Abstract
Background
Immune checkpoint inhibitors (ICIs) are indicated for various cancers and are the mainstay of cancer immunotherapy. They are often associated with ICI-related pneumonitis (CIP), however, hindering a favorable clinical course. Recently, non-oncology concomitant drugs have been reported to affect the efficacy and toxicity of ICIs; however, the association between these drugs and the risk for CIP is uncertain. The aim of this study was to assess the impact of baseline concomitant drugs on CIP incidence in ICI-treated advanced cancer patients.
Patients and methods
This was a single-center retrospective study that included a cohort of 511 patients with advanced cancer (melanoma and non-small-cell lung, head and neck, genitourinary, and other types of cancer) treated with ICIs. Univariable analysis was conducted to identify baseline co-medications associated with CIP incidence. A propensity score matching analysis was used to adjust for potential CIP risk factors, and multivariable analysis was carried out to assess the impact of the identified co-medications on CIP risk.
Results
Forty-seven (9.2%) patients developed CIP. In these patients, the organizing pneumonia pattern was the dominant radiological phenotype, and 42.6% had grade ≥3 CIP, including one patient with grade 5. Of the investigated baseline co-medications, the proportion of antiplatelet drugs (n = 50, 9.8%) was higher in patients with CIP (23.4% versus 8.4%). After propensity score matching, the CIP incidence was higher in patients with baseline antiplatelet drugs (22% versus 6%). Finally, baseline antiplatelet drug use was demonstrated to increase the risk for CIP incidence regardless of cancer type (hazard ratio, 3.46; 95% confidence interval 1.21-9.86).
Conclusions
An association between concomitant antiplatelet drug use at baseline and an increased risk for CIP was seen in our database. This implies the importance of assessing concomitant medications for CIP risk management.
Key words: advanced cancer, antiplatelet drug, immune checkpoint inhibitor, pneumonitis, propensity score matching
Highlights
-
•
We studied the association between baseline drugs and CIP in ICI-treated advanced cancer patients with multiple cancer types.
-
•
Baseline antiplatelet drugs were associated with an increased risk for CIP incidence (HR, 3.46; 95% CI 1.21-9.86).
-
•
This study suggested the importance of evaluating concomitant drugs for CIP risk stratification in ICI therapy.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer chemotherapy in recent years. The first anti-cytotoxic T-lymphocyte-associated antigen-4 antibody, ipilimumab, was approved for metastatic melanomas in 2011, followed by the launch of novel anti-programmed death-1 and anti-programmed death-ligand 1 (PD-L1) antibodies for various cancer types.1 To date, ICIs are commonly used in multiple clinical settings because of less toxicity than conventional cytotoxic chemotherapeutic agents and their evident efficacies. However, ICIs do induce a specific and annoying toxicity of this drug class, an immune-related adverse event (irAE) in various organs.2
Among irAEs, ICI-related pneumonitis (CIP) is symptomatic immune-mediated lung toxicity associated with ICI administration, which is of clinical significance. CIP is a relatively common irAE, developing in ∼10% of ICI-treated patients in the real-world clinical setting.3 CIP often disrupts the favorable clinical course of patients. Severe CIP (grade ≥3) is reported in 40% of all CIP cases,4,5 which requires immediate ICI discontinuation and systemic anti-inflammatory treatment, thus rendering patients unable to receive anticancer drug therapy. Indeed, several studies have shown that the development of CIP is associated with poor survival outcomes in patients with ICI-treated non-small-cell lung cancer (NSCLC).6,7 Thus, CIP risk stratification before ICI induction is essential in improving a patient’s prognosis.
Recently, there has been growing clinical interest in the relationship between baseline concomitant drugs and irAEs. Concomitant use of several non-oncology drugs with ICIs is reported to increase the risk for specific irAEs. For example, proton pump inhibitors (PPIs),8 antibiotics,9 and non-steroidal anti-inflammatory drugs (NSAIDs)10 are reported to be associated with an increased risk for developing ICI-related colitis and acute kidney injuries.11,12 These etiologies might be correlated with mechanisms beyond conventional pharmacologic interactions in combination with ICIs. They could be better explained by immune modulation within the tumor microenvironment through the involvement of the peripheral immune response, alteration of the gut microbiota, and drug-specific aggressiveness to target organs.13, 14, 15 The association between baseline concomitant drugs and CIP incidence has rarely been investigated, although any drug can potentially induce inherent pulmonary toxicity. Currently, known CIP risk factors are mostly restricted to factors related to deteriorating lung conditions, such as age,16 sex,17 smoking history,18 baseline underlying lung diseases,19, 20, 21, 22 and history of thoracic radiotherapy (TRT).18,23 Therefore, providing information about the impact of baseline drugs on the risk of CIP incidence would be beneficial.
We conducted this retrospective observational study to address the unexplored relationship between baseline concomitant drugs and CIP incidence. In this study, we aimed to examine whether non-oncology concomitant drugs commonly prescribed for comorbidities and toxicities of anticancer therapy affect the risk of CIP incidence in patients with advanced cancer treated with ICIs.
Material and methods
Patients and settings
This study was approved by the Institutional Review Board of Shinshu University School of Medicine (approval number: 5550). The patient informed consent was waived off because this was a retrospective observational study. Instead, an opt-out document for this study has been posted on the website of Shinshu University School of Medicine. We retrospectively reviewed cancer patients treated with ICIs at Shinshu University Hospital (700 beds; Nagano, Japan) between 30 April 2014 and 30 October 2021. To determine a minimum observation period of at least 6 months, the data cut-off was set on 30 June 2022. The ICI-treated patients’ list was extracted from the pharmacy registry database in our hospital. The eligibility criteria were defined as follows: (i) receiving at least one cycle of ICIs, including not only monotherapy but also combinatorial treatment; (ii) receiving ICIs for advanced or recurrent disease (i.e. excluding those receiving ICIs as adjuvant therapy); (iii) patients who underwent ICI therapy in our hospital and for whom baseline (defined within 2 weeks before ICI induction) clinical information was available; and (iv) patients who did not receive TRT within 3 months of ICI therapy. A total of 511 patients were eligible for the analysis (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.102030); 464 had not developed CIP (defined as the ‘non-CIP group’), and 47 had developed CIP (defined as the ‘CIP group’) in their clinical course.
Data collection
All baseline and subsequent clinical data were extracted from patients’ electronic medical records. Along with the demographic data, information on comorbidities (i.e. hypertension, dyslipidemia, diabetes, coronary artery disease, cerebrovascular disease, and peripheral vascular disease) and concomitant non-oncology drugs at baseline (i.e. angiotensin receptor blocker/angiotensin-converting enzyme inhibitor, hypoglycemic, antilipidemic, PPI, NSAIDs, antibiotics, antiplatelet drugs, and oral corticosteroids) was collected.
Radiological assessment
The findings of chest computed tomography (CT) scans were reviewed by two pulmonologists (MK and KS) and one radiologist (SK), who were blinded to the patient’s background, clinical course, and outcomes. CT images were reconstructed with a slice thickness of <2.5 mm. The findings of baseline emphysema and fibrosis (interstitial changes) were evaluated from baseline CT images. In addition, CT phenotypes of CIP were assessed according to the Japanese Respiratory Society classification of drug-induced lung injuries, as follows: organizing pneumonia (OP-like; Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2023.102030), diffuse alveolar damage (DAD; Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2023.102030), non-specific interstitial pneumonia (Supplementary Figure S2C, available at https://doi.org/10.1016/j.esmoop.2023.102030), hypersensitivity pneumonia (Supplementary Figure S2D, available at https://doi.org/10.1016/j.esmoop.2023.102030), or acute eosinophilic pneumonia (Supplementary Figure S2E, available at https://doi.org/10.1016/j.esmoop.2023.102030); those who could not be categorized into these categories were classified as not otherwise specified.24 The decision was made through a comprehensive discussion in case of conflicting diagnostic opinions for baseline lung abnormalities and CT phenotypes.
Toxicity evaluation
The toxicity grades of irAEs were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events.25 CIP was defined as a chest radiological abnormality based on CT scans that newly appeared after ICI administration. Pneumonitis events that developed during other anticancer drug therapy following ICIs were not included in CIP events. The medical record-based CIP diagnosis was confirmed when other causes, such as pulmonary infection or cardiogenic pulmonary edema, could be excluded by a review. Two researchers (TA and KT) reviewed and confirmed irAEs based on previous diagnoses in the medical records; for CIP, only its development and grading were evaluated, not radiological findings (described in ‘Radiological assessment’).
Statistical analyses
The first objective of this study was to identify factors associated with CIP incidence, encompassing baseline co-medications as well as other clinical factors. For survival time analyses, mortality events before CIP incidence were considered competing risks. Univariable logistic regression analyses were carried out to identify factors associated with CIP incidence. The identified risk factors and clinically important factors for CIP assessment were included as explanatory variables in a multivariable analysis. Multivariable logistic regression analysis was used to estimate the odds ratios (ORs) with a 95% confidence interval (CI) for these factors affecting CIP incidence. A Fine-Gray type regression analysis was used to estimate the hazard ratios (HRs) with a 95% CI for these factors affecting CIP incidence.
The second objective was to estimate the effect of a baseline co-medication identified as a risk factor for CIP incidence. To adjust for potential confounders, we carried out a propensity score matching (PSM) analysis in a 2 : 1 ratio using a standardized deviation width of 0.20 for the logit transformation of the estimated propensity score. The following factors that are considered clinically important and reported to be associated with CIP risk according to previous reports16, 17, 18, 19, 20, 21, 22, 23,26 were included as covariates in the PSM: age, sex, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), findings of pulmonary emphysema and fibrosis on baseline chest CT, prior TRT history, NSCLC patients, and receiving ICI plus platinum chemotherapy combination. A Fine-Gray type regression analysis in the matched cohort was further conducted to assess the risk for CIP incidence.
The time to CIP incidence was determined from ICI induction to CIP onset or censored observation at the last follow-up. The cumulative incidence for CIP was estimated using the cumulative incidence function method considering competing risk, and intergroup comparison was conducted using Gray’s test. All statistical analyses were carried out using the EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Statistical significance was set at P < 0.05 (two-sided).27
Results
Patient characteristics
The patients’ characteristics of the non-CIP and CIP groups are presented in Table 1. The total cohort comprised 375 male (73.4%) and 136 female (26.6%) patients with NSCLCs (35.6%), melanomas (18.1%), head and neck cancers (HNCs; 14.3%), or genitourinary cancers (GCs; 13.9%). Among them, 199 (38.9%) developed irAEs, and 47 (9.2%) developed CIP (information on any irAEs is presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102030). The proportion of patients with poor ECOG-PS (≥2) was lower in the CIP group than in the non-CIP group (4.3% versus 15.1%). Among the baseline concomitant drugs, the frequency of antiplatelet drug use was higher in the CIP group than in the non-CIP group (23.4% versus 8.4%). The frequency of pulmonary fibrosis at baseline CT (23.4% versus 11.6%) and TRT history (17.0% versus 7.1%) was also higher in the CIP group than in the non-CIP group. The relationship between any irAE and each baseline drug is presented in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102030. The irAE incidence was lower among PPI users (42% versus 29.1%). The incidence of total irAEs between antiplatelet drug users and non-users was comparable (9.6% versus 10.1%).
Table 1.
Patients’ characteristics
| Characteristics, n (%) | Total n = 511 | Non-CIP n = 464 | CIP n = 47 |
|---|---|---|---|
| Median age, years (IQR) | 70 (63-76) | 70 (62.75-76) | 71 (66-76) |
| Sex | |||
| Male | 375 (73.4) | 337 (72.6) | 38 (80.9) |
| Female | 136 (26.6) | 127 (27.4) | 9 (19.1) |
| Smoking history, yes | 339 (66.3) | 304 (65.5) | 35 (74.5) |
| ECOG-PS | |||
| 0 | 184 (36.0) | 160 (34.5) | 24 (51.1) |
| 1 | 255 (49.9) | 234 (50.4) | 21 (44.7) |
| ≥2 | 72 (14.1) | 70 (15.1) | 2 (4.3) |
| Cancer type | |||
| Non-small-cell lung cancer | 182 (35.6) | 164 (35.3) | 18 (38.3) |
| Melanoma | 93 (18.1) | 81 (17.5) | 12 (25.5) |
| Head and neck cancer | 73 (14.3) | 69 (14.9) | 4 (8.5) |
| Genitourinary cancera | 71 (13.9) | 65 (14.0) | 6 (12.8) |
| Other types of cancerb | 92 (18.0) | 85 (18.3) | 7 (14.9) |
| Comorbidity | |||
| Hypertension | 208 (40.7) | 187 (40.3) | 21 (44.7) |
| Diabetes | 81 (15.9) | 75 (16.2) | 6 (12.8) |
| Dyslipidemia | 85 (16.6) | 76 (16.4) | 9 (19.2) |
| Cerebrovascular disease | 37 (7.2) | 33 (7.1) | 4 (8.5) |
| Coronary artery disease | 24 (4.7) | 21 (4.5) | 3 (6.4) |
| Peripheral vascular disease | 14 (2.7) | 12 (2.6) | 2 (4.3) |
| Baseline concomitant drug | |||
| ARB/ACE-I | 116 (22.7) | 105 (22.6) | 11 (23.4) |
| Hypoglycemic drug | 56 (11.0) | 52 (11.2) | 4 (8.5) |
| Antilipidemic drug | 94 (18.4) | 84 (18.1) | 10 (21.3) |
| Proton pump inhibitor | 189 (37.0) | 174 (37.5) | 15 (31.9) |
| NSAIDs | 178 (34.8) | 166 (35.8) | 12 (25.5) |
| Antibiotic | 26 (5.1) | 24 (5.2) | 2 (4.3) |
| Antiplatelet drug | 50 (9.8) | 39 (8.4) | 11 (23.4) |
| Oral corticosteroid | 24 (4.7) | 23 (5.0) | 1 (2.1) |
| Baseline radiological information | |||
| Emphysema | 165 (32.3) | 144 (31.0) | 21 (44.7) |
| Fibrosis | 65 (12.7) | 54 (11.6) | 11 (23.4) |
| Prior TRT history | 41 (8.0) | 33 (7.1) | 8 (17.0) |
| ICI therapy | |||
| Monotherapy | 382 (74.8) | 348 (75.0) | 34 (72.3) |
| ICI + platinum chemotherapy | 71 (13.9) | 62 (13.4) | 9 (19.2) |
| ICI + ICI | 31 (6.1) | 27 (5.8) | 4 (8.5) |
| ICI + other anticancer agent | 27 (5.3) | 27 (5.8) | 0 (0) |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CIP, checkpoint inhibitor-related pneumonitis; CT, computed tomography; ECOG-PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; TRT, thoracic radiotherapy.
Genitourinary cancers include urothelial cancers (n = 42) and renal cell cancers (n = 29).
Other cancers include hepatic cell cancer (n = 26), gastric cancer (n = 16), esophageal cancer (n = 14), small-cell lung cancer (n = 9), malignant pleural mesothelioma (n = 9), uterine cancer (n = 4), breast cancer (n = 2), Hodgkin’s lymphoma (n = 2), pancreatic cancer (n = 2), cancer of unknown primary (n = 2), thymic cancer (n = 2), Merkel cell cancer (n = 2), ovarian cancer (n = 1), and cholangiocellular cancer (n = 1).
Characteristics of CIP
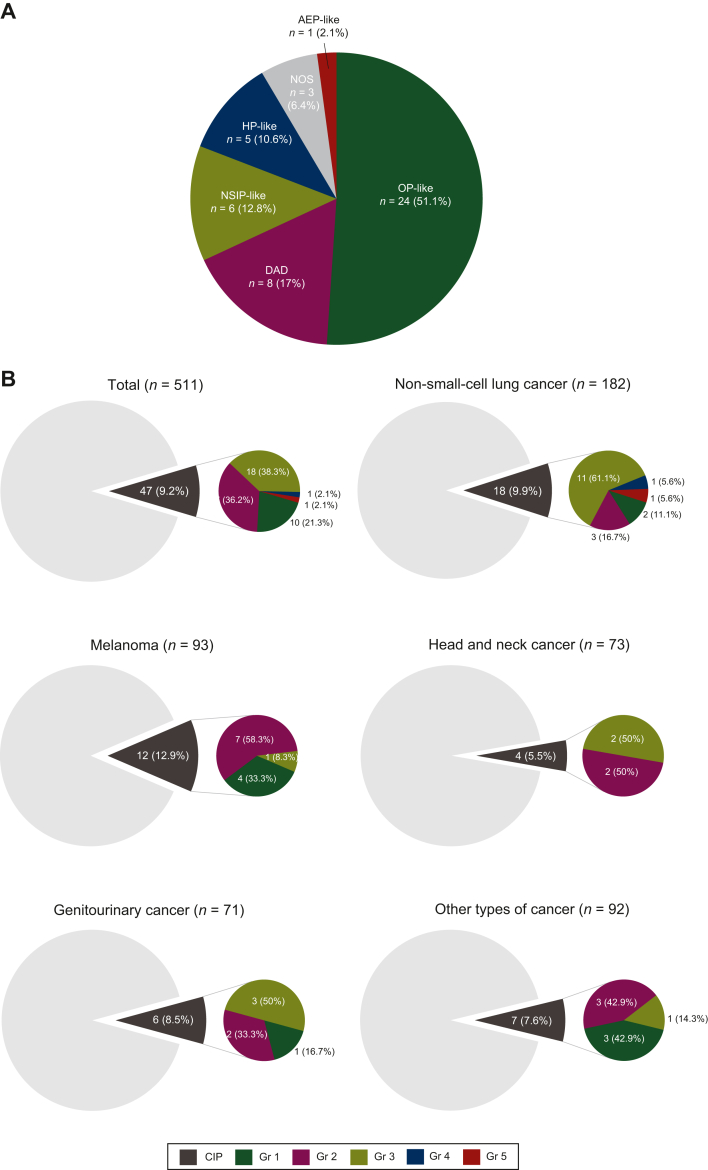
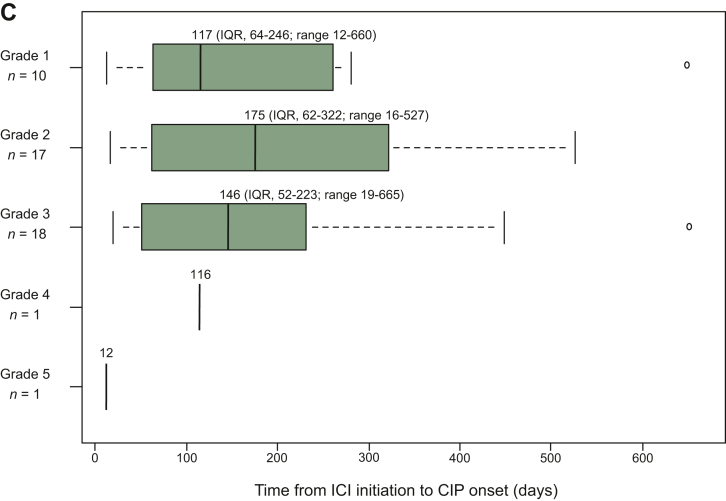
Among patients with CIP (n = 47), OP-like was the most common CT phenotype (51.1%), followed by DAD (17.0%) (Figure 1A), and 42.5% had CIP with a grade ≥3. The incidence of CIP was 9.9%, 12.9%, 5.5%, 8.5%, or 7.6% in patients with NSCLC, melanoma, HNCs, GCs, or other cancers, respectively. In patients with NSCLC, those with grade ≥3 CIP accounted for 72.2% (Figure 1B). The median follow-up period for the total cohort was 335 days (5-3010 days). The median duration from ICI initiation to CIP onset was 146 days (interquartile range, 53-271; range, 12-665), occurring within 6 months in all severity strata (Figure 1C). Three hundred and eight (60.3%) patients received ICIs as second- or later-line therapy, and 63 had a history of tyrosine kinase inhibitor therapy. Of those patients, CIP developed in only two cases. Thirty patients (63.8%) received corticosteroids for CIP. One of the 17 patients who did not receive corticosteroids for CIP died of a stroke immediately after CIP development. One patient with NSCLC died due to CIP (grade 5) despite intravenous methylprednisolone pulse therapy.
Figure 1.
Clinical characteristics of checkpoint inhibitor-related pneumonitis in this study. (A) Description of computed tomography phenotype classification of immune checkpoint inhibitor-related pneumonitis. (B) Frequency and severity of immune checkpoint inhibitor-related pneumonitis per cancer type. (C) Time to the onset and severity of immune checkpoint inhibitor-related pneumonitis per grade. In the box plots, the centerline represents the median, the box limits represent the lower and upper quartile values, and the whiskers extend to the most extreme values within a 1.5 × quartile range. AEP, acute eosinophilic pneumonia; CIP, checkpoint inhibitor-related pneumonitis; DAD, diffuse alveolar damage; Gr, grade; HNCs, head and neck cancers; HP, hypersensitivity pneumonia; ICI, immune checkpoint inhibitor; IQR, interquartile range; NOS, not otherwise specified; NSCLC, non-small cell lung cancer; NSIP, non-specific interstitial pneumonia; OP, organizing pneumonia.
Association of antiplatelet drugs and CIP incidence
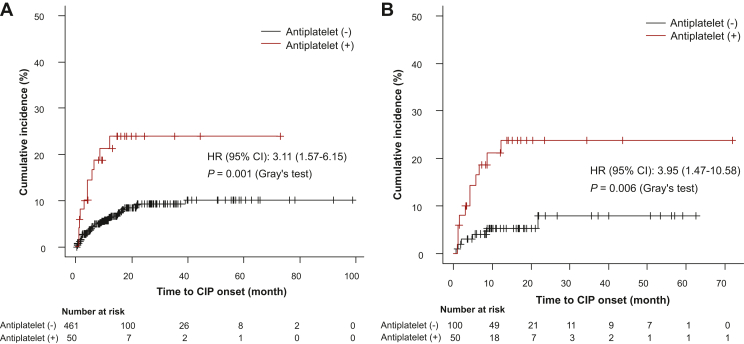
Univariable logistic regression analysis showed that baseline antiplatelet drug use (OR, 3.33; 95% CI 1.57-7.05), pulmonary fibrosis (OR, 2.28; 95% CI 1.10-4.74), and TRT history (OR, 2.68; 95% CI 1.16-6.20) were associated with an increased risk for CIP (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102030). Multivariable logistic regression analysis showed that baseline antiplatelet drug use (OR, 3.18; 95% CI 1.47-6.88; P = 0.003), pulmonary fibrosis (OR, 2.22; 95% CI 1.03-4.78; P = 0.04), and TRT history (OR, 2.50; 95% CI 1.03-6.07, P = 0.04) were independent risk factors for CIP (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.102030). The cumulative incidence for CIP was higher in the patients treated with antiplatelet drugs at baseline than in the patients without (HR, 3.11; 95% CI 1.57-6.15; P = 0.001; Figure 2A). A multivariable Fine-Gray regression analysis showed that the risk for CIP incidence was significantly higher among patients receiving baseline antiplatelet drugs (HR, 2.93; 95% CI 1.45-5.93; P = 0.003) after controlling for ECOG-PS, NSCLC, baseline pulmonary fibrosis, and TRT history (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.102030). The description of baseline antiplatelet drugs and underlying diseases requiring antiplatelet therapy is shown in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2023.102030. Aspirin (n = 26, 5.1%) and clopidogrel (n = 14, 2.7%) were commonly used at baseline. Two patients were receiving two different antiplatelet drugs in combination. The clinical course of CIP patients receiving antiplatelet drugs is described in Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2023.102030. Among the 11 patients, 5 received corticosteroid therapy; all improved, except one with a severe stroke who died shortly after the onset of grade 2 CIP (Case Number 11).
Figure 2.
Cumulative incidence for CIP with or without baseline antiplatelet drugs. (A) Total cohort. (B) Cohort after propensity score matching. CI, confidence interval; CIP, checkpoint inhibitor-related pneumonitis; HR, hazard ratio.
To assess the effect of baseline antiplatelet drugs on CIP incidence, patients not receiving baseline antiplatelet drugs (‘non-antiplatelet group’) and patients receiving baseline antiplatelet drugs (‘antiplatelet group’) were matched by a PSM analysis; consequently, 150 patients were identified. After PSM, the CIP incidence was higher in the ‘antiplatelet group’ than in the ‘non-antiplatelet group’ (22% versus 6%; Table 2). The cumulative incidence for CIP was also higher in the ‘antiplatelet group’ than in the ‘non-antiplatelet group’ (HR, 3.95; 95% CI 1.47-10.58; P = 0.006; Figure 2B). Multivariable analysis with a Fine-Gray regression model showed that the risk for CIP incidence was higher in the ‘antiplatelet group’ (HR, 3.46; 95% CI 1.21-9.86; P = 0.02) after controlling for melanomas and other types of cancer (Table 3).
Table 2.
Patients’ characteristics before and after propensity score matching
| Characteristic, n (%) | Before matchinga (n = 511) |
After matchinga (n = 150) |
||||
|---|---|---|---|---|---|---|
| Non-antiplatelet n = 461 | Antiplatelet n = 50 | SMD | Non-antiplatelet n = 100 | Antiplatelet n = 50 | SMD | |
| Age ≥70 years | 226 (49) | 40 (80) | 0.70 | 82 (82) | 40 (80) | 0.05 |
| Male | 332 (72) | 43 (86) | 0.35 | 83 (83) | 43 (86) | 0.08 |
| Female | 129 (28) | 7 (14) | 17 (17) | 7 (14) | ||
| Smoking history | 297 (64.4) | 42 (84) | 0.46 | 82 (82) | 42 (84) | 0.05 |
| ECOS-PS ≥2 | 70 (15.2) | 2 (4) | 0.39 | 4 (4) | 2 (4) | <0.001 |
| Non-small-cell lung cancer | 163 (35.4) | 19 (38) | 0.06 | 36 (36) | 19 (38) | 0.04 |
| Melanoma | 78 (16.9) | 15 (30) | 0.31 | 17 (17) | 15 (30) | 0.31 |
| Head and neck cancer | 67 (14.5) | 6 (12) | 0.08 | 14 (14) | 6 (12) | 0.06 |
| Genitourinary cancer | 67 (14.5) | 4 (8) | 0.21 | 12 (12) | 4 (8) | 0.13 |
| Other types of cancer | 86 (18.7) | 6 (12) | 0.19 | 21 (21) | 6 (12) | 0.24 |
| Emphysema at baseline | 143 (31.5) | 22 (44) | 0.26 | 44 (44) | 22 (44) | <0.001 |
| Fibrosis at baseline | 57 (12.6) | 8 (16) | 0.07 | 13 (13) | 8 (16) | 0.09 |
| Prior TRT history | 39 (8.5) | 2 (4) | 0.19 | 4 (4) | 2 (4) | <0.001 |
| ICI + platinum chemotherapy | 63 (13.7) | 8 (16) | 0.07 | 15 (15) | 8 (16) | 0.03 |
| CIP incidence | 36 (7.8) | 11 (22) | 0.41 | 6 (6) | 11 (22) | 0.47 |
CIP, checkpoint inhibitor-related pneumonitis.; ECOG-PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; SMD, standardized mean difference; TRT, thoracic radiotherapy.
Matched (2 : 1) with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score; matched variables: age, sex, smoking history, Eastern Cooperative Oncology Group performance status, non-small cell lung cancer, baseline emphysema, baseline fibrosis, prior thoracic radiotherapy, and immune checkpoint inhibitor plus platinum chemotherapy.
Table 3.
Multivariable analysis for time to checkpoint inhibitor-related pneumonitis event with Fine-Gray competing risk modela after propensity score matching analysisb
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Melanoma (versus all others) | 1.72 | 0.61-4.82 | 0.30 |
| Other types of cancer (versus all others) | 0.41 | 0.05-3.11 | 0.38 |
| Baseline antiplatelet drugs (versus non-user) | 3.46 | 1.21-9.86 | 0.02 |
CI, confidence interval; HR, hazard ratio.
A mortality event before a checkpoint inhibitor-related pneumonitis event was defined as competing risk.
Including 17 checkpoint inhibitor-related pneumonitis events in the matched cohort.
Discussion
This study examined the relationship between the baseline concomitant drugs and CIP incidence in a cohort of advanced cancer patients treated with ICIs, revealing that baseline antiplatelet drug use increases the risk for CIP. The overall incidence of CIP in our cohort was 9.2%, which was generally consistent with previously reported values in the real-world clinical setting.3,28 Despite its retrospective nature, this was the first study to implicate the impact of baseline antiplatelet drugs on CIP risks with a relatively large sample size. It highlights the importance of attending to concomitant baseline drugs for CIP risk assessment before ICI induction.
One strength of this study was to estimate the impact of baseline antiplatelet drugs on CIP incidence in different multivariable models. In the total cohort, baseline pulmonary fibrosis and TRT history as well as antiplatelet drug use were independent risk factors for CIP incidence. These are known and prominent CIP risk factors, suggesting that our findings might be generalizable to ICI-treated patients. Since the relatively few CIP events in the study cohort restricted the number of explanatory variables in the multivariable analyses, we further controlled for confounding in the PSM analysis. The covariates in the PSM partially included factors that could be associated with baseline antiplatelet drug use, such as age, sex, and smoking history. Importantly, the HRs for CIP incidence were comparably high among patients with antiplatelet drug use both in the total cohort and the matched cohort. These findings suggest a robust association between baseline antiplatelet drug use and CIP incidence in the cohort of this study.
Among the investigated baseline non-oncological drugs in this study, only antiplatelet drugs were indicated to be associated with CIP incidence. Several drugs, such as PPIs,29 NSAIDs,30 antibiotics,31 and corticosteroids,32 reportedly influence the gut microbiota among ICI-treated patients, thereby increasing the risk for incidence of a particular irAE,8, 9, 10, 11, 12 which were not associated with CIP incidence in the univariable analysis. It is not prudent to discuss this alongside other drugs that have not been examined in a multivariable analysis; however, solely antiplatelet drugs may be involved in the potential mechanisms underlying CIP incidence.
In this study, there is no experimental evidence underlying an association between antiplatelet drugs and CIP incidence. We considered, however, that several hypothetical mechanisms inferred from previous findings include two key aspects: an altering immune landscape within the tumor microenvironment mediated by the concomitant use of antiplatelet drugs and ICIs and the specific role of platelets within the pulmonary circulation and immune systems.
Firstly, some preliminary data suggest that antiplatelet drugs could enhance antitumor immunity during ICI treatment. Platelet-derived PD-L1 has been reported to promote immune evasion and contribute to tumor growth by inhibiting CD4+ and CD8+ lymphocytes.33 Of note, preclinical studies have shown that the co-administration of antiplatelet drugs and ICIs decreases cancer cell PD-L1 concentrations,34 increases tumor-infiltrating CD4+ and CD8+ lymphocyte numbers,35 and enhances their antitumor efficacy.33,35,36 These findings can be supported by data from several clinical investigations. A retrospective analysis in ICI-treated patients by Cortellini et al.37 reported an improved tumor response rate and progression-free survival among patients receiving aspirin at baseline. Moreover, a systematic review investigating the association between ICI efficacy and baseline co-medications showed that low-dose aspirin was associated with improved progression-free survival.38 Although these findings primarily involved the investigation of the effects of aspirin, they would support the idea that concomitant antiplatelet drug use enhances antitumor immunity in ICI-treated patients. Given that a disordered immune system induced by ICI administration has been implicated as a potential mechanism of irAEs,2 antiplatelet drugs might promote an immune environment more prone to developing irAEs, including CIP.
In addition, the unique role of platelets within the lungs derived from several fundamental types of research could partly explain the etiology of antiplatelet drugs and CIP incidence. The lungs are enriched in megakaryocyte reserves, and platelets continuously accumulate within the pulmonary circulation.39 Platelets also have an essential effector activity in hemostasis and inflammation within the lung, contributing to the integrity of the alveolar capillaries’ basal barrier, regulating vascular permeability, and contributing to tissue repair and remodeling of alveoli and pulmonary vessels.39 Moreover, it has been reported that platelets interact with activated leukocytes under inflammatory conditions to reduce the levels of mediators such as tumor necrosis factor-α and neutrophil elastase and increase the level of transforming growth factor-β that helps counteract an inflammatory response, thereby protecting against lung injury and contributing to wound healing.40 Therefore, the concomitant antiplatelet drugs might have interfered with the process of repairing potential lung tissue damage caused by ICIs, leading to CIP incidence. Altogether, in patients receiving ICIs, the impact of antiplatelet drugs on antitumor immunity and the potential role of platelets in lung wound healing might support an association between antiplatelet drugs and the increased risk of CIP. Antiplatelet drug-induced interstitial lung diseases, however, have been previously reported on a case-report basis with unknown etiology.41, 42, 43 Therefore, it is still uncertain whether the hypothetical etiology of pneumonitis in the present study is unique to ICI-treated patients and warrants further investigation.
There are several limitations to this study. First, because this is a retrospective nature, any potential selection bias or the effect of missing data is difficult to eliminate. The CIP diagnosis was based on an independent medical record-based review by multiple investigators, including an experienced radiologist and pulmonologist; however, lung injury induced by other causes cannot be ruled out completely. Moreover, this study failed to examine information on the treatment duration of ICIs. The study cohort comprised multiple cancer types with varying treatment patterns, including ICI regimens. Additionally, in some cases, treatment periods involved interruptions and resumptions, making it difficult to define a uniform treatment duration. To address this bias, time-dependent outcome analyses considering competing risks were conducted, though not all competing risks could be accounted for. Second, the mechanisms underlying the effect of antiplatelet drugs on CIP incidence remain unclear. Bronchoalveolar lavage and lung biopsy were rarely carried out for CIP diagnosis in this study. Therefore, the immunological and pathological details of the results of this study should be elucidated in further research. Third, the limited sample size may have affected the power for significance. It was impossible to estimate the impact of the investigated concomitant drugs other than antiplatelet drugs on CIP risk. It was also difficult to analyze CIP risk depending on the status of antiplatelet drugs and other concomitant medications. Furthermore, these factors made it difficult to carry out a risk analysis for each type of antiplatelet drug. Thus, it is uncertain which type of antiplatelet drug is associated with an increased risk of CIP. Fourth, information on concomitant drugs was only available at baseline and was not examined for subsequent changes; the unknown status of concomitant drugs after ICI initiation might be a residual confounding in this study.
Conclusions
In conclusion, this study is the first to specifically address the relationship between baseline antiplatelet drugs and CIP incidence among ICI-treated advanced cancer patients. It cannot be concluded that antiplatelet medications should be discontinued during ICI therapy to avoid the risk for CIP incidence. We believe, however, that avoiding potential adverse events by reviewing low-priority prescriptions and preventing polypharmacy may benefit cancer patients treated with ICIs. Our findings should be validated in future studies with larger cohorts of patients.
Acknowledgements
We thank Editage (www.editage.com) for English language editing.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The datasets generated and/or analyzed during the current study are available from the corresponding author (SK) on reasonable request.
Supplementary data
References
- 1.Twomey J.D., Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23(2):39. doi: 10.1208/s12248-021-00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morad G., Helmink B.A., Sharma P., Wargo J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi: 10.1016/j.cell.2021.09.020. Erratum in: Cell. 2022;185(3):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuss J.E., Suresh K., Naidoo J. Checkpoint inhibitor pneumonitis: Mechanisms, characteristics, management strategies, and beyond. Curr Oncol Rep. 2020;22(6):56. doi: 10.1007/s11912-020-00920-z. [DOI] [PubMed] [Google Scholar]
- 4.Khunger M., Jain P., Rakshit S., et al. Safety and efficacy of PD-1/PD-L1 inhibitors in treatment-naive and chemotherapy-refractory patients with non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer. 2018;19(3):e335–e348. doi: 10.1016/j.cllc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Nishino M., Giobbie-Hurder A., Hatabu H., Ramaiya N.H., Hodi F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607–1616. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 6.Fukihara J., Sakamoto K., Koyama J., et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20(6):442–450.e4. doi: 10.1016/j.cllc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Suresh K., Psoter K.J., Voong K.R., et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14(3):494–502. doi: 10.1016/j.jtho.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Yin J., Elias R., Peng L., et al. Chronic use of proton pump inhibitors is associated with an increased risk of immune checkpoint inhibitor colitis in renal cell carcinoma. Clin Genitourin Cancer. 2022;20(3):260–269. doi: 10.1016/j.clgc.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohiuddin J.J., Chu B., Facciabene A., et al. Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy. J Natl Cancer Inst. 2021;113(2):162–170. doi: 10.1093/jnci/djaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marthey L., Mateus C., Mussini C., et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395–401. doi: 10.1093/ecco-jcc/jjv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seethapathy H., Zhao S., Chute D.F., et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14(12):1692–1700. doi: 10.2215/CJN.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meraz-Muñoz A., Amir E., Ng P., et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee S., Ibrahimi S., Khalid B., Roman D., Zhao D., Aljumaily R. Do proton pump inhibitors modulate the efficacy of anti-PD-1/PD-L1 therapy? A retrospective study. J Oncol Pharm Pract. 2019;25(3):762–764. doi: 10.1177/1078155218771152. [DOI] [PubMed] [Google Scholar]
- 14.Chalabi M., Cardona A., Nagarkar D.R., et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Fessler J., Matson V., Gajewski T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108. doi: 10.1186/s40425-019-0574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho J.Y., Kim J., Lee J.S., et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–156. doi: 10.1016/j.lungcan.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Suresh K., Voong K.R., Shankar B., et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 18.Voong K.R., Hazell S., Hu C., et al. MA 09.08 receipt of chest radiation and immune-related pneumonitis in patients with NSCLC treated with anti-PD-1/PD-L1. J Thorac Oncol. 2017;12:S1837. [Google Scholar]
- 19.Shibata Y., Murakami S., Kato T. Overview of checkpoint inhibitor pneumonitis: incidence and associated risk factors. Expert Opin Drug Saf. 2021;20(5):537–547. doi: 10.1080/14740338.2021.1898584. [DOI] [PubMed] [Google Scholar]
- 20.Sul J., Blumenthal G.M., Jiang X., He K., Keegan P., Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016;21(5):643–650. doi: 10.1634/theoncologist.2015-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi T., Shimizu J., Hasegawa T., et al. Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: a retrospective analysis. BMC Cancer. 2021;21(1):924. doi: 10.1186/s12885-021-08661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibaki R., Murakami S., Matsumoto Y., et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother. 2020;69(1):15–22. doi: 10.1007/s00262-019-02431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrón F., Sánchez R., Arroyo-Hernández M., et al. Risk of developing checkpoint immune pneumonitis and its effect on overall survival in non-small cell lung cancer patients previously treated with radiotherapy. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.570233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo K., Azuma A., Kanazawa M., et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51(4):260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health–National Cancer Institute.
- 26.Cui P., Liu Z., Wang G., et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med. 2018;7(8):4115–4120. doi: 10.1002/cam4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin J., Wu Y., Yang X., Gan L., Xue J. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.830631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins A.M., Kichenadasse G., McKinnon R.A., et al. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: post hoc analysis of IMpower150. Br J Cancer. 2022;126(1):42–47. doi: 10.1038/s41416-021-01606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasetya R.A., Metselaar-Albers M., Engels F. Concomitant use of analgesics and immune checkpoint inhibitors in non-small cell lung cancer: a pharmacodynamics perspective. Eur J Pharmacol. 2021;906 doi: 10.1016/j.ejphar.2021.174284. [DOI] [PubMed] [Google Scholar]
- 31.Hakozaki T., Richard C., Elkrief A., et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. 2020;8(10):1243–1250. doi: 10.1158/2326-6066.CIR-20-0196. [DOI] [PubMed] [Google Scholar]
- 32.Colard-Thomas J., Thomas Q.D., Viala M. Comedications with immune checkpoint inhibitors: involvement of the microbiota, impact on efficacy and practical implications. Cancers (Basel) 2023;15(8):2276. doi: 10.3390/cancers15082276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinterleitner C., Strähle J., Malenke E., et al. Platelet PD-L1 reflects collective intratumoral PD-L1 expression and predicts immunotherapy response in non-small cell lung cancer. Nat Commun. 2021;12(1):7005. doi: 10.1038/s41467-021-27303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asgari A., Lesyk G., Poitras E., et al. Platelets stimulate programmed death-ligand 1 expression by cancer cells: inhibition by anti-platelet drugs. J Thromb Haemost. 2021;19(11):2862–2872. doi: 10.1111/jth.15478. [DOI] [PubMed] [Google Scholar]
- 35.Riesenberg B.P., Ansa-Addo E.A., Gutierrez J., Timmers C.D., Liu B., Li Z. Cutting edge: targeting thrombocytes to rewire anticancer immunity in the tumor microenvironment and potentiate efficacy of PD-1 blockade. J Immunol. 2019;203(5):1105–1110. doi: 10.4049/jimmunol.1900594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaslavsky A.B., Adams M.P., Cao X., et al. Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-76351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortellini A., Tucci M., Adamo V., et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Chen H., Chen S., Li Z., Chen J., Li W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1) doi: 10.1080/2162402X.2021.1957605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weyrich A.S., Zimmerman G.A. Platelets in lung biology. Annu Rev Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo S., Wang Y., An Q., et al. Platelets protect lung from injury induced by systemic inflammatory response. Sci Rep. 2017;7 doi: 10.1038/srep42080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto K., Nakao S., Hasegawa S., et al. Vol. 8. SAGE Open Med; 2020. (Analysis of drug-induced interstitial lung disease using the Japanese Adverse Drug Event Report database). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abro S., Bikeyeva V., Naqvi W.A., et al. Clopidogrel-associated interstitial lung disease: a case report and literature review. Cureus. 2022;14(8) doi: 10.7759/cureus.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An J., Lee S.H., Chang B. Clopidogrel-induced interstitial lung disease: a case report. Ther Clin Risk Manag. 2021;17:711–715. doi: 10.2147/TCRM.S319077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.