Abstract
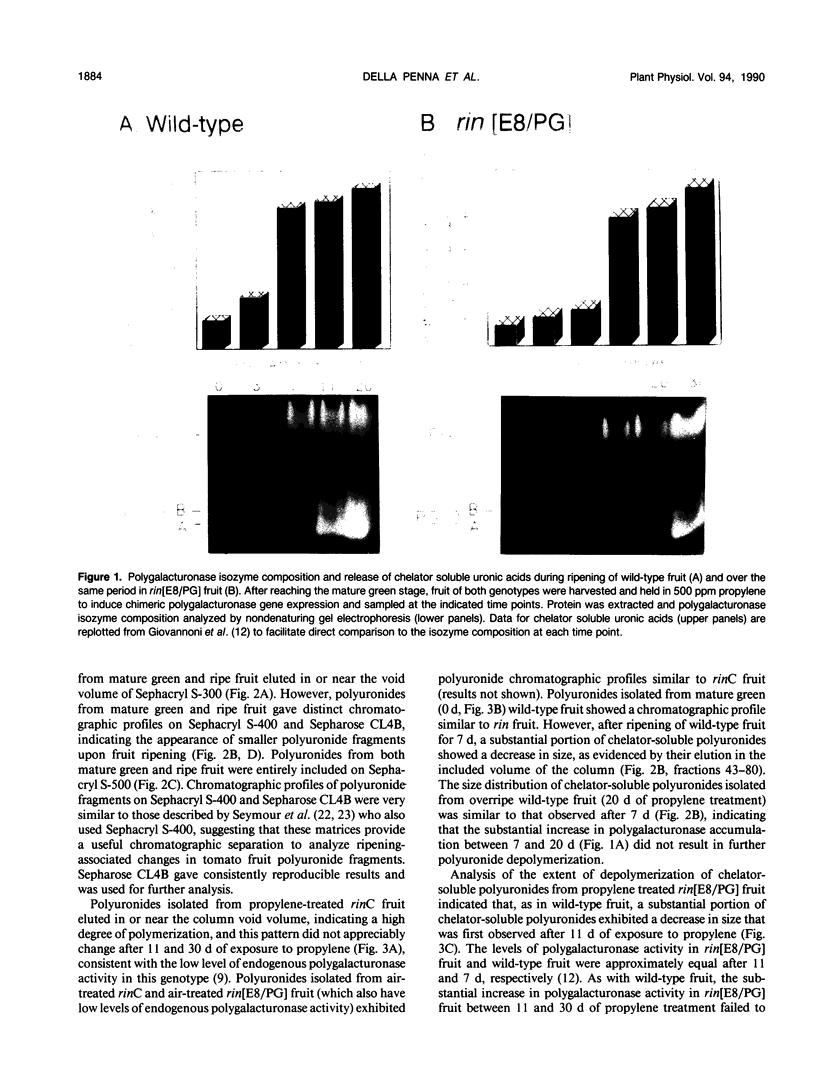
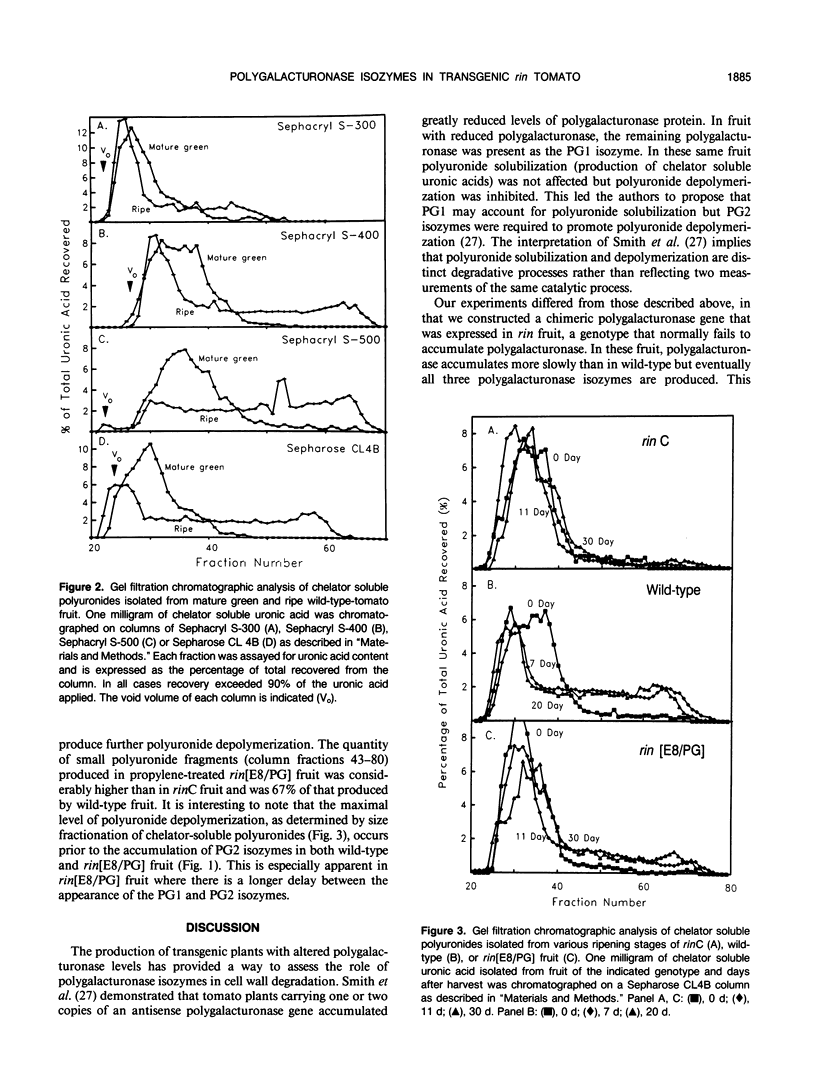
We have previously described the construction and expression of a chimeric gene that allows developmentally regulated expression of tomato (Lycopersicon esculentum) polygalacturonase in ripening-impaired, mutant (rin) tomato fruit (JJ Giovannoni, D DellaPenna, AB Bennett, RL Fischer [1989] The Plant Cell 1: 53-63). We now show that expression of the chimeric polygalacturonase gene in rin tomato fruit resulted in the accumulation of all three polygalacturonase isozymes (PG1, PG2A, and PG2B). Polyuronide solubilization and polyuronide depolymerization both reached their maximal levels in transgenic rin fruit prior to the appearance of PG2 isozymes. These results demonstrate that PG1, PG2A, and PG2B all arise by differential processing of a single gene product and further suggest that the PG1 isozyme is sufficient to carry out both polyuronide solubilization and depolymerization in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggs M. S., Harriman R. W., Handa A. K. Changes in Gene Expression during Tomato Fruit Ripening. Plant Physiol. 1986 Jun;81(2):395–403. doi: 10.1104/pp.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Bennett A. B. In vitro synthesis and processing of tomato fruit polygalacturonase. Plant Physiol. 1988 Apr;86(4):1057–1063. doi: 10.1104/pp.86.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Kates D. S., Bennett A. B. Polygalacturonase Gene Expression in Rutgers, rin, nor, and Nr Tomato Fruits. Plant Physiol. 1987 Oct;85(2):502–507. doi: 10.1104/pp.85.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Lincoln J. E., Fischer R. L., Bennett A. B. Transcriptional Analysis of Polygalacturonase and Other Ripening Associated Genes in Rutgers, rin, nor, and Nr Tomato Fruit. Plant Physiol. 1989 Aug;90(4):1372–1377. doi: 10.1104/pp.90.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J. J., DellaPenna D., Bennett A. B., Fischer R. L. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989 Jan;1(1):53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson D., Tucker G. A., Keen J., Ray J., Bird C. R., Schuch W. Sequencing and identification of a cDNA clone for tomato polygalacturonase. Nucleic Acids Res. 1986 Nov 11;14(21):8595–8603. doi: 10.1093/nar/14.21.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J. E., Cordes S., Read E., Fischer R. L. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci U S A. 1987 May;84(9):2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshrefi M., Luh B. S. Carbohydrate composition and electrophoretic properties of tomato polygalacturonase isoenzymes. Eur J Biochem. 1983 Oct 3;135(3):511–514. doi: 10.1111/j.1432-1033.1983.tb07681.x. [DOI] [PubMed] [Google Scholar]
- Pressey R., Avants J. K. Two forms of polygalacturonase in tomatoes. Biochim Biophys Acta. 1973 Jun 6;309(2):363–369. doi: 10.1016/0005-2744(73)90035-1. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Seymour G. B., Harding S. E. Analysis of the molecular size of tomato (Lycopersicon esculentum Mill) fruit polyuronides by gel filtration and low-speed sedimentation equilibrium. Biochem J. 1987 Jul 15;245(2):463–466. doi: 10.1042/bj2450463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy R. E., Kramer M., Hiatt W. R. Reduction of polygalacturonase activity in tomato fruit by antisense RNA. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8805–8809. doi: 10.1073/pnas.85.23.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Watson C. F., Morris P. C., Bird C. R., Seymour G. B., Gray J. E., Arnold C., Tucker G. A., Schuch W., Harding S. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol. 1990 Mar;14(3):369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- Tucker G. A., Robertson N. G., Grierson D. Changes in polygalacturonase isoenzymes during the 'ripening' of normal and mutant tomato fruit. Eur J Biochem. 1980 Nov;112(1):119–124. doi: 10.1111/j.1432-1033.1980.tb04993.x. [DOI] [PubMed] [Google Scholar]