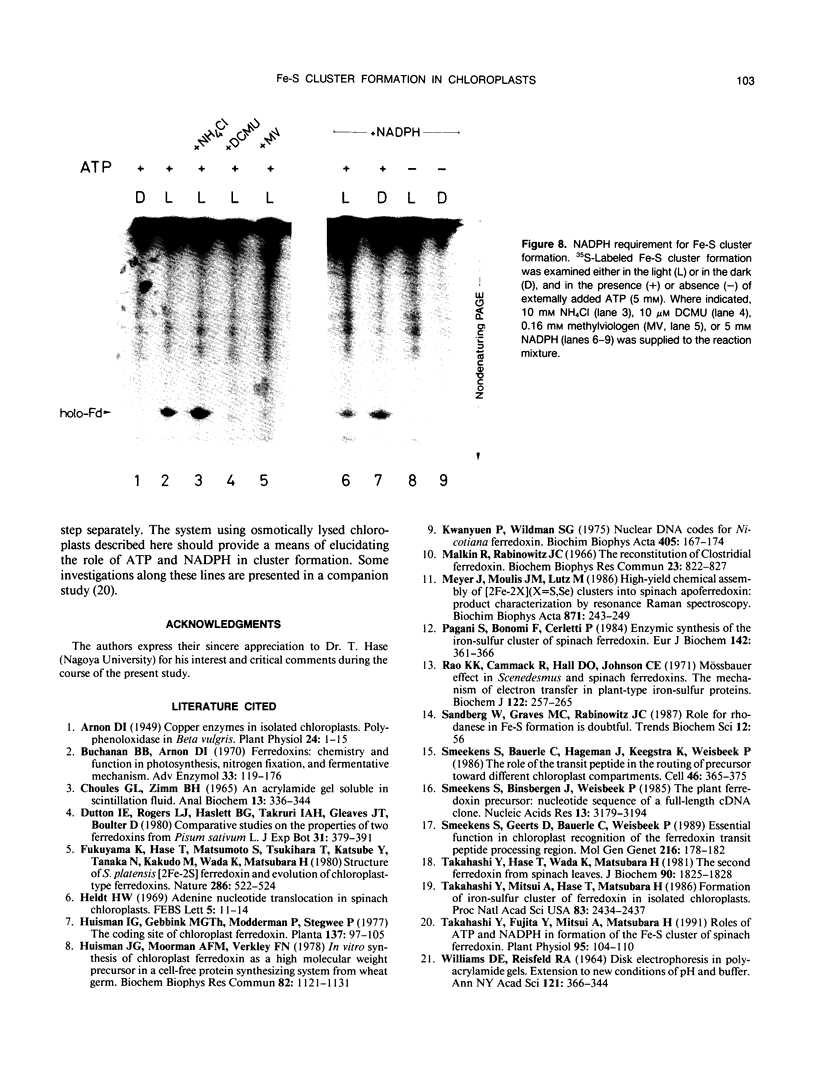
Abstract
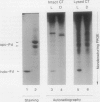
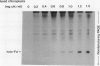
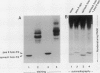
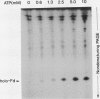
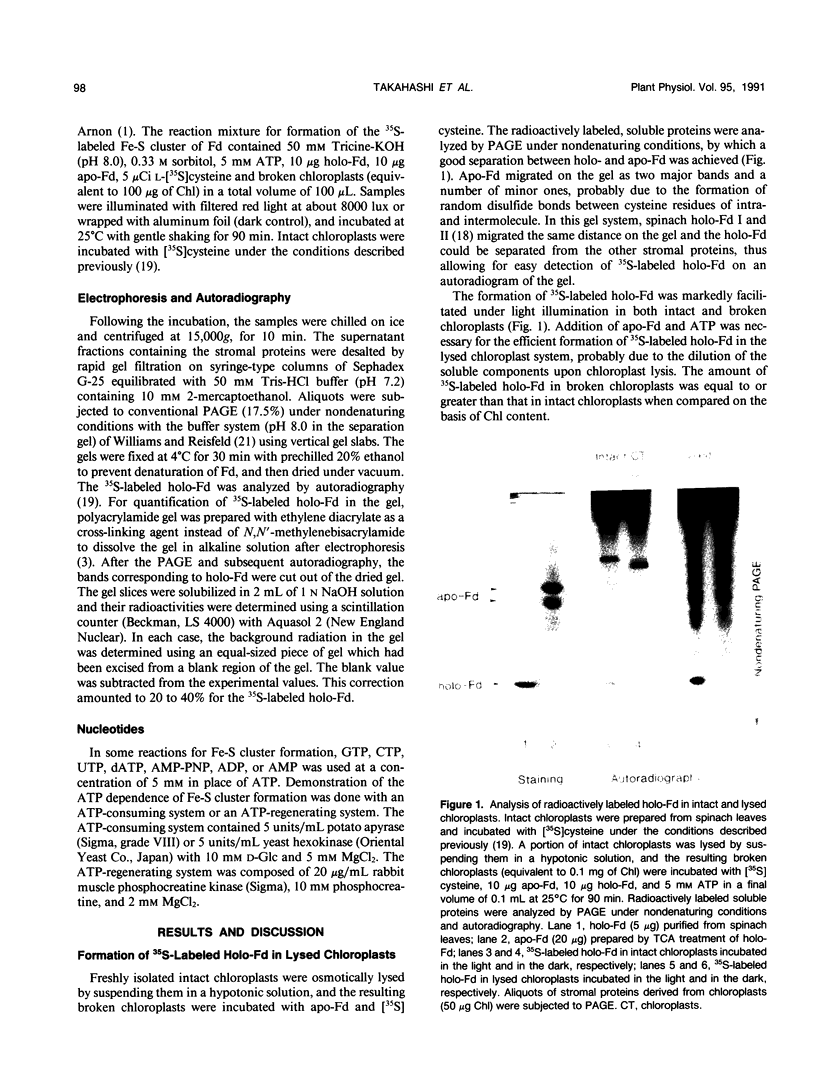
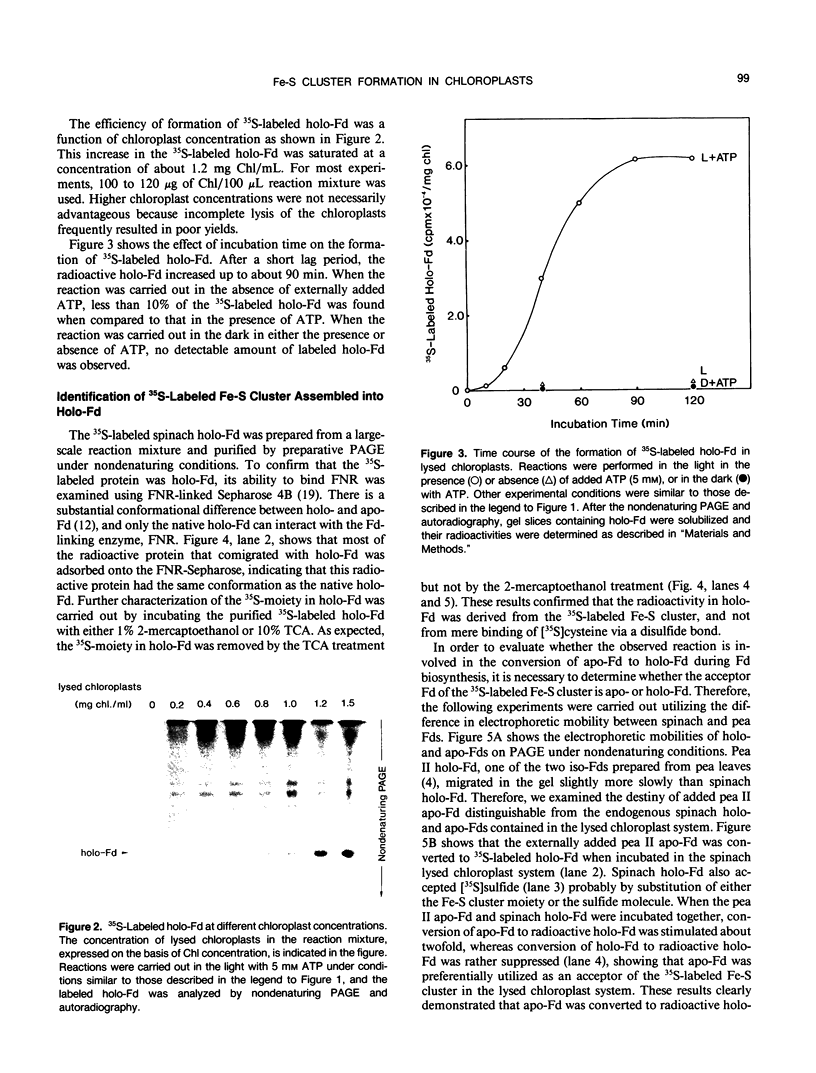
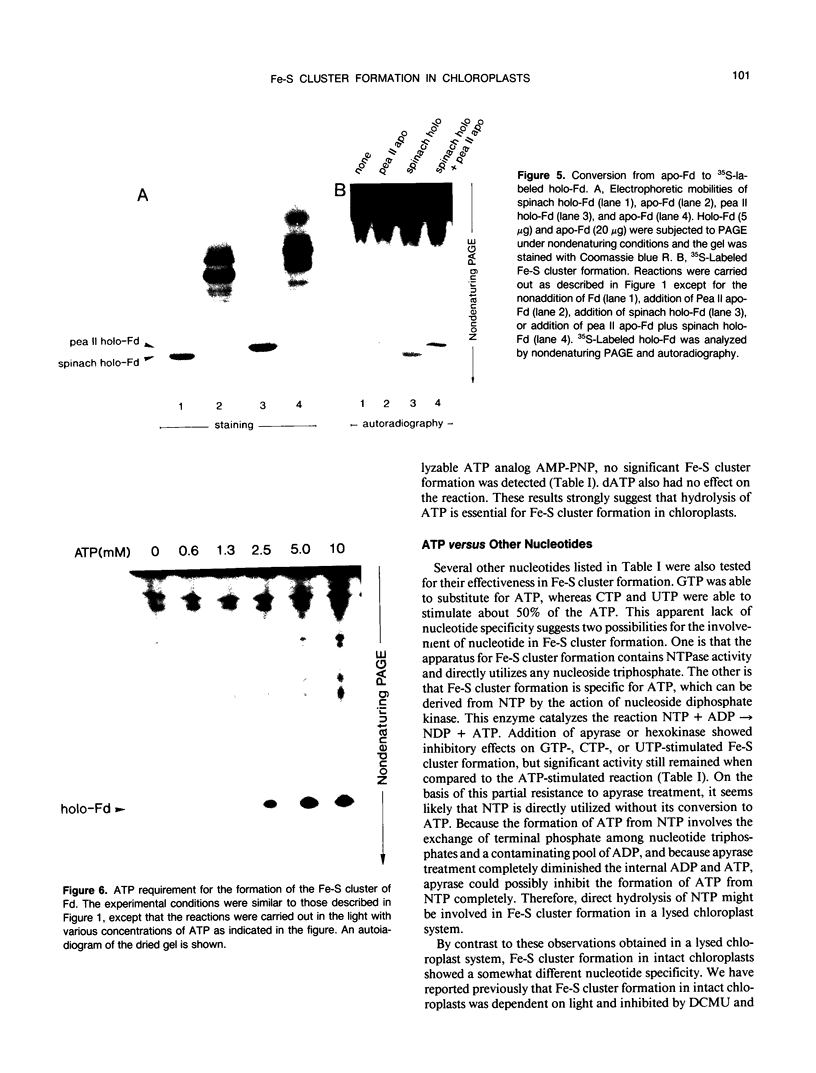
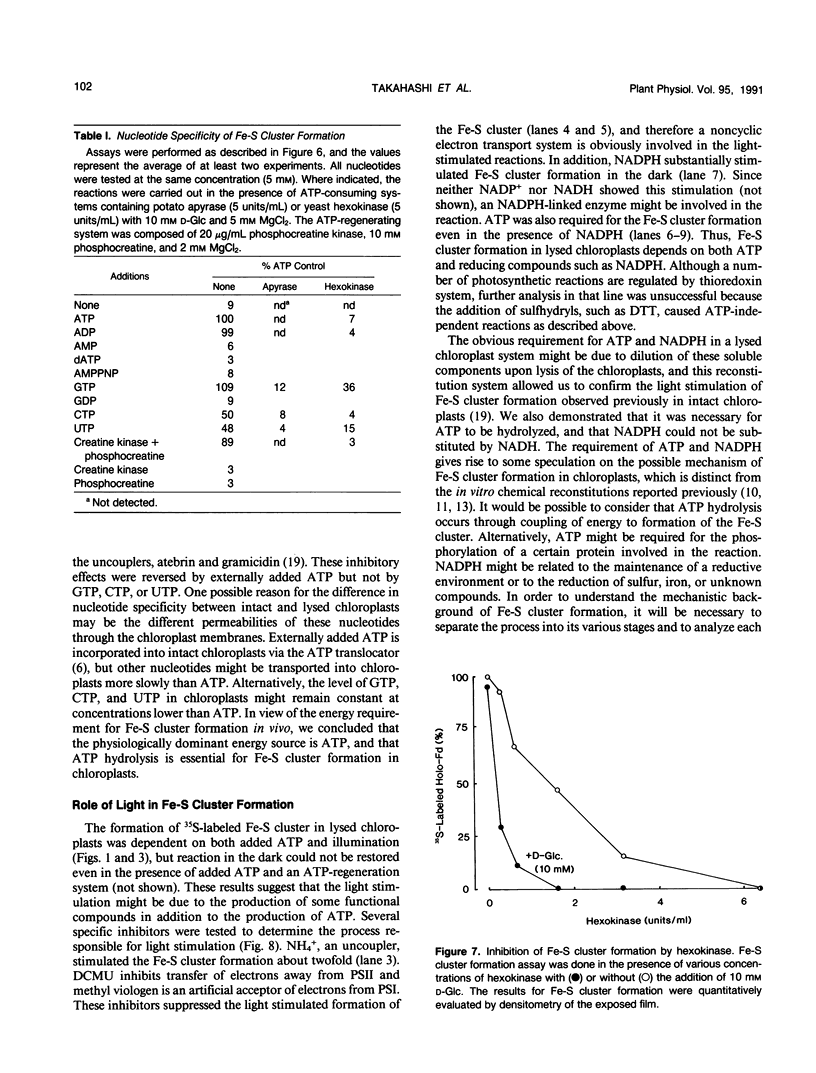
In vitro formation of the 35S-labeled Fe-S cluster of ferredoxin (Fd) has been achieved by incubating apo-Fd and [35S]cysteine with osmotically lysed chloroplasts of spinach (Spinacia oleracea). Correct integration of the 35S-labeled Fe-S cluster into Fd was verified on the basis of the following: (a) Under nondenaturing conditions, 35S-labeled holo-Fd showed the same electrophoretic mobility as authentic holo-Fd; (b) 35S-labeled holo-Fd showed an ability to bind Fd-NADP+ reductase; (c) the 35S-labeled moiety was removed from the Fd polypeptide by TCA treatment but not by 2-mercaptoethanol treatment; (d) externally added pea II apo-Fd was converted to 35S-labeled holo-Fd. This reconstitution was dependent on both ATP and light, and formation of the 35S-labeled Fe-S cluster was observed upon addition of ATP or when an ATP generation-system was constructed in the light. In contrast, ATP-consuming systems abolished the Fe-S cluster formation. A non-hydrolyzable ATP analog was unable to serve as an ATP substitute, indicating the requirement of ATP hydrolysis for cluster formation. GTP was able to substitute for ATP, but CTP and UTP were less effective. Fe-S cluster formation in lysed chloroplasts was stimulated by light even in the presence of added ATP. Light stimulation was inhibited by DCMU or methyl viologen but not by NH4+. NADPH was able to substitute for light, indicating that light energy is required for the production of reducing compounds such as NADPH in addition to the generation of ATP. These results confirm the requirement of light for the Fe-S cluster formation observed previously in intact chloroplasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Choules G. L., Zimm B. H. An acrylamide gel soluble in scintillation fluids: its application to electrophoresis at neutral and low pH. Anal Biochem. 1965 Nov;13(2):336–344. doi: 10.1016/0003-2697(65)90202-2. [DOI] [PubMed] [Google Scholar]
- Giles I. G., Poat P. C., Munday K. A. An investigation of the interactions of the allosteric modifiers of pyruvate kinase with the enzyme from Carcinus maenas hepatopancreas. Biochem J. 1977 Jul 1;165(1):97–105. doi: 10.1042/bj1650097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Huisman J. G., Moorman A. F., Verkley F. N. In vitro synthesis of chloroplast ferredoxin as a high molecular weight precursor in a cell-free protein synthesizing system from wheat germs. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1121–1131. doi: 10.1016/0006-291x(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Kwanyuen P., Wildman S. G. Nuclear DNA codes for Nicotiana ferredoxin. Biochim Biophys Acta. 1975 Sep 9;405(1):167–174. doi: 10.1016/0005-2795(75)90327-x. [DOI] [PubMed] [Google Scholar]
- Malkin R., Rabinowitz J. C. The reconstitution of clostridial ferredoxin. Biochem Biophys Res Commun. 1966 Jun 21;23(6):822–827. doi: 10.1016/0006-291x(66)90561-4. [DOI] [PubMed] [Google Scholar]
- Pagani S., Bonomi F., Cerletti P. Enzymic synthesis of the iron-sulfur cluster of spinach ferredoxin. Eur J Biochem. 1984 Jul 16;142(2):361–366. doi: 10.1111/j.1432-1033.1984.tb08295.x. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Hase T., Wada K., Matsubara H. The second ferredoxin from spinach leaves. J Biochem. 1981 Dec;90(6):1825–1828. doi: 10.1093/oxfordjournals.jbchem.a133662. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Mitsui A., Fujita Y., Matsubara H. Roles of ATP and NADPH in formation of the fe-s cluster of spinach ferredoxin. Plant Physiol. 1991 Jan;95(1):104–110. doi: 10.1104/pp.95.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Mitsui A., Hase T., Matsubara H. Formation of the iron-sulfur cluster of ferredoxin in isolated chloroplasts. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2434–2437. doi: 10.1073/pnas.83.8.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEME R. J. A PROCEDURE FOR HIGH VOLTAGE ELECTROPHORESIS IN AGAR GEL, WITH A NOTE ON ITS APPLICATION TO ACRYLAMIDE AND STARCH GEL. Ann N Y Acad Sci. 1964 Dec 28;121:366–372. doi: 10.1111/j.1749-6632.1964.tb14209.x. [DOI] [PubMed] [Google Scholar]