. 2019 Jun;10:658. doi: 10.3389/fneur.2019.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zager EL, Shaver EG, Hurst RW, Flamm ES. Distal anterior inferior cerebellar artery aneurysms. J Neurosurg. 2002 Sep;97(3):692–6. doi: 10.3171/jns.2002.97.3.0692. [DOI] [PubMed] [Google Scholar]