Abstract
Objective: To explore a possible connection between active viral infections and manifestation of dermatomyositis (DM).
Methods: Skeletal muscle biopsies were analyzed from patients diagnosed with juvenile (n=10) and adult (n=12) DM. Adult DM patients harbored autoantibodies against either TIF-1γ (n=7) or MDA5 (n=5). Additionally, we investigated skeletal muscle biopsies from non-diseased controls (NDC, n=5). We used an unbiased high-throughput RNA sequencing (HTS) approach to detect viral sequences. To further increase sequencing depth, a host depletion approach was applied.
Results: In this observational study, no relevant viral sequences were detected either by native sequencing or after host depletion. The absence of detectable viral sequences makes an active viral infection of the muscle tissue unlikely to be the cause of DM in our cohorts.
Discussion: Type I interferons (IFN) play a major role in the pathogenesis of both juvenile and adult DM. The IFN response is remarkably conserved between DM subtypes classified by specific autoantibodies. Certain acute viral infections are accompanied by a prominent type I IFN response involving similar downstream mechanisms as in DM. Aiming to elucidate the pathogenesis of DM in skeletal muscle tissue, we used deep RNA sequencing and a host depletion approach to detect possible causative viruses.
Keywords: Dermatomyositis (DM), Interferon (IFN), Viral signature, Next generation sequencing
Introduction
Although the pathogenesis of DM is not completely understood, it is well established that type I IFN plays a key role in both juvenile and adult DM. The IFN response can be defined by a specific up-regulation of IFN-stimulated molecules such as ISG15 or MxA and is comparable to that found in lupus erythematosus (SLE) and certain inherited interferonopathies, but also in viral infections [10]. In fact, parvovirus B19, coxsackie virus, polyomavirus, Epstein-Barr virus (EBV), influenza virus, human immunodeficiency virus (HIV), and SARS-CoV-2 have been associated with DM onset [3]. Since the detection of specific autoantibodies, such as anti-Mi-2, anti-TIF-1γ, anti-NXP2, anti-SAE, and anti-MDA5, the spectrum of DM can be defined more precisely regarding prognosis and clinical course such as risk of cancer development [27]. DM subtype-specific investigation of type I IFN-regulated transcripts identified a set of significantly dysregulated genes in muscle biopsies derived from anti-TIF-1γ+ patients [4]. Moreover, MDA5, RIG1, and TRIM33 (TIF1γ) are specifically involved in downstream signaling of viral infections [13]. MDA5 is a key protein sensor for viral double-stranded RNA (dsRNA) motifs to induce expression of IFN1 in certain viral diseases [6], and TRIM33 inhibits endogenous retrovirus (ERV) gene transcription [21]. Recent studies have demonstrated that myositis autoantibodies, most commonly anti-TIF-1γ, anti-NXP2, and anti-MDA5, are also found in more than 50% of juvenile myositis patients, with different frequencies in different populations, but again associated with specific clinical manifestations and prognosis [12, 15, 22, 29].
It is well described, especially in children, that DM can occur with an acute onset of general viral infection-like symptoms such as fever, fatigue, and apathy [20].
Hence, the aim of our project was the identification of any viral signatures (in terms of an active virus replication) in skeletal muscle samples using an unbiased high-throughput sequencing approach. In contrast to other targeted approaches such as virus-specific PCRs, we aimed at viral genome detection without prior restrictions to probable pathogens to investigate any - including previously unknown - possible viral species.
Material and Methods
Patient cohort
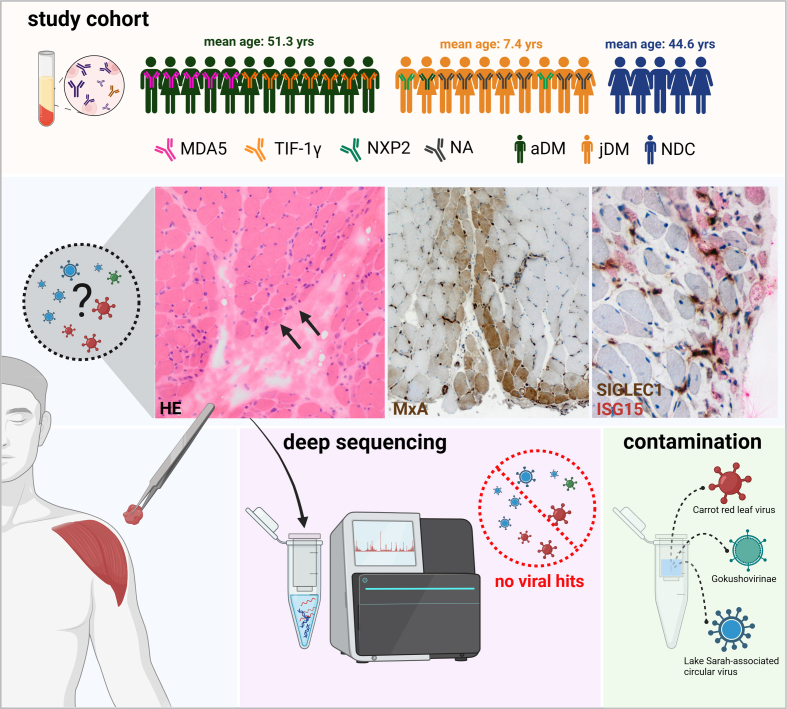
Skeletal muscle biopsies were analyzed from treatment-naive patients diagnosed directly after symptom onset with juvenile or adult DM according to ENMC diagnostic criteria [14]. We included n=22 DM cases from three institutions between 2008 and 2018, in which the serum of adult patients was either positive for autoantibodies against TIF-1γ (n=7) or MDA5 (n=5). The morphological muscle involvement was related to the underlying autoantibody with perifascicular atrophy and inflammation in TIF-1γ+ patients and less severe muscle damage in MDA5+ patients [1]. The serum of n=2 juvenile patients was positive for autoantibodies against NPX2. The other juvenile patients were diagnosed before autoantibody test-ing was widely available and retroactive information on autoantibodies was not available. Additionally, we investigated skeletal muscle biopsies (n=5) from non-diseased controls (NDC) with nonspecific complaints, without overt muscle weakness, absence of any morphologic abnormalities in the skeletal muscle biopsies, elevated creatine kinase (CK) levels, or laboratory evidence of any systemic inflammation including negativity of autoantibody testing. Informed consent was obtained from all patients at each institution involved. Procedures were approved by the official ethical standards committee (EA2/163/17) at the Charité – Universitätsmedizin Berlin. The experimental setup is shown in Figure 1.
Figure 1. Experimental study design.
The study cohort, including subgroups and information on autoantibodies, is given in the upper panel. Histological work-up of DM skeletal muscle samples shows perifascicular atrophy (HE, arrows). Immunohistochemical staining of type I IFN-inducible proteins ISG15 and MxA demonstrates a perifascicular staining pattern with SIGLEC1-positive macrophages (brown) in close proximity to ISG15 positive muscle fibers (red). Deep sequencing of DM patients’ skeletal muscle biopsies only revealed viral reads derived from column-based extraction and library preparation kit contaminations. (aDM: adult dermatomyositis, jDM: juvenile dermatomyositis, NDC: non-diseased controls, NA: No autoantibody testing, yrs: years).
High throughput-sequencing of RNA
Total RNA was extracted from fresh-frozen, cryopreserved (at -80°C) skeletal muscle specimens using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. RNA extraction was checked by testing for Beta-2 microglobulin (B2M) mRNA using a RT-qPCR as previously described [23]. For the detection of viral sequences two strategies were applied: (i) unbiased native sequencing of the nucleic acids and (ii) a more sensitive sequencing approach after the specific removal of ribosomal RNA (rRNA). The detailed steps are given in Supplementary material and methods.
Statistics and HTS data analyses
For virus detection, we applied two approaches for viral read identification. First, a computational pipeline that consists of a collection of bash shell (C. Ramey, GNU bash, available at https://www.gnu.org/software/bash/) scripts that invoke third-party and custom in-house programs. Overall execution is coordinated by a Slurm Pipeline Python package (T. C. Jones, Slurm Pipeline, available at https://github.com/acorg/slurm-pipeline). Second, we classified sequences with Kraken2 (version 2.1.3) and the Kraken2 [28] ‘standard’ database (available at https://benlangmead.github.io/aws-indexes/k2, revision date 10/9/23). The results were visualized using KRONA [19] and Pavian [5] and independently analyzed by two virologists. No special cut-off value was applied, but rather each individual virus hit was examined and evaluated individually. The detailed steps for both analyses are given in Supplementary material and methods.
Data availability
The sequencing data (non-human reads) of the samples generated in this study have been deposited in the Sequence Read Archive (SRA) under the accession numbers SAMN39051134 - SAMN39051187.
Results
Sequencing results before and after rRNA removal
Successful RNA extraction was confirmed by the detection of beta-2-microglobulin mRNA in all samples (Supplementary table 1). All samples were processed in the two sequencing approaches: Using native sequencing, a total of 661.6 Mio reads (Range: 7.1-32.8 Mio per sample, mean: 24.5 Mio, median: 25.5 Mio) were generated for all patients analyzed. Roughly, 90% of the resulting reads could be mapped to rRNA references (NR_003287.1, NR_145820.4) and less than 1% of the reads mapped against a globin reference sequence (HBA1: NM_000558.5, HBA2: NM_000517.6, HBB: NM_000518.5, HBD: NM_000519.4) (Table 1).
Table 1.
Overview of samples, PCR, and sequencing results. The table includes number of generated reads and proportion of rRNA and globin reads for native sequencing and sequencing after rRNA depletion.
| Native sequencing | rRNA removal | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Cohort | ⚥ | age | Reads [R1+R2] | reads against rRNA* | % rRNA | reads against Globin** | % globin | Reads [R1+R2] | reads against rRNA* | % rRNA | reads against Globin** | % globin |
| 1 | MDA 5 | m | 34 | 25,451,212 | 23,454,672 | 92.2 | 1 | 0.000004 | 23,752,226 | 700,27 | 2.95 | 2,965 | 0.012483 |
| 2 | MDA 5 | f | 32 | 16,964,910 | 15,449,997 | 91.1 | 100 | 0.000589 | 11,763,512 | 115,577 | 0.98 | 116 | 0.000986 |
| 3 | MDA 5 | f | 39 | 25,805,868 | 24,592,381 | 95.3 | 192 | 0.000744 | 23,635,252 | 349,242 | 1.48 | 54,525 | 0.230694 |
| 4 | MDA 5 | m | 31 | 27,640,030 | 26,338,410 | 95.3 | 97 | 0.000351 | 23,982,524 | 530,301 | 2.21 | 1,507 | 0.006284 |
| 5 | MDA 5 | f | 38 | 26,560,152 | 24,391,028 | 91.8 | 6.780 | 0.025527 | 12,321,160 | 142,752 | 1.16 | 269 | 0.002183 |
| 6 | TIF-1γ | f | 72 | 24,181,444 | 22,604,310 | 93.5 | 101 | 0.000418 | 32,040,902 | 1,823,768 | 5.69 | 388 | 0.001211 |
| 7 | TIF-1γ | m | 69 | 25,030,838 | 22,695,134 | 90.7 | 102 | 0.000407 | 32,634,054 | 839,161 | 2.57 | 1,214 | 0.003720 |
| 8 | TIF-1γ | f | 47 | 27,551,144 | 25,511,050 | 92.6 | 11 | 0.000040 | 37,285,322 | 877,105 | 2.35 | 225 | 0.000603 |
| 9 | TIF-1γ | f | 64 | 26,854,504 | 22,726,155 | 84.6 | 53 | 0.000197 | 38,565,498 | 969,885 | 2.51 | 3,156 | 0.008183 |
| 10 | TIF-1γ | f | 38 | 27,316,894 | 24,417,085 | 89.4 | 6 | 0.000022 | 19,416,276 | 233,351 | 1.20 | 293 | 0.001509 |
| 11 | TIF-1γ | f | 83 | 27,684,124 | 24,577,672 | 88.8 | 122 | 0.000441 | 38,328,838 | 681,366 | 1.78 | 548 | 0.001430 |
| 12 | TIF-1γ | m | 69 | 29,464,600 | 27,636,300 | 93.8 | 83 | 0.000282 | 21,102,676 | 388,385 | 1.84 | 22 | 0.000104 |
| 13 | NDC | f | 70 | 30,284,074 | 26,373,635 | 87.1 | 55 | 0.000182 | 28,112,926 | 388,141 | 1.38 | 9,949 | 0.035389 |
| 14 | NDC | f | 51 | 25,886,910 | 25,146,497 | 97.1 | 0 | 0.000000 | 25,898,230 | 711,277 | 2.75 | 332 | 0.001282 |
| 15 | NDC | m | 53 | 22,971,962 | 20,643,167 | 89.9 | 590 | 0.002568 | 6,997,044 | 33,46 | 0.48 | 233 | 0.003330 |
| 16 | NDC | f | 17 | 32,842,356 | 28,922,129 | 88.1 | 34 | 0.000104 | 20,534,850 | 363,574 | 1.77 | 107 | 0.000521 |
| 17 | NDC | f | 32 | 19,838,176 | 18,580,234 | 93.7 | 532 | 0.002682 | 14,686,946 | 338,501 | 2.30 | 1,31 | 0.008919 |
| 18 | jDM | m | 20 | 26,934,130 | 25,226,685 | 93.7 | 10 | 0.000037 | 10,881,868 | 421,412 | 3.87 | 257 | 0.002362 |
| 19 | jDM | f | 15 | 24,941,254 | 22,494,587 | 90.2 | 98 | 0.000393 | 28,547,838 | 314,633 | 1.10 | 3,078 | 0.010782 |
| 20 | jDM | m | 2 | 21,805,986 | 19,930,688 | 91.4 | 22 | 0.000101 | 5,877,104 | 17,646 | 0.30 | 13 | 0.000221 |
| 21 | jDM | f | 2 | 22,330,238 | 20,058,021 | 89.8 | 284 | 0.001272 | 6,781,752 | 117,288 | 1.73 | 99 | 0.001460 |
| 22 | jDM | m | 6 | 7,149,280 | 4,980,683 | 69.7 | 26 | 0.000364 | 5,332,966 | 236,384 | 4.43 | 119 | 0.002231 |
| 23 | jDM | f | 5 | 23,297,262 | 21,965,327 | 94.3 | 62 | 0.000266 | 13,150,292 | 128,447 | 0.98 | 101 | 0.000768 |
| 24 | jDM | m | 7 | 23,303,882 | 21,816,633 | 93.6 | 59 | 0.000253 | 11,615,248 | 31,92 | 0.27 | 111 | 0.000956 |
| 25 | jDM | m | 10 | 20,889,528 | 19,197,167 | 91.9 | 13 | 0.000062 | 12,207,356 | 468,646 | 3.84 | 68 | 0.000557 |
| 26 | jDM | m | 2 | 19,364,716 | 16,680,717 | 86.1 | 141 | 0.000728 | 32,487,164 | 53,126 | 0.16 | 2,411 | 0.007421 |
| 27 | jDM | f | 5 | 29,292,446 | 27,326,249 | 93.3 | 816 | 0.002786 | 20,397,658 | 386,63 | 1.90 | 6,248 | 0.030631 |
* NR_003287.1, NR_145820.4
** HBA1: NM_000558.5, HBA2: NM_000517.6, HBB: NM_000518.5, HBD: NM_000519.4
For removal of rRNA and globin sequences, we initially applied the QIAseq FastSelect -rRNA and Globin HMR Kit for four samples. This resulted in a nearly complete removal of rRNA (<1% remaining) and a similar reduction of globin sequences (data not shown). As the initial number of globin mRNA reads was already low, we proceeded with the removal of only rRNA for all samples.
After rRNA depletion, a total of 558.3 Mio reads (Range: 5.3-38.6 Mio per sample, mean: 20.7 Mio, median: 20.5 Mio) were generated. A maximum of 5.69% rRNA reads and well below 1% globin reads remained.
As expected, Kraken2 classification of reads from both approaches resulted in many reads classified as being of human, bacterial, or archaea origin (Supplementary table 2). Although not within the scope of the present study, which focuses on viruses, the detection of these reads can be used to ensure consistent quality and comparability of the sequencing data for all samples. The matched bacterial reads mainly correspond to environmental bacteria and are likely explained by contamination from the environment or the skin microbiome (e.g. Cutibacterium acnes).
Matches against virus sequences
For both sequencing approaches and both sequence classification approaches, no relevant human virus findings were detected. Both classification approaches resulted in overall similar results (Supplementary figure 1). A common finding were retroviral reads (Murine leukemia virus) which were previously described as common contamination from library preparation kit reagents [8]. Remarkably, after rRNA removal, a higher number of previously described contaminations from extraction columns and library preparation kit reagents were found that were not detected in the native sequencing approach. These mainly included plant- and algae-infecting viruses such as Carrot red leaf virus, Lake Sarah-associated circular virus, and Gokushovirinae. In one sample, viral reads against Kadipiro virus were identified, which was also previously shown to be a potential kit contamination [11, 18]. Additionally, we detected Circovirus-like viral reads that were previously associated with RNeasy MinElute columns used for RNA extraction [2]. In all depleted samples, including the control subjects, we found reads mapping against two short (<150 bp) genome regions of AAV-5, which we therefore did not count as a virus finding. We were not able to identify the origin of these two short sequences, but an origin in the used kits or reagents are also likely. Although only RNA library preparation was performed, it was also possible to detect DNA viruses, e.g., via transcripts or replication intermediates as shown by the detection of the DNA virus Gokushovirinae, Cycloviruses, and Chrysochromulina ericina virus.
Discussion
The aim of the project was to explore a putative viral pathogenesis of DM using an unbiased methodology. This was based on obvious evidence concerning molecular links between relevance and presence of IFN-related gene expression, and known autoantibody functions such as MDA5/RIG1 and TRIM33, as well as epitope homology of TRIM proteins with specific viral species including poxviruses [16].
Here, we detected viral sequences, especially after rRNA removal, in most of the samples. Nevertheless, the few viral reads concerning Murine leukemia virus, Carrot red leaf virus, Lake Sarah-associated circular virus, Gokushovirinae, Parvovirus NIH-CQV, and Kadipiro virus are very likely derived from column-based extraction and library preparation kit contamination [2] instead of being the cause of direct viral muscle infections, which have been reported for influenza viruses, enteroviruses, HIV, and hepatitis viruses [7, 17, 25] among others.
The increase in such matches in the rRNA- depleted samples indicates the higher sensitivity of this approach and that the sequencing depth was sufficient to detect potential causative virus sequences including RNA transcripts from active replicating DNA viruses, had they been present in the muscle samples. Therefore, it can be assumed that no viral pathogens were present at the times and sites of sample collection.
However, the absence of evidence of active viral infection in the muscle tissues at the time of sampling does not preclude previous viral infections.
Autoimmunity and the detection and accumulation of antibodies are well established to be at the root of the pathogenesis of DM [9]. Viral infections and virus exposure in turn contribute to accumulation of virus-specific antibodies and generation of autoantibodies, which may lead to autoimmunity via several pathways including molecular mimicry, epitope spreading, and bystander activation even after complete viral clearance [9, 24]. Multiple other factors such as genetic predisposition, host immune response, and viral strain may also play an important role in disease occurrence, progression, and prognosis. As antibodies recognizing viruses demonstrate a high abundance in DM patients, further studies using next generation sequencing techniques will be needed to investigate the viral exposure patterns in DM patients at different time points during the course of the disease [16, 26]. This may also help to detect new, previously unknown DM-specific autoantibodies to further prognostically stratify DM patients.
Author contributions
Werner Stenzel, Corinna Preusse, Victor Corman, and Josefine Radke designed the study concept, performed data analysis, and wrote the manuscript. Terry C. Jones and Julia Melchert performed data analysis and revised the manuscript. Randi Koll performed data analysis. Olivier Benveniste, Ulrike Schara-Schmidt, and Sarah Leonard-Louis provided muscle biopsy samples and revised the manuscript. Christian Drosten and Hans-Hilmar Goebel revised the manuscript and contributed to data analysis.
Funding
This work was supported by the Deutsche Gesellschaft für Muskelkranke (DGM) e.V.
Acknowledgements
We gratefully thank P. Matylewski and S. Stefaniak for excellent technical assistance and advice. We thank Nikolai Zaki for help with Kraken2 analyses. Cartoon images were created with Biorender.com.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
References
- Allenbach Y, Leroux G, Suarez-Calvet X, Preusse C, Gallardo E, Hervier B, Rigolet A, Hie M, Pehl D, Limal N et al (2016) Dermatomyositis With or Without Anti-Melanoma Differentiation-Associated Gene 5 Antibodies: Common Interferon Signature but Distinct NOS2 Expression. Am J Pathol 186: 691-700. DOI: 10.1016/j.ajpath.2015.11.010 [DOI] [PubMed]
- Asplund M, Kjartansdottir KR, Mollerup S, Vinner L, Fridholm H, Herrera JAR, Friis-Nielsen J, Hansen TA, Jensen RH, Nielsen IB et al (2019) Contaminating viral sequences in high-throughput sequencing viromics: a linkage study of 700 sequencing libraries. Clin Microbiol Infect 25: 1277-1285. DOI: 10.1016/j.cmi.2019.04.028 [DOI] [PubMed]
- Bax CE, Maddukuri S, Ravishankar A, Pappas-Taffer L, Werth VP (2021) Environmental triggers of dermatomyositis: a narrative review. Ann Transl Med 9: 434. DOI: 10.21037/atm-20-3719 [DOI] [PMC free article] [PubMed]
- Bolko L, Jiang W, Tawara N, Landon-Cardinal O, Anquetil C, Benveniste O, Allenbach Y (2021) The role of interferons type I, II and III in myositis: A review. Brain Pathol 31: e12955. DOI: 10.1111/bpa.12955 [DOI] [PMC free article] [PubMed]
- Breitwieser FP, Salzberg SL (2020) Pavian: interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics 36: 1303-1304. DOI: 10.1093/bioinformatics/btz715 [DOI] [PMC free article] [PubMed]
- Brisse M, Ly H (2019) Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front Immunol 10: 1586. DOI: 10.3389/fimmu.2019.01586 [DOI] [PMC free article] [PubMed]
- Crum-Cianflone NF (2008) Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev 21: 473-494. DOI: 10.1128/CMR.00001-08 [DOI] [PMC free article] [PubMed]
- Erlwein O, Robinson MJ, Dustan S, Weber J, Kaye S, McClure MO (2011) DNA extraction columns contaminated with murine sequences. PLoS One 6: e23484. DOI: 10.1371/journal.pone.0023484 [DOI] [PMC free article] [PubMed]
- Halilu F, Christopher-Stine L (2022) Myositis-specific Antibodies: Overview and Clinical Utilization. Rheumatol Immunol Res 3: 1-10. DOI: 10.2478/rir-2022-0001 [DOI] [PMC free article] [PubMed]
- Higgs BW, Zhu W, Richman L, Fiorentino DF, Greenberg SA, Jallal B, Yao Y (2012) Identification of activated cytokine pathways in the blood of systemic lupus erythematosus, myositis, rheumatoid arthritis, and scleroderma patients. Int J Rheum Dis 15: 25-35. DOI: 10.1111/j.1756-185X.2011.01654.x [DOI] [PubMed]
- Holmes EC (2019) Reagent contamination in viromics: all that glitters is not gold. Clin Microbiol Infect 25: 1167-1168. DOI: 10.1016/j.cmi.2019.06.019 [DOI] [PubMed]
- Li D, Tansley SL (2019) Juvenile Dermatomyositis-Clinical Phenotypes. Curr Rheumatol Rep 21: 74. DOI: 10.1007/s11926-019-0871-4 [DOI] [PMC free article] [PubMed]
- Lozhkov AA, Yolshin ND, Baranovskaya IL, Plotnikova MA, Sergeeva MV, Gyulikhandanova NE, Klotchenko SA, Vasin AV (2023) Kinetics of interferon-lambda and receptor expression in response to in vitro respiratory viral infection. Acta Virol 67: 99-108. DOI: 10.4149/av_2023_110 [DOI] [PubMed]
- Mammen AL, Allenbach Y, Stenzel W, Benveniste O, Group EtWS (2020) 239th ENMC International Workshop: Classification of dermatomyositis, Amsterdam, the Netherlands, 14-16 December 2018. Neuromuscul Disord 30: 70-92. DOI: 10.1016/j.nmd.2019.10.005 [DOI] [PubMed]
- McCann LJ, Livermore P, Wilkinson MGL, Wedderburn LR (2022) Juvenile dermatomyositis. Where are we now? Clin Exp Rheumatol 40: 394-403. DOI: 10.55563/clinexprheumatol/56ilob [DOI] [PubMed]
- Megremis S, Walker TDJ, He X, O'Sullivan J, Ollier WER, Chinoy H, Pendleton N, Payton A, Hampson L, Hampson I, Lamb JA (2021) Analysis of human total antibody repertoires in TIF1gamma autoantibody positive dermatomyositis. Commun Biol 4: 419. DOI: 10.1038/s42003-021-01932-6 [DOI] [PMC free article] [PubMed]
- Narayanappa G, Nandeesh BN (2021) Infective myositis. Brain Pathol 31: e12950. DOI: 10.1111/bpa.12950 [DOI] [PMC free article] [PubMed]
- Ngoi CN, Siqueira J, Li L, Deng X, Mugo P, Graham SM, Price MA, Sanders EJ, Delwart E (2017) Corrigendum: The plasma virome of febrile adult Kenyans shows frequent parvovirus B19 infections and a novel arbovirus (Kadipiro virus). J Gen Virol 98: 517. DOI: 10.1099/jgv.0.000762 [DOI] [PMC free article] [PubMed]
- Ondov BD, Bergman NH, Phillippy AM (2011) Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 12: 385. DOI: 10.1186/1471-2105-12-385 [DOI] [PMC free article] [PubMed]
- Papadopoulou C, Wedderburn LR (2017) Treatment of Juvenile Dermatomyositis: An Update. Paediatr Drugs 19: 423-434. DOI: 10.1007/s40272-017-0240-6 [DOI] [PubMed]
- Rajsbaum R, Garcia-Sastre A, Versteeg GA (2014) TRIMmunity: the roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. J Mol Biol 426: 1265-1284. DOI: 10.1016/j.jmb.2013.12.005 [DOI] [PMC free article] [PubMed]
- Schanzer A, Rager L, Dahlhaus I, Dittmayer C, Preusse C, Della Marina A, Goebel HH, Hahn A, Stenzel W (2021) Morphological Characteristics of Idiopathic Inflammatory Myopathies in Juvenile Patients. Cells 11:. DOI: 10.3390/cells11010109 [DOI] [PMC free article] [PubMed]
- Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7: 33. DOI: 10.1186/1471-2199-7-33 [DOI] [PMC free article] [PubMed]
- Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM (2019) Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 11:. DOI: 10.3390/v11080762 [DOI] [PMC free article] [PubMed]
- Smuts H, Kew M, Khan A, Korsman S (2014) Novel hybrid parvovirus-like virus, NIH-CQV/PHV, contaminants in silica column-based nucleic acid extraction kits. J Virol 88: 1398. DOI: 10.1128/JVI.03206-13 [DOI] [PMC free article] [PubMed]
- Su R, Yan H, Li N, Ding T, Li B, Xie Y, Gao C, Li X, Wang C (2022) Application value of blood metagenomic next-generation sequencing in patients with connective tissue diseases. Front Immunol 13: 939057. DOI: 10.3389/fimmu.2022.939057 [DOI] [PMC free article] [PubMed]
- Turnier JL, Kahlenberg JM (2022) Using autoantibody signatures to define cancer risk in dermatomyositis. J Clin Invest 132:. DOI: 10.1172/JCI156025 [DOI] [PMC free article] [PubMed]
- Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20: 257. DOI: 10.1186/s13059-019-1891-0 [DOI] [PMC free article] [PubMed]
- Wu JQ, Lu MP, Reed AM (2020) Juvenile dermatomyositis: advances in clinical presentation, myositis-specific antibodies and treatment. World J Pediatr 16: 31-43. DOI: 10.1007/s12519-019-00313-8 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data (non-human reads) of the samples generated in this study have been deposited in the Sequence Read Archive (SRA) under the accession numbers SAMN39051134 - SAMN39051187.