Abstract
Background
Despite increasing evidence on the safety of pregnancy after anticancer treatments in breast cancer survivors, many physicians and patients remain concerned about a potential risk of pregnancy specifically in the case of hormone receptor-positive breast cancer.
Materials and methods
A systematic literature search of Medline, Embase and Cochrane library with no language or date restriction up to 31 March 2023 was carried out. To be included, articles had to be retrospective and prospective case-control and cohort studies as well as clinical trials comparing survival outcomes of premenopausal women with or without a pregnancy after prior diagnosis of hormone receptor-positive breast cancer. Disease-free survival (DFS) and overall survival (OS) were the outcomes of interest. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Study protocol is registered in PROSPERO (n. CRD42023394232).
Results
Out of 7796 screened studies, 8 were eligible to be included in the final analysis. A total of 3805 patients with hormone receptor-positive invasive early breast cancer were included in these studies, of whom 1285 had a pregnancy after breast cancer diagnosis. Median follow-up time ranged from 3.8 to 15.8 years and was similar in the pregnancy and non-pregnancy cohorts. In three studies (n = 987 patients) reporting on DFS, no difference was observed between patients with and those without a subsequent pregnancy (HR 0.96, 95% CI 0.75-1.24, P = 0.781). In the six studies (n = 3504 patients) reporting on OS, patients with a pregnancy after breast cancer had a statistically significant better OS than those without a pregnancy (HR 0.46, 95% CI 0.27-0.77, P < 0.05).
Conclusions
This systematic review and meta-analysis of retrospective cohort studies provides updated evidence that having a pregnancy in patients with prior history of hormone receptor-positive invasive early breast cancer appears safe without detrimental effect on prognosis.
Key words: pregnancy, breast cancer, hormone receptor-positive disease, premenopausal patients, oncofertility
Highlights
-
•
Many young women with breast cancer wish to complete their families following completion of anticancer therapies.
-
•
Many physicians are concerned that pregnancy after hormone receptor-positive breast cancer may increase recurrences.
-
•
Our meta-analysis showed that pregnancy after hormone receptor-positive breast cancer did not impact prognosis.
-
•
Pregnancy after breast cancer did not impact DFS and was associated with improved OS.
-
•
Pregnancy following diagnosis and treatments of hormone receptor-positive breast cancer should not be discouraged.
Introduction
Breast cancer is the most frequent malignancy diagnosed in women of reproductive age; today, thanks to improvements in breast cancer treatments, patients with previous breast cancer are the largest group of young-onset cancer survivors.1, 2, 3 Hence, survivorship has acquired a crucial role in the care plan of patients with breast cancer.4,5 In young women with childbearing plans, premature ovarian insufficiency (POI) and impaired fertility are potential long-term side-effects of anticancer treatments with a major negative impact on their life after cancer,6,7 and result in relatively few women becoming pregnant after breast cancer.8
Considering the rise in the age at first pregnancy and the growing incidence of young-onset breast cancer in many countries, an increased number of women are diagnosed with breast cancer before completing their childbearing plans.9 Discussion of POI risk and available options to preserve fertility is a key component of the oncofertility counseling for all women diagnosed at reproductive age.5
In recent years, several studies have provided important evidence on the safety of conceiving, naturally or through fertility preservation techniques, in young women following diagnosis and treatment of breast cancer.8,10,11 However, some concerns in special patient populations remain partially unresolved. As an example, patients and physicians continue to have doubts about the safety of pregnancy after hormone receptor-positive early breast cancer. Firstly, these patients are candidates to receive endocrine therapy for up to 5-10 years after diagnosis and having a pregnancy during endocrine therapy is contraindicated. Thus, patients have been advised historically to delay pregnancy until the end of adjuvant endocrine therapy, further reducing the time window for fertility and thus the chances of successful pregnancy.12, 13, 14, 15, 16, 17, 18 Moreover, as breast cancer is a hormone-driven tumor and considering the very high concentrations of female hormones during pregnancy, there is concern that pregnancy might increase the risk of recurrence.19,20 Finally, hormone receptor-positive breast cancer is a tumor subtype characterized by a constant long-term risk of recurrence.21,22 Hence, long-term follow-up is required to fully investigate the safety of pregnancy in these patients.
In recent years, several retrospective cohort studies have tried to address the safety of pregnancy after breast cancer in patients with prior hormone receptor-positive early breast cancer.23,24 The prospective ‘Pregnancy Outcome and Safety of Interrupting Therapy for Women with Endocrine Responsive Breast Cancer (POSITIVE)’ trial has recently reported its first results showing that a temporary interruption of adjuvant endocrine therapy to attempt pregnancy appears to be safe in early follow-up in young breast cancer patients.25 However, findings from POSITIVE are limited by enrollment of women with predominantly stage I or II disease (only 6% had stage III disease) and short-term follow-up (41 months), which is currently too short to capture the long-term risk of recurrence in patients affected by hormone receptor-positive breast cancer.
Thus, concerns remain about a potential detrimental effect of pregnancy in women with prior hormone receptor-positive breast cancer with patients still being counseled against attempting to conceive despite the growing body of evidence for safety.19,26
The present systematic review of the literature and meta-analysis aims to provide updated evidence on the safety of pregnancy following diagnosis and treatment of hormone receptor-positive early breast cancer.
Materials and methods
Search strategy and selection criteria
The present systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.27
A systematic literature search of Medline, Embase and Cochrane library with no language or date restriction up to 31 March 2023 was carried out using the following terms: (“breast neoplasms” [MeSH Terms]) AND ((“pregnancy” [MeSH Terms]) OR (“pregnancies” [MeSH Terms]) OR (“conception” [MeSH Terms]) OR (“gestation” [MeSH Terms])).
Relevant articles were cross-referenced to confirm that all possible pertinent records were identified.
Eligible studies had to satisfy the following criteria: (i) retrospective or prospective case-control or cohort studies and clinical trials comparing survival outcomes of patients with (pregnancy cohort) or without (non-pregnancy cohort) a pregnancy after diagnosis and treatment of hormone receptor-positive early breast cancer; (ii) studies with available information on one or more of the outcomes of interest, i.e. overall survival (OS), disease-free survival (DFS), breast cancer recurrences; (iii) availability or possibility to estimate the hazard ratio (HR) with 95% confidence intervals (CIs).
Exclusion criteria were: (i) case reports and case series including fewer than 10 patients; (ii) studies reporting on breast cancer diagnosed during pregnancy or within 1 year after pregnancy with no data on pregnancies following breast cancer diagnosis; and (iii) ongoing studies with results not presented or published at the time of the literature search.
This work is registered with the PROSPERO registration number CRD42023394232, and the full protocol is available on the PROSPERO website.
Study objectives
The main objective of the analysis was to compare survival outcomes (in terms of OS, DFS and/or breast cancer recurrences) of young women with or without a pregnancy following diagnosis and treatment of hormone receptor-positive invasive early breast cancer.
Data analysis
The systematic literature search was carried out independently by two authors (LA and MML), and any discrepancies were solved by discussion with a third author (EB).
The following variables were extracted independently by two authors (LA and MML) from all included studies, if available: first author, year of publication, study design and methodology, number of women included in each cohort, number of women with a pregnancy after hormone receptor-positive breast cancer; survival outcomes; matching criteria for choosing controls or controlling factors. For studies with more than one publication, only the most updated was included.
Risk of bias assessment
Quality assessment and risk of bias were carried out using the Newcastle–Ottawa Assessment Scale (NOS).28 The NOS assigns a maximum of 9 points according to three risks of bias domain for case-control or cohort study. Studies were classified as follows: low, moderate and high risk of bias according to the different score obtained.
Statistical methods
HR and 95% CI were extracted from the included studies. When these measures were not reported but the number of events for each group could be derived, HRs were estimated using the method reported by Watkins and Bennett.29 Studies for which HRs were not available or could not be computed for a specific outcome were excluded from that analysis.
Pooled HRs with their 95% CI were calculated using the random-effects model using the method of DerSimonian and Laird.30 The Higgins I2 index was used to evaluate the quantitative measure of the degree of inconsistency in the results of the included studies.31 Egger’s asymmetry test was used to assess the probability of publication bias.32
Pooled estimates were considered statistically significant with a P value of <0.05 (two-sided).
Sensitivity analyses were conducted to assess whether the pooled estimates were stable or dependent on one single included study.
Statistical analyses were carried out by MB and EB using Stata 13.1 (StataCorp, 2013; Stata Statistical Software: Release 13, StataCorp LP, College Station, TX).
Results
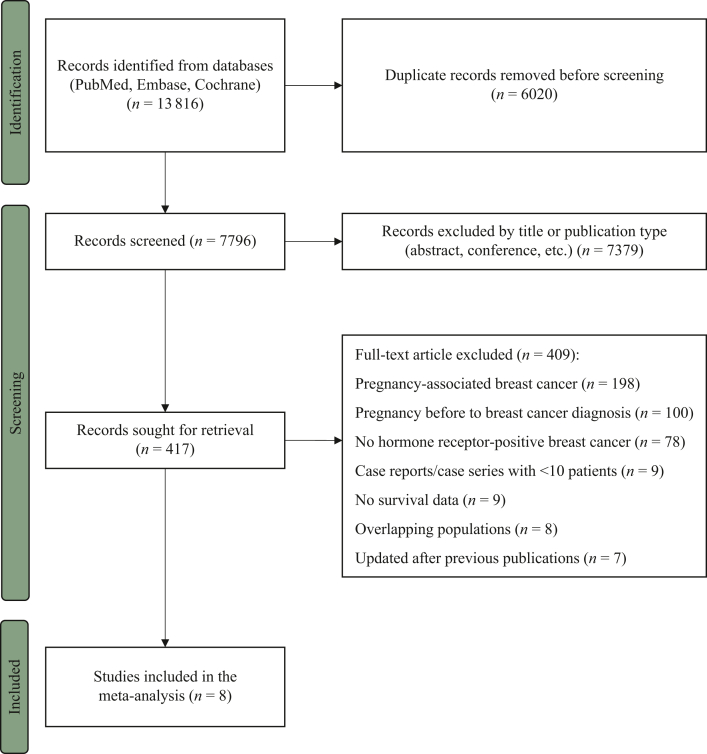
Of the 7796 identified records, after applying all the inclusion and exclusion criteria, 8 studies were included in the meta-analysis (Figure 1).10,23,24,33, 34, 35, 36, 37 A total of 3805 patients with hormone receptor-positive invasive early breast cancer were included in these studies, of whom 1285 had a post-treatment pregnancy and 2520 did not.
Figure 1.
PRISMA flow diagram of study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The main characteristics of the included studies are reported in Table 1. All the included studies were retrospective cohort studies. All the studies reported results correcting for guarantee-time bias.
Table 1.
Main characteristics of the studies included in the meta-analysis
| Reference | Country | Years | Study design | Mean/median age at diagnosis in pregnancy versus no pregnancy cohort | Patients with pregnancy after HR+ BC, n | Patients without pregnancy after BC, n | Mean/median time from diagnosis to pregnancy | Mean/median follow-up time in pregnancy versus no pregnancy cohort | Matching criteria for choosing controls/controlling factors | Outcomes | Risk of biasa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Valentini et al., 201333 | Canada, USA, Europe, Asia | 1985-2010 | Retrospective cohort study | 32.5 versus 33.8 years (not only HR+) | 21 | 66 | Mean: 2.4 years (not only HR+) | 10.2 years (not only HR+) | Age, BRCA mutation, country of residency, date of breast cancer diagnosis, date of completion of baseline questionnaire | 15 years survival rate | High |
| Nye et al., 201734 | USA | 2000-2010 | Retrospective cohort study | 34.2 versus 36.1 years | 32 | 29 | Within 5 years | 9.2 versus 6.5 years | Age, stage at diagnosis | DFS | Moderate |
| Lambertini et al., 201823 | Europe | Before 2007 | Retrospective cohort study | 32.0 versus 35.0 years (not only HR+) | 194 | 492 | Median: 4.7 years (not only HR+) | 9.6 years | ER status, nodal status, adjuvant treatments, age, year of diagnosis | DFS in HR+ (primary) DFS in HR−, OS (secondary) |
Low |
| Lambertini et al., 202010 | World | 2000-2012 | Retrospective cohort study | 31.0 versus 36.0 years (not only HR+) | 60 | 180 | Median: 6.3 years | 8.3 years | Time to pregnancy/DFS event, year at diagnosis, nodal status, hormone receptor status, type of BRCA mutation | Pregnancy rate, DFS (primary) Pregnancy/fetal/obstetric outcomes, OS (secondary) |
Low |
| Chuang et al., 202035 | Taiwan | 2002-2014 | Retrospective cohort study | 31.0 versus 32.3 years (not only HR+) | 87 | 311 | Median: 3.31 years (not only HR+) | 4.3 versus 3.8 years (not only HR+) | Age at diagnosis, year at diagnosis, propensity score for pregnancy, time to pregnancy/DFS event | OS | Moderate |
| Rauh-Hain et al., 202236 | USA | 2000-2012 | Retrospective cohort study | 32 versus 33 years (not only HR+) | 240 | 273 | Median: 2.72 years (not only HR+) | 9.3 years (not only HR+) | Age, year at diagnosis, stage, grade, ER/PgR/HER2 status, CT/RT/surgery, race/ethnicity, median household income, insurance at diagnosis, marital status, Charlson comorbidity score | OS | Moderate |
| Anderson et al., 202224 | Scotland | 1981-2018 | Retrospective cohort study | 31.0 versus 32.0 years (not only HR+) | 102 | 612 | Median: 4.1 years (not only HR+) | 15.8 versus 14.7 years (not only HR+) | Year of diagnosis | OS | Low |
| Bae et al., 202237 | Korea | 2004-2014 | Retrospective cohort study | 32.2 versus 40.0 years (not only HR+) | 549 | 557 | Median: 3.3 years (not only HR+) | 8.2 years | Age at diagnosis, adjuvant ET/CT/RT | OS | High |
CT, chemotherapy; DFS, disease-free survival; ER, estrogen receptors; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR+, hormone receptor-positive breast cancer; OS, overall survival; PgR, progesterone receptors; RT, radiotherapy.
Quality assessment and risk of bias were carried out using the Newcastle–Ottawa Assessment Scale.
In one included study, the entire patient population had hormone receptor-positive breast cancer.34 The other seven studies included patients with all breast cancer subtypes; however, for the purpose of the present meta-analysis, only data deriving from patients with hormone receptor-positive breast cancer were extracted. In two of the included studies, biological characteristics of breast cancer were not reported, and the tumor subtype was established according to the use of adjuvant endocrine therapy.33,37
The mean age at breast cancer diagnosis ranged from 31.0 to 34.2 years in patients with pregnancy after breast cancer and from 32.0 to 40.0 years in patients without a subsequent pregnancy.
Median follow-up time ranged from 4.3 to 15.8 years and from 3.8 to 14.7 years in the pregnancy and non-pregnancy cohorts, respectively.
Information on use of adjuvant endocrine therapy was reported in more than half of the included studies,10,33, 34, 35,37 while only three studies specified the type of administered treatment.10,33,34 In two studies, all patients on adjuvant endocrine therapy received tamoxifen alone,33,34 while in one study 28.9% of patients in the pregnancy cohort received tamoxifen alone, 57.7% tamoxifen in combination with a gonadotropin hormone-releasing hormone agonist (GnRHa) and 7.7% of patients received GnRHa in combination with an aromatase inhibitor.10
The duration of adjuvant endocrine therapy was reported in two studies. In both studies, the duration of adjuvant endocrine treatment was significantly shorter in the pregnancy cohort than in the non-pregnancy cohort (50 versus 60 months, P < 0.001 and 20.9 versus 42.3 months, P = 0.008, respectively).10,34
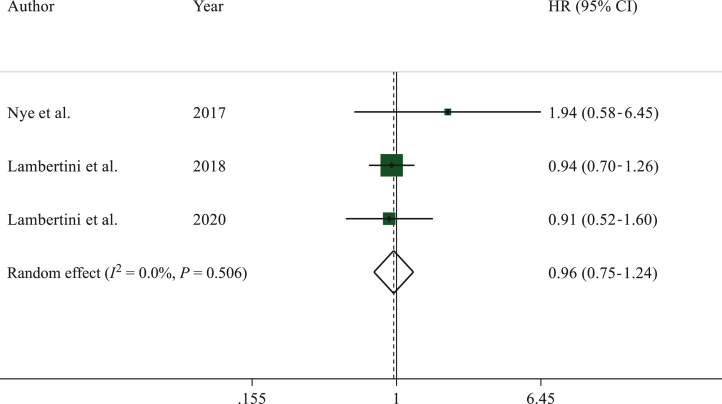
A total of three studies reported DFS.10,23,34 Overall, 987 patients were included in DFS analysis, of whom 286 had a subsequent pregnancy.
No difference in DFS was observed between patients in pregnancy and non-pregnancy cohorts, respectively (HR 0.96, 95% CI 0.75-1.24, P = 0.781) (Figure 2).
Figure 2.
Forest plot describing event-free survival of patients who had a pregnancy after hormone receptor-positive breast cancer as compared to the non-pregnancy cohort.
CI, confidence interval; HR, hazard ratio.
No heterogeneity (I2 = 0.0%, P = 0.506) or publication bias (P = 0.406) were observed. Sensitivity analyses are reported in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102031.
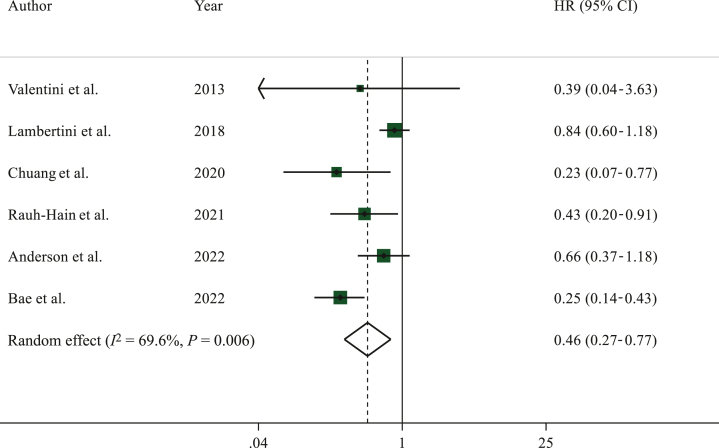
Data on OS were reported in six studies.23,24,33,35, 36, 37 Among 3504 patients included in this analysis, 1193 had a subsequent pregnancy.
Patients in the pregnancy cohort had better OS than those in the non-pregnancy cohort (HR 0.46, 95% CI 0.27-0.77, P < 0.005) (Figure 3).
Figure 3.
Forest plot describing overall survival of patients who had a pregnancy after hormone receptor-positive breast cancer as compared to the non-pregnancy cohort.
CI, confidence interval; HR, hazard ratio.
Significant heterogeneity was observed in this analysis (I2 = 69.6%, P = 0.006) with no publication bias (P = 0.259). Sensitivity analyses are reported in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102031.
Discussion
The present systematic review of the literature and meta-analysis including 8 studies and a total of 3805 young patients showed that pregnancy in women with previous diagnosis and treatment of hormone receptor-positive breast cancer can be considered safe. There was no detrimental effect in terms of DFS (HR 0.96, 95% CI 0.75-1.24, P = 0.781) and better OS (HR 0.46, 95% CI 0.27-0.77, P < 0.005) in patients with a subsequent pregnancy as compared to those who did not conceive following diagnosis and treatment of hormone receptor-positive breast cancer.
Previous data on the safety of pregnancy in the specific group of patients with hormone receptor-positive breast cancer derived from a meta-analysis that included only two studies.8 Compared to previous results, the present meta-analysis provides updated data by including six additional studies with a much larger cohort of patients with hormone receptor-positive breast cancer. The sensitivity analyses for both DFS and OS confirmed the consistency of the overall findings, indicating that no individual study had significantly influenced the overall results.
One of the main aspects to be considered in the oncofertility counseling of patients with hormone receptor-positive breast cancer is the management of adjuvant endocrine therapy, during which pregnancy is contraindicated. Only half of the included studies reported details on the adjuvant endocrine therapy and two studies on the duration of the treatment.
In the studies by Nye et al. and Lambertini et al., reporting on the duration of endocrine therapy, patients in the pregnancy cohort had a significantly shorter exposure to adjuvant endocrine therapy than those in the non-pregnancy cohort.10,34 Median time from breast cancer diagnosis to subsequent pregnancy in patients with hormone receptor-positive breast cancer was 6.3 years in the study reporting this information.10
Historically, patients with breast cancer who wished to conceive were counseled to wait for at least 2 years from the time of breast cancer diagnosis.38 This recommendation derived from observational retrospective studies based on the risk of tumor recurrence being higher during the first 2 years after diagnosis; this is also the time window required to recover from chemotherapy-induced ovarian toxicity.39 Specifically in the cohort of patients with hormone receptor-positive breast cancer, counseling has been historically to wait until the completion of adjuvant endocrine therapy, and thus delaying the pregnancy by at least 5 years, considering the lack of data regarding the safety of a temporarily discontinuation of treatment to attempt a pregnancy and the benefit deriving from adjuvant endocrine therapy, particularly in patients at higher risk of recurrence who are candidates to receive also ovarian function suppression for 5 years.38,40
Today, results from the prospective POSITIVE trial (ClinicalTrials.gov identifier: NCT02308085) have become available.25 POSITIVE is the first prospective clinical trial assessing the safety of a temporary interruption of adjuvant endocrine therapy to attempt pregnancy in 518 young patients (<42 years) after hormone receptor-positive early breast cancer. Patients enrolled had received at least 18-30 months of adjuvant endocrine therapy before attempting pregnancy. From this first analysis, breast cancer-free interval (BCFI) of the patients in the POSITIVE trial did not differ significantly from the BCFI in a cohort of patients derived from the SOFT and TEXT trials (HR 0.81, 95% CI 0.57-1.15).25 The 3-year incidence of breast cancer events was 8.9% (95% CI 6.3% to 11.6%) and 9.2% (95% CI 7.6% to 10.8%) in the treatment-interruption group and control cohort, respectively. Importantly, despite the reassuring first data of the POSITIVE trial, the follow-up duration (median of 41 months) of that study is relatively short to capture late recurrences, which can occur in patients with hormone receptor-positive disease even after more than 10 years from diagnosis.41,42 In our analysis, the included studies had a median follow-up in the pregnancy cohort of 4.3-15.8 years thus allowing to capture also some late recurrences.
Another important strength of our analysis is that all the included studies corrected for the potential ‘healthy mother effect’. The ‘healthy mother effect’ has led to the hypothesis over the years that only women who feel healthy give birth and those with poorer prognosis would not try to conceive. For this reason, more recent studies have generally reported analyses that take this effect into account and correct for the guarantee-time bias. The guarantee-time bias exists whenever an analysis that is timed from random assignment or enrollment (i.e. DFS or OS) is compared across groups defined by a classifying event occurring during follow-up.43
In our work, all studies corrected for this potential risk of bias, strengthening the overall result of the meta-analysis that pregnancy after diagnosis may not only be safe but also potentially confer a survival benefit.44,45
Regarding the potential adverse effects of the hormonal environment during pregnancy, it can be questioned whether high levels of estrogens, progesterone and human gonadotropin during pregnancy are in fact adverse as they can induce apoptosis in breast cancer cells expressing hormone receptors, and have been used to treat breast cancer historically.46 Additionally, some studies have suggested that maternal immunity is stimulated against cancer cells during pregnancy in the so-called ‘fetal antigen hypothesis’.47 All these hypotheses seem to be in line with the results of our analysis that having a pregnancy after hormone receptor-positive breast cancer appears to be protective in terms of OS.
There are several limitations in our study that should be considered in interpreting the results. All data in this meta-analysis derived from retrospective, observational studies, which had different matching criteria. This may explain the heterogeneity observed in one of the analyses. Moreover, data extracted to investigate the safety of pregnancy in the specific cohort of women with hormone receptor-positive breast cancer derived mostly from a subgroup of patients within larger cohort studies including women affected by all breast cancer subtypes. This is the main reason why baseline characteristics of patients with hormone receptor-positive disease were often not separately reported, nor the type and duration of adjuvant endocrine therapy were specified.
Moreover, other planned analyses such as evaluating the safety of pregnancy after hormone receptor-positive breast cancer according to the time of pregnancy after breast cancer, stage at diagnosis, type of administered adjuvant hormone therapy and germline BRCA status could not be carried out due to lack of available data from the collected studies.
Additional research efforts are still needed in this area. Longer follow-up of the POSITIVE trial will be critical to provide evidence on the safety of a temporary interruption of adjuvant endocrine therapy in patients with hormone receptor-positive early breast cancer.48 Moreover, there is lack of evidence on the safety of having multiple pregnancies following treatment completion and on conceiving after prior history of breast cancer diagnosed during pregnancy. Limited data so far have been reported on the safety of pregnancy obtained through assisted reproductive technologies in the specific cohort of patients with hormone receptor-positive breast cancer.11
Conclusions
The present systematic review and meta-analysis of retrospective cohort studies showed that having a pregnancy in patients with prior history of hormone receptor-positive early breast cancer appears safe without detrimental effect on prognosis.
Following adequate treatment and follow-up, pregnancy should not be discouraged in this patient population.
Acknowledgments
Funding
This work was partially supported by the Italian Association for Cancer Research (‘Associazione Italiana per la Ricerca sul Cancro’, AIRC) [grant number MFAG 2020 ID 24698] and by Italian Ministry of Health—5 x 1000 funds (years 2021-2022).
Disclosure
EB reports research support (to the institution) from Gilead outside the submitted work. EM reports honoraria from Roche, Sevie, Novartis. IVL reports honoraria from AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst), Novartis (Inst), Sandoz (Inst); research funding from Resilience (Inst) and travel support from Novartis outside the submitted work. Executive board member BIG. CS reports grants from AstraZéneca, Daiichi Sankyo, Eisai Europe Ltd., Gilead, Novartis, Pharmalex, Pfizer Inc, Phillips Health Works, Pierre Fabre, PintPharma, Puma Biotechnology Inc, Roche Farma, SA Seagen Int, Solti, Synthon Biopharmaceuticals, Zymeworks; consulting fees from AstraZéneca, Daiichi Sankyo, Eisai Europe Ltd., Gilead, Novartis, Pharmalex, Pfizer Inc, Phillips Health Works, Pierre Fabre, PintPharma, Puma Biotechnology Inc, Roche Farma, SA Seagen Int., Solti, Synthon Biopharmaceuticals, Zymeworks; honoraria from AstraZéneca, Daiichi Sankyo, Eisai Europe Ltd., Gilead, Novartis, Pharmalex, Pfizer Inc, Phillips Health Works, Pierre Fabre, PintPharma, Puma Biotechnology Inc, Roche Farma, SA Seagen Int., Solti, Synthon Biopharmaceuticals, Zymeworks. RAA reports personal fees and non-financial support from Roche Diagnostics, personal fees from Ferring Pharmaceuticals, IBSA and Merck Serono, outside the submitted work. ID reports trial grant and reagents (to the institution) from Roche Diagnostics; academic trial grant from Ferring; speaker’s honoraria from Novartis; support for attending meetings from Ferring and Theramex. EdA reports financial honoraria and/or advisory board from Roche/GNE, Novartis, SeaGen, Zodiac, Libbs, Pierre Fabre, Lilly, Astra-Zeneca, MSD, Gilead Sciences. Travel grants from Roche/GNE and Astra-Zeneca. Research grant to my institution from Roche/GNE, Astra-Zeneca, GSK/Novartis, Gilead Sciences. Non-financial: ESMO director of membership 2023-2024; BSMO President 2023-2026. LDM reports grants from Eli lilly, Novartis, Roche, Daiichi Sankyo, Seagen, Astrazeneca, Gilead, Pierre Fabre; consulting fees from Eli lilly, Gilead, Daiichi Sankyo; honoraria from Roche, Novartis, Pfizer, Eli lilly, Astrazeneca, MSD, Seagen, Gilead, Pierre Fabre, Eisai, Exact Sciences, Ipsen, GSK, Agendia, Stemline. AHP reports research grants from Breast Cancer Research Foundation, Susan G. Komen, US NIH/NCI, PCORI; royalties from UpToDate. ML reports advisory role for Roche, Lilly, Novartis, Astrazeneca, Pfizer, Seagen, Gilead, MSD and Exact Sciences, speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo and Takeda, travel grants from Gilead and Daiichi Sankyo, and research support (to the institution) from Gilead outside the submitted work. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Fidler M.M., Gupta S., Soerjomataram I., Ferlay J., Steliarova-Foucher E., Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579–1589. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Fidler-Benaoudia M., Keegan T.H., Hipp H.S., Jemal A., Siegel R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro C.L. Cancer survivorship. N Engl J Med. 2018;379(25):2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
- 5.Lambertini M., Peccatori F.A., Demeestere I., et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Arecco L., Perachino M., Damassi A., et al. Burning questions in the oncofertility counseling of young breast cancer patients. Breast Cancer (Auckl) 2020;14 doi: 10.1177/1178223420954179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondeaux E., Massarotti C., Fontana V., et al. The PREgnancy and FERtility (PREFER) study investigating the need for ovarian function and/or fertility preservation strategies in premenopausal women with early breast cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.690320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambertini M., Blondeaux E., Bruzzone M., et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2021;39:3293–3305. doi: 10.1200/JCO.21.00535. [DOI] [PubMed] [Google Scholar]
- 9.Ely D.M., Hamilton B.E. Trends in fertility and mother’s age at first birth among rural and metropolitan counties: United States, 2007-2017. NCHS Data Brief. 2018;(323):1–8. [PubMed] [Google Scholar]
- 10.Lambertini M., Ameye L., Hamy A.S., et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J Clin Oncol. 2020;38(26):3012–3023. doi: 10.1200/JCO.19.02399. [DOI] [PubMed] [Google Scholar]
- 11.Arecco L., Blondeaux E., Bruzzone M., et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum Reprod. 2022;37(5):954–968. doi: 10.1093/humrep/deac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddy K.J., Gelber S.I., Tamimi R.M., et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–1156. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein H.J., Lacchetti C., Anderson H., et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol. 2016;34(14):1689–1701. doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 14.Lambertini M., Goldrat O., Clatot F., Demeestere I., Awada A. Controversies about fertility and pregnancy issues in young breast cancer patients: current state of the art. Curr Opin Oncol. 2017;29(4):243–252. doi: 10.1097/CCO.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 15.Shandley L.M., Spencer J.B., Fothergill A., et al. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril. 2017;107(1):243–252.e5. doi: 10.1016/j.fertnstert.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambertini M., Fontana V., Massarotti C., et al. Prospective study to optimize care and improve knowledge on ovarian function and/or fertility preservation in young breast cancer patients: results of the pilot phase of the PREgnancy and FERtility (PREFER) study. Breast. 2018;41:51–56. doi: 10.1016/j.breast.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Buonomo B., Brunello A., Noli S., et al. Tamoxifen exposure during pregnancy: a systematic review and three more cases. Breast Care. 2020;15:148–156. doi: 10.1159/000501473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paluch-Shimon S., Cardoso F., Partridge A.H., et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4) Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 19.Lambertini M., Di Maio M., Pagani O., et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. 2018;42:41–49. doi: 10.1016/j.breast.2018.08.099. [DOI] [PubMed] [Google Scholar]
- 20.Razeti M.G., Spinaci S., Spagnolo F., Massarotti C., Lambertini M. How I perform fertility preservation in breast cancer patients. ESMO Open. 2021;6(3) doi: 10.1016/j.esmoop.2021.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzger-Filho O., Sun Z., Viale G., et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31(25):3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambertini M., Campbell C., Gelber R.D., et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res Treat. 2019;177(1):103–114. doi: 10.1007/s10549-019-05284-y. [DOI] [PubMed] [Google Scholar]
- 23.Lambertini M., Kroman N., Ameye L., et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110(4):426–429. doi: 10.1093/jnci/djx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson R.A., Lambertini M., Hall P.S., Wallace W.H., Morrison D.S., Kelsey T.W. Survival after breast cancer in women with a subsequent live birth: influence of age at diagnosis and interval to subsequent pregnancy. Eur J Cancer. 2022;173:113–122. doi: 10.1016/j.ejca.2022.06.048. [DOI] [PubMed] [Google Scholar]
- 25.Partridge A.H., Niman S.M., Ruggeri M., et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388(18):1645–1656. doi: 10.1056/NEJMoa2212856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senkus E., Gomez H., Dirix L., et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3-98: fertility attitudes in young patients with breast cancer. Psychooncology. 2014;23(2):173–182. doi: 10.1002/pon.3384. [DOI] [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
- 28.Wells G.A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at.
- 29.Watkins C., Bennett I. A simple method for combining binomial counts or proportions with hazard ratios for evidence synthesis of time-to-event data. Res Syn Meth. 2018;9(3):352–360. doi: 10.1002/jrsm.1301. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentini A., Lubinski J., Byrski T., et al. The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res Treat. 2013;142(1):177–185. doi: 10.1007/s10549-013-2729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nye L., Rademaker A., Gradishar W.J. Breast cancer outcomes after diagnosis of hormone-positive breast cancer and subsequent pregnancy in the tamoxifen era. Clin Breast Cancer. 2017;17(4):e185–e189. doi: 10.1016/j.clbc.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang S.C., Lin C.H., Lu Y.S., Hsiung C.A. Mortality of pregnancy following breast cancer diagnoses in Taiwanese women. Oncologist. 2020;25(2):e252–e258. doi: 10.1634/theoncologist.2019-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauh-Hain J.A., Zubizarreta J., Nitecki R., et al. Survival outcomes following pregnancy or assisted reproductive technologies after breast cancer: a population-based study. Cancer. 2022;128(17):3243–3253. doi: 10.1002/cncr.34371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae S.Y., Lee J., Lee J.S., et al. Prognosis of pregnancy after breast cancer diagnosis according to the type of treatment: a population-based study in Korea by the SMARTSHIP group. Breast. 2022;63:46–53. doi: 10.1016/j.breast.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paluch-Shimon S., Cardoso F., Partridge A.H., et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5) Ann Oncol. 2022;33(11):1097–1118. doi: 10.1016/j.annonc.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Azim H.A., Peccatori F.A., de Azambuja E., Piccart M.J. Motherhood after breast cancer: searching for la dolce vita. Expert Rev Anticancer Ther. 2011;11(2):287–298. doi: 10.1586/era.10.208. [DOI] [PubMed] [Google Scholar]
- 40.Sella T., Ruddy K.J., Carey L.A., Partridge A.H. Optimal endocrine therapy in premenopausal women: a pragmatic approach to unanswered questions. JCO Oncol Pract. 2022;18(3):211–216. doi: 10.1200/OP.21.00482. [DOI] [PubMed] [Google Scholar]
- 41.Pan H., Gray R., Braybrooke J., et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen R.N., Esen B.Ö., Mellemkjær L., et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. 2022;114(3):391–399. doi: 10.1093/jnci/djab202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giobbie-Hurder A., Gelber R.D., Regan M.M. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sankila R., Heinävaara S., Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: “healthy mother effect”. Am J Obstet Gynecol. 1994;170(3):818–823. doi: 10.1016/s0002-9378(94)70290-x. [DOI] [PubMed] [Google Scholar]
- 45.Valachis A., Tsali L., Pesce L.L., et al. Safety of pregnancy after primary breast carcinoma in young women: a meta-analysis to overcome bias of healthy mother effect studies. Obstet Gynecol Surv. 2010;65(12):786–793. doi: 10.1097/OGX.0b013e31821285bf. [DOI] [PubMed] [Google Scholar]
- 46.Rajkumar L., Guzman R.C., Yang J., Thordarson G., Talamantes F., Nandi S. Prevention of mammary carcinogenesis by short-term estrogen and progestin treatments. Breast Cancer Res. 2004;6(1):R31–R37. doi: 10.1186/bcr734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janerich D.T. The fetal antigen hypothesis: cancers and beyond. Med Hypotheses. 2001;56(1):101–103. doi: 10.1054/mehy.2000.1119. [DOI] [PubMed] [Google Scholar]
- 48.Arecco L., Lambertini M. Safety of interrupting adjuvant endocrine therapy to conceive: early data are POSITIVE. Nat Rev Clin Oncol. 2023;20:662–663. doi: 10.1038/s41571-023-00797-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.