Abstract
Patent foramen ovale (PFO) is the most common congenital cardiac abnormality and is usually considered a benign finding. This case series suggests a potential link between PFO and vasospastic angina. It also demonstrates PFO closure as a potential therapeutic intervention for individuals with PFO who suffer from refractory vasospastic angina.
Key Words: coronary vasospasm, migraine, patent foramen ovale, vasospastic angina
Central Illustration
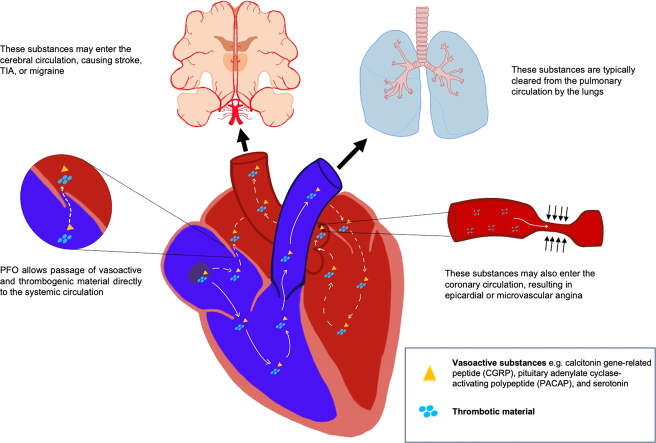
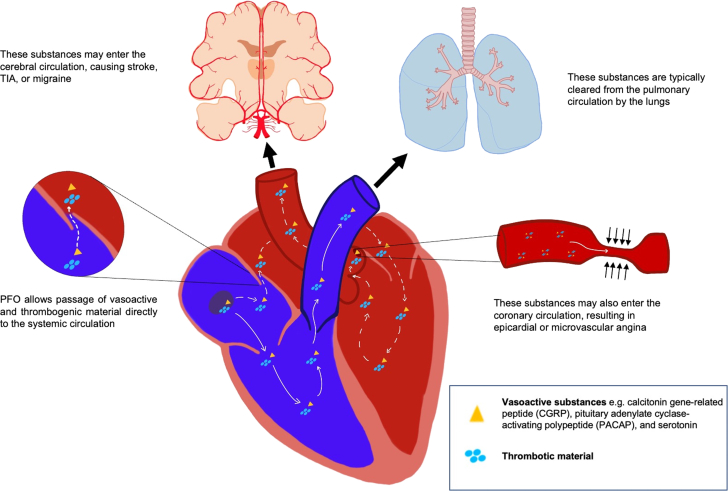
Patent foramen ovale (PFO) is the most common congenital cardiac abnormality and is present in 20% to 34% of the population. It occurs when the septum primum of the atria fails to fuse with the septum secundum after birth, leaving a flap that permits intermittent right-to-left blood flow under certain hemodynamic conditions.1 Although PFO is often discovered incidentally, it has been associated with hypoxemia, paradoxical emboli, cryptogenic stroke, and migraine with aura because of the shunting of venous blood with thrombotic material or vasoactive peptides to the systemic circulation.2 Patients who have experienced cryptogenic stroke in the setting of PFO benefit from percutaneous or surgical closure, which reduces the risk of recurrent stroke.3 Based on a meta-analysis of 2 randomized clinical trials, PFO closure also reduces the frequency of migraine attacks and migraine days per month, particularly in patients with migraine with aura, of whom 50% have PFOs.4 A small subset of patients with PFO appear to experience angina at rest, which we hypothesize is mediated by epicardial or microvascular spasm. In this case series, we report 9 female patients with PFO who had anginal chest pain, 7 of whom had PFO closure and experienced improvement in symptoms. Many of these patients also had migraine, supporting a mechanistic link between PFO, vasospastic angina, and migraines (Figure 1).
Learning Objectives
-
•
To establish the role of PFO in migraine and vasospastic angina.
-
•
To describe the presentation of patients with the constellation of PFO, migraine and vasospastic angina.
-
•
To discuss potential treatment strategies for patients with the syndrome of PFO, migraine and vasospastic angina.
Figure 1.
Mechanism by Which PFO Permits Vasoactive Substances to Left Sided Organs
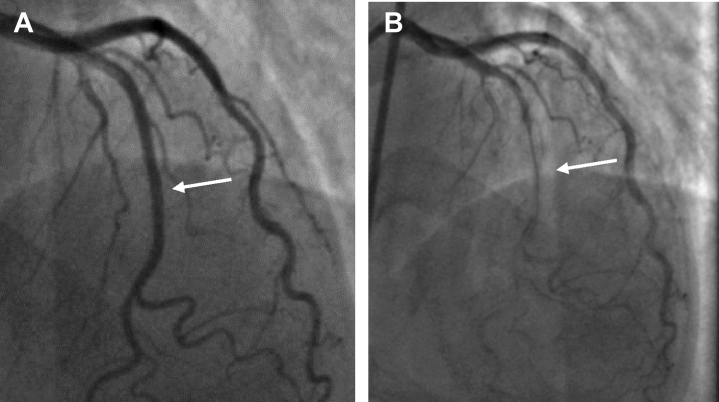
(A) Baseline angiogram demonstrating angiographically normal left anterior descending artery (arrow). (B) Angiogram after 100 μg acetylcholine intracoronary demonstrating diffuse narrowing of the left anterior descending artery (arrow).
Selected Case
A 68-year-old woman developed angina 4 months after an infection with COVID-19. Her medical history was notable for migraine with aura that began in her 30s and occurred approximately 6 days per month. Her family history was notable for vasospastic angina and migraine in both of her sisters, which resolved with PFO closure. Her prior workup findings for chest pain were negative for acute coronary syndrome, pulmonary embolism, or pulmonary parenchymal disease. A coronary computed tomography angiogram demonstrated no evidence of atherosclerosis or arterial narrowing but was notable for a small PFO. Given that she had recently recovered from COVID-19, pericarditis was the presumed diagnosis, and she was treated with a nonsteroidal anti-inflammatory agent and colchicine at an outside facility. The patient developed recurrent angina 1 month later, prompting another visit to the emergency department. She again was treated with a course of nonsteroidal anti-inflammatory drugs and colchicine, with some improvement in her symptoms but without complete resolution.
At our institution, transcranial Doppler study was obtained given the findings of her coronary computed tomography angiogram and demonstrated a large right-to-left shunt (5/5) as classified by the Spencer logarithmic scale (0-5, with 5+ being most significant), which is used to quantify the degree of shunting on transcranial Doppler. A decision was made to pursue provocative testing for coronary vasospasm and potential PFO closure. The baseline angiogram was negative for atherosclerotic coronary artery disease (Figure 2A). With administration of intracoronary acetylcholine 50 μg, the patient developed angina, showed ischemic electrocardiographic changes, and had angiographic evidence of coronary vasospasm with reduction in luminal diameter segmentally in the left anterior descending artery (LAD) (Figure 2B). This was relieved with the administration of 200 μg intracoronary nitroglycerin. PFO closure was performed with delivery of a 25-mm Gore Cardioform occluder device. An agitated saline bubble study from the femoral vein demonstrated no residual shunting. At the 11-month follow up, the patient had no recurrent angina or migraines.
Figure 2.
Angiogram Demonstrating Coronary Vasospasm With Acetylcholine Testing
(A) Baseline angiogram demonstrating angiographically normal LAD (arrow). (B) Angiogram after 100ug acetylcholine I.C. demonstrating diffuse narrowing of the LAD (arrow).
In Table 1, we report 9 female patients with PFO and coronary vasospasm (either spontaneously noted on angiography or with provocative acetylcholine testing). Eight of these patients also had migraine with aura. The 7 patients who underwent PFO closure had relief from chest pain and migraine.
Table 1.
Clinical Vignettes of Patients With Vasospastic Angina, PFO, and Migraine
| Patient # | Presentation | Migraines | Chest Pain | PFO Evaluation | Angiography | Provocative Testing | PFO Closure | Medical Therapy | Residual Symptoms/Length of Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A 49-year-old woman with long-standing history of migraine with aura and intermittent angina for 7 years who presented with chest pain and ventricular fibrillation | Yes | Yes | TEE | Index study with focal narrowing of the distal LAD with repeat study demonstrating resolution of previously seen focal stenosis | No | Yes | Clopidogrel and ASA for 1 month; ASA thereafter | No symptoms at the 15-year follow-up; closed 2008 |
| 2 | A 68-year-old woman (sister of patient 1) with several years of migraine with aura and recent-onset angina after COVID-19 infection | Yes | Yes | TCD with 5/5 right-to-left shunt | No epicardial coronary artery disease | +Ach with narrowing of the LAD (Figure 1) | Yes | Clopidogrel and ASA for 1 month; ASA thereafter | No symptoms at the 11-month follow-up |
| 3 | A 38-year-old woman with long-standing migraine history and 10 years of intermittent angina, found to have nocturnal hypoxemia to 80% | Yes | Yes | TEE right-to-left shunt TCD with 5/5 right-to-left shunt |
No epicardial coronary artery disease | +Ach with no epicardial narrowing but with reproduction of anginal chest pain but without ECG changes | Yes | Clopidogrel and ASA for 1 month; ASA thereafter | No symptoms at the 6-month follow-up |
| 4 | A 51-year-old woman (sister of patients 1 and 2) with migraine and history of 2v CABG for SCAD, with recurrent angina | Yes | Yes, after CABG for presumed SCAD (initial diagnosis made at OSH) | TCD with 4/5 right-to-left shunt | Mild luminal irregularities in the epicardial coronary arteries with atretic LIMA-LAD and absent radial LCX | +Ach with narrowing of the LAD | Yes | Clopidogrel and ASA for 1 month; none since | No symptoms at the 13-year follow-up; closed 2010 |
| 5 | A 50-year-old woman with migraines with visual aura, TND (right arm numbness and weakness, with right paralysis) and several months of angina | Yes, with visual aura | Yes | TEE with right-to-left shunt | N/A | N/A | Yes | Metoprolol | No angina at the 4-year follow-up Reduced visual auras (from 20-30 episodes/year to fewer than 6 episodes/year) |
| 6 | A 34-year-old woman with mixed connective tissue disorder, recurrent migraines, and several months of angina | Yes, with aura | Yes | TCD with 5/5 left-to-right shunt TEE with left-to-right shunt |
No epicardial atherosclerosis; mild myocardial bridge in the distal LAD | +Ach with >90% narrowing | Deferred in favor of clopidogrel therapy, now pending PFO closure | Clopidogrel | Angina (15 days per month) despite clopidogrel use at the 5-month follow-up with skin bruising Mild improvement in migraines with aura |
| 7 | A 43-year-old woman with migraines with visual aura, TND symptoms, factor V Leiden, and recurrent episodes of angina (3-5 times per week, improved with nitroglycerin) | Yes, with visual aura | Yes, nitrate responsive | TEE with right-to-left shunt and atrial septal aneurysm | N/A | N/A | Pending | Clopidogrel Nitroglycerin spray as needed |
Improvement in angina with nitroglycerin and clopidogrel with no improvement in migraine Has transitioned to ticagrelor with improvement in migraine |
| 8 | A 47-year-old woman with hypertension, obesity, IDDM, OSA, migraine, hypoxemia, and recurrent angina | Yes | Yes | TEE with right-to-left shunt Large shunt reported on TCD |
No epicardial coronary artery disease | +Ach with narrowing of the LAD | Yes | Clopidogrel + ASA followed by ASA monotherapy Currently on diltiazem, losartan, and rosuvastatin |
Occasional angina subsequently (2-3 times per month) Improvement in migraine (now with 20/year) 4-year follow up; closed 2019 |
| 9 | A 65-year-old woman with supraventricular tachycardia, asthma, and HCV with several years of angina | No | Yes | TTE bubble study with right-to-left shunt | No epicardial coronary artery disease | +Ach with narrowing of the diagonal artery | Surgical closure | ASA, isosorbide mononitrate, SL nitroglycerin as needed | Minimal angina with antianginal therapy Reports significant improvement in functional capacity 4-year follow up; closed 2019 |
2v = 2-vessel; Ach = acetylcholine; ASA = acetylsalicylic acid; CABG = coronary artery bypass graft; ECG = electrocardiogram; HCV = hepatitis C virus; IDDM = insulin dependent diabetes mellitus; LAD = left anterior descending artery; LCX = left circumflex artery; LIMA = left internal mammary artery; N/A = not applicable; OSA = obstructive sleep apnea; OSH = outside hospital; PFO = patent foramen ovale; SCAD = spontaneous coronary artery dissection; TCD = transcranial doppler; TEE = transesophageal echocardiography; TND = transient neurologic deficits; TTE = transthoracic echocardiography.
Discussion
Although usually considered a benign finding, the presence of a PFO increases the risk of stroke and paradoxical embolus. PFO is also associated with migraine with aura. By shunting venous blood to the systemic circulation, PFO may facilitate the passage of thrombotic or vasoactive substances that elicit cerebral and vascular responses, resulting in migraine and vasospastic angina in susceptible individuals (Figure 1). Closure of the PFO would instead shunt venous blood through the pulmonary circulation, where vasoactive substances are metabolized by the capillary endothelium, reducing the risk of vasospastic angina and migraine.
PFO may contribute to the development of migraine with aura by permitting vasoactive substances to pass to the brain. Several neuropeptides, including calcitonin gene–related peptide, pituitary adenylate cyclase–activating polypeptide, and serotonin have been implicated in the pathophysiology of migraine and are under investigation as potential therapeutic targets.5 Paradoxical microembolization of platelet aggregates into the systemic circulation may further promote migraine onset by producing focal transient ischemia.6 These substances, which otherwise would be filtered or metabolized during passage through the pulmonary circulation, may enter the arterial system and induce cortical spreading depression, leading to a migraine attack.7
This case series suggests that PFO may also predispose a subset of individuals to vasospastic angina by permitting the passage of vasoactive substances into the coronary circulation, inducing epicardial or microvascular coronary vasospasm. Although the exact mechanism of vasospasm has not been elucidated, several factors, including smooth muscle hypercontractility, autonomic dysfunction, and endothelial dysfunction, have been identified. As with migraines, vasoactive substances including ergonovine, histamine, acetylcholine, and serotonin may provoke coronary vasospasm in susceptible people.8
Coronary vasospasm has also been implicated in the pathogenesis of takotsubo cardiomyopathy. As described by Angelini9 in a case series of 4 patients, acetylcholine provocative testing resulted in severe narrowing of the coronary arteries in patients with left ventricular apical ballooning. One patient was noted to have reproduction of left ventricular apical ballooning during acetylcholine administration. Given the potential link between coronary vasospasm and PFO, evaluation for the presence of PFO may identify individuals at risk for development of takotsubo cardiomyopathy. In one case report, Takafuji et al10 reported a case of a 16-year-old patient with embolic stroke and reverse takotsubo cardiomyopathy who was found to have a PFO. Further investigation is warranted to elucidate the potential relationship between PFO and takotsubo syndrome.
This case series suggests that there is a mechanistic link between right-to-left shunting in the setting of a PFO and coronary artery spasm in a small minority of patients. Although the 9 patients described in this study represent <1% of the patients we have seen with PFO-related clinical symptoms, their coronary and cerebral arteries may be particularly susceptible to vasoactive substances that are typically able to bypass metabolism in the pulmonary circulation because of right-to-left shunt at the atrial level. The observation that all 7 patients with vasospastic angina who underwent PFO closure had relief of their symptoms further strengthens this hypothesis. Improvement in migraine symptoms with antiplatelet therapy such as thienopyridines, which inhibit the release of serotonin, may identify patients with PFO who may respond to closure. This is currently being tested in a randomized clinical trial (RELIEF [Gore Cardioform Septal Occluder Migraine Clinical Study]; NCT04100135). Given the potential for significant reduction in symptom burden with PFO closure, we believe that further research into the mechanistic link between PFO, coronary vasospasm, and migraine is important and that the therapeutic use of PFO closure in such patients should be considered.
Funding Support and Author Disclosures
Dr Parikh has received research support from Bayer, Infraredx, and Abbott Vascular; and consulting fees from Abbott Vascular. Dr Tobis consults and provides lectures for WL Gore. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Calvert P.A., Rana B.S., Kydd A.C., Shapiro L.M. Patent foramen ovale: anatomy, outcomes, and closure. Nat Rev Cardiol. 2011;8(3):148–160. doi: 10.1038/nrcardio.2010.224. [DOI] [PubMed] [Google Scholar]
- 2.Giblett J.P., Williams L.K., Kyranis S., Shapiro L.M., Calvert P.A. Patent foramen ovale closure: state of the art. Interv Cardiol. 2020;15:e15. doi: 10.15420/icr.2019.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Søndergaard L., Kasner S.E., Rhodes J.F., et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033–1042. doi: 10.1056/NEJMoa1707404. [DOI] [PubMed] [Google Scholar]
- 4.Saver J.L., Carroll J.D., Thaler D.E., et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022–1032. doi: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 5.Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174–182. doi: 10.1016/S1474-4422(17)30435-0. [DOI] [PubMed] [Google Scholar]
- 6.Finocchi C., Del Sette M. Migraine with aura and patent foramen ovale: myth or reality? Neurol Sci. 2015;36(1):61–66. doi: 10.1007/s10072-015-2163-8. [DOI] [PubMed] [Google Scholar]
- 7.Tietjen G.E., Collins S.A. Hypercoagulability and migraine. Headache. 2018;58(1):173–183. doi: 10.1111/head.13044. [DOI] [PubMed] [Google Scholar]
- 8.Matta A., Bouisset F., Lhermusier T., et al. Coronary artery spasm: new insights. J Interv Cardiol. 2020;2020 doi: 10.1155/2020/5894586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini P. Transient left ventricular apical ballooning: a unifying pathophysiologic theory at the edge of Prinzmetal angina. Catheter Cardiovasc Interv. 2008;71(3):342–352. doi: 10.1002/ccd.21338. [DOI] [PubMed] [Google Scholar]
- 10.Takafuji H., Arai J., Saigusa K., Obunai K. Reverse takotsubo cardiomyopathy caused by patent foramen ovale-related cryptogenic stroke: a case report. Eur Heart J Case Rep. 2020;4(6):1–6. doi: 10.1093/ehjcr/ytaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]