Abstract
Objective
To evaluate fertility treatment outcomes among transgender (TG) men with a history of gender-affirming hormone therapy with exogenous testosterone.
Design
Descriptive, retrospective cohort study.
Patients
Transgender men with a history of gender-affirming hormone therapy with exogenous testosterone underwent fertility treatments, including embryo cryopreservation, in vitro fertilization (IVF), co-IVF, oocyte cryopreservation, and intrauterine insemination (IUI), between 2013 and 2021.
Intervention
Gender-affirming hormone therapy with testosterone.
Main Outcome Measures
Live births (LBs), number of frozen embryos, and number of frozen oocytes. Other outcome measures included total gonadotropin used, peak estradiol levels, oocytes retrieved, oocyte maturity rate, fertilization rate, and embryo grade.
Results
A total of 77 TG men self-presented or were referred to care at a single academic fertility center, of which 46 (59.7%) TG men underwent fertility preservation and/or family-building counseling, with 16 (20.8%) patients proceeding to fertility treatment. Of those patients who underwent treatment, 11 (68.8%) had a history of gender-affirming hormone therapy with exogenous testosterone use. Cohort 1 included IVF (n = 1), co-IVF (n = 1), embryo cryopreservation (n = 2), cohort 2 included oocyte cryopreservation (n = 4), and cohort 3 included IUI (n = 3). In cohort 1, both the patients who underwent IVF and the patients who underwent co-IVF achieved LBs. All embryo cryopreservation cycles froze three or more embryos. In cohort 2, the average number of frozen mature oocytes was 19.3 ± 16.2 (range 6–43). All patients who underwent IUI cycles achieved LB.
Conclusion
In this study, no correlation existed between patient age, time on or off gender-affirming hormone therapy with exogenous testosterone, total gonadotropin used, and number of oocytes retrieved. All patients who completed IVF or embryo cryopreservation produced high-quality blastocytes, and this is the first study to show successful IUI cycles in patients with a history of gender-affirming hormone therapy with exogenous testosterone. This study demonstrates that TG men who have used gender-affirming hormone therapy previously can successfully undergo fertility treatments to attain oocyte and embryo cryopreservation, pregnancy, and LBs.
Key Words: transgender male, assisted reproductive technology, gender-affirming hormone therapy, exogenous testosterone
Transgender (TG) people are individuals whose gender identity does not align with the sex assigned at birth. There are 1.6 million trans and gender-diverse (TGD) people across the United States from an array of ethnic, socioeconomic, and religious backgrounds (1). Members of the TGD community have identities that span the gender spectrum. Although some trans individuals identify as solely female and feminine or male and masculine, other gender-diverse people may identify with both feminine and masculine aspects of gender. Comprehensive gender-affirming care for the TGD population encompasses medical, social, and legal assistance across many aspects of an individual’s life. Some TGD individuals choose to undergo medical gender-affirming care as part of their journey toward actualizing their true selves. Two of the largest areas of gender-affirming care are gender-affirming surgery (GAS) and gender-affirming hormone therapy (GAHT).
For TG men, GAS is grouped into two primary domains: gender-affirming chest surgery and gender-affirming genital surgery. Chest surgeries include bilateral mastectomy, chest reduction, and breast augmentation, whereas genital surgeries include hysterectomy, bilateral salpingo-oophorectomy, vaginectomy, phalloplasty, and metoidioplasty. Transgender men may elect to have some or none of these procedures performed. In TG men, GAHT typically involves exogenous testosterone supplementation to induce masculinization of physical features and suppression of more stereotypical feminine physical features. Common effects of exogenous testosterone include virilization of hair patterns, increased muscle growth, redistribution of fat, deepening of voice, changes in sweat and odor patterns, gonadal effects such as increased libido, and menopausal symptoms including vaginal dryness or menstrual cessation. Although gender-affirming genital surgeries (i.e., hysterectomy and bilateral salpingo-oophorectomy), can permanently impact the future reproductive potential of TG men, the effects of GAHT on fertility are less clear.
In 2021, Pirtea et al. (2) summarized the existing literature on the impact of testosterone therapy on the ovarian histology of TG men. Most studies reported polycystic ovary syndrome-like changes to ovarian architecture both macroscopically and microscopically (2). However, a study by Ikeda et al. (3), published in 2013, countered those findings by reporting that exogenous testosterone changes in the ovarian cortex and stroma occurred without inducing full polycystic ovarian changes. Ikeda et al. (3) study suggested that the numbers of primordial, early, and antral follicles were similar when comparing the ovaries of patients on high-dose androgen therapy compared with controls with no history of GAHT. A study by De Roo et al. (4) found similar results by demonstrating that after more than a year of testosterone therapy, follicle cortical distribution was similar to that of controls.
Trans and gender-diverse individuals seek care with reproductive endocrinologists for numerous family-building and fertility goals. Some desire fertility preservation before GAHT or GAS, whereas others desire assistance with family building in a manner that would minimize the risk of triggering gender dysphoria (5). In light of inconclusive data on the impact of GAHT on the ovaries, the World Professional Association for Transgender Health, the American Society for Reproductive Medicine (ASRM), and the Endocrine Society recommend stopping GAHT for at least 3 months before starting fertility treatments that involve ovarian stimulation (6, 7, 8). However, a known major barrier to care for TG men seeking fertility care and family-building services is the fear that stopping GAHT could result in the reversal of androgen-induced changes that align with their gender identity. Some of the potential physical changes experienced with the cessation of exogenous testosterone include reversal of virilization and resumption of menses (9). In 2017, Armuand et al. (10) reported that discontinuation of GAHT triggered gender incongruence and dysphoria because of resulting physical changes during GAHT hiatus at the time of fertility preservation among TG men (10). Therefore, it is essential to focus on the impact of GAHT on the fertility potential of TGD individuals. The aim of this study was to provide reassuring data on fertility treatment outcomes in the setting of historical GAHT and contribute to the growing body of literature about TG men in the fertility care setting.
Materials and methods
This descriptive, retrospective cohort study included all TG men who presented for care at a single academic fertility center between January 2013 and December 2021. Patients are presented to care by self-referral, referral from their general obstetrician-gynecologist, or the affiliated academic hospital’s center for transgender medicine. Using natural language processing within the computerized database of the electronic medical record, the patient cohort was identified using the following keywords: “transgender,” “trans,” “trans male,” “transmale,” “trans man,” “transman,” “FTM,” “female to male,” and “natal female.” Additionally, the electronic medical record was queried for chart alerts that indicated to the provider that the patient was TG or was a TG partner of a cisgender patient undergoing fertility consultation or treatment. All identified patient charts were reviewed by two independent reviewers for assessment for inclusion and subsequent data collection.
Baseline demographics were collected on all patients and included the following: age, body mass index, and partner status. A detailed medical history of prior and planned gender-affirming care was obtained via a review of the identified patient’s medical records. Gender-affirming care data included GAS, GAHT with testosterone, exogenous testosterone dose range, serum testosterone level at intake, and time on and off testosterone therapy. Additionally, ovarian reserve characteristics, including antimüllerian hormone (AMH) levels, day 3 follicle-stimulating hormone (FSH) levels, and basal antral follicle count (BAFC), were recorded. Patients were subsequently divided into three different cohorts on the basis of planned utilization of fertility services and assisted reproductive technology (ART). Cohort 1 included patients who underwent embryo cryopreservation, in vitro fertilization (IVF), or co-IVF, with their cisgender female partner being the planned embryo recipient (11). Cohort 2 included patients undergoing oocyte cryopreservation. Cohort 3 included patients undergoing intrauterine insemination (IUI). Per ASRM and the World Professional Association for Transgender Health guidelines (7), all TG men ceased GAHT with exogenous testosterone before proceeding to treatment. However, patients were not instructed to await the resumption of menses before the initiation of treatment. Nor was treatment delayed until the serum testosterone level was within the normal female range.
This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai, with a waiver of consent for retrospective analysis of deidentified data.
Cohort 1: IVF, Co-IVF, and Embryo Cryopreservation Cycles
Patients underwent controlled ovarian stimulation, which was performed as previously described (12, 13). Ovarian follicle growth was measured with transvaginal ultrasonography. Recombinant or purified human chorionic gonadotropin, leuprolide acetate, or a combination was used to induce final oocyte maturation once two or more follicles reached a mean diameter of ≥18 mm. Oocyte retrieval was performed 36 hours later under transvaginal ultrasound guidance. All metaphase II (MII) oocytes were fertilized either using intracytoplasmic sperm injection (ICSI) or conventional insemination. All embryos were cultured to the blastocyst stage as described previously (14). For cycles with preimplantation genetic testing for aneuploidy, trophectoderm biopsy was performed on days 5, 6, or 7 of embryo development once embryos achieved an adequate morphologic grade (modified Gardner morphologic score 4CC or higher), and then embryos were vitrified as described previously (15). Embryos were transferred in either a fresh or frozen cycle to the intended recipient with luteal support by vaginal and/or intramuscular progesterone. The total number of embryos transferred was done in accordance with the ASRM practice committee guidelines (16).
Only the first stimulation, cryopreservation, and first transfer cycles were evaluated to capture the stimulation outcome of the cycle in the closest proximity to discontinuation of testosterone therapy.
Cohort 2: Oocyte Cryopreservation Cycles
Patients underwent similar treatment as cohort 1, but treatment ceased after vitrification of all MII oocytes, as described previously (17). Only the first stimulation and cryopreservation cycles were evaluated to capture the stimulation outcome of the cycle in the closest proximity to the discontinuation of testosterone therapy.
Cohort 3: IUI Cycles
Ovarian stimulation and IUI were performed as previously described (18). Ovarian stimulation was performed using clomiphene citrate for five consecutive days, beginning on cycle day 3 and continuing until cycle day 7. Monitoring using transvaginal ultrasound was performed starting on cycle day 11 or 12 until a dominant follicle was identified, at which point ovulation was triggered with recombinant purified human chorionic gonadotropin hormone. Intrauterine insemination was performed 36 hours after the ovulation trigger was administered (19). A single IUI was performed using processed and prepared fresh or frozen ejaculate. Once the sperm and its suspension media were aspirated into a syringe and attached to an insemination catheter, the specimen was injected into the uterine cavity in a sterile fashion. All IUI cycles were evaluated for all patients.
Cycle Evaluation
The cycle data were collected depending on the course of treatment. Most study patients (cohorts 1 and 2) underwent ovarian stimulation, and the following cycle characteristics were collected: total gonadotropin dose used, surge estradiol (E2) levels, number of oocytes retrieved, number of mature oocytes, type of fertilization, maturity rate, number fertilized, fertilization rate, number of blastocysts, blastulation rate, embryo grade, and pregnancy outcome. Within cohort 3, the type of IUI cycle (medicated vs. natural) and number of follicles at trigger were recorded.
The primary outcome for patients undergoing IVF, co-IVF, or IUI was clinical pregnancy as defined by visualization of an intrauterine gestational sac on transvaginal ultrasonography in the setting of a positive pregnancy test result. The primary outcome for patients undergoing embryo or oocyte cryopreservation was the number of embryos and oocytes frozen, respectively.
Results
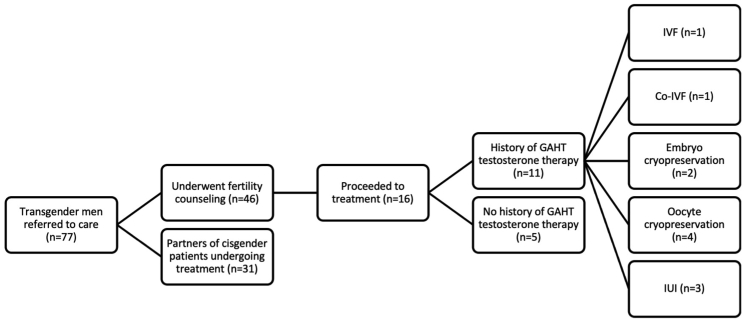
Between 2013 and 2021, 77 TG men self-presented or were referred to care at a single academic fertility center. Figure 1 illustrates the care pathway of patients who presented for care in this study. Of the 77 TG men captured, 31 (40.3%) were partners of cisgender patients undergoing treatment. The remaining 46 (59.7%) TG men underwent fertility preservation and/or family-building counseling, with 16 (20.8%) patients proceeding to fertility treatment. Of those patients who underwent treatment, 11 (68.8%) had a history of GAHT with exogenous testosterone use. Of these 11 patients, fertility treatment types included IVF, co-IVF, embryo cryopreservation, oocyte cryopreservation, and IUI. Cohort 1 included patients undergoing IVF, co-IVF, and embryo cryopreservation (n = 4); cohort 2 included patients undergoing oocyte cryopreservation (n = 4); and cohort 3 included patients undergoing IUI (n = 3).
Figure 1.
A flow diagram of patient care. This figure illustrates the care pathway of patients who presented for care in this study. Of the 77 transgender (TG) men, 31 (40.3%) were partners of cisgender patients undergoing treatment. The remaining 46 (59.7%) TG men underwent fertility preservation and/or family-building counseling, with 16 (20.8%) patients proceeding to fertility treatment. Of those who underwent treatment, 11 (68.8%) had a history of gender-affirming hormone therapy (GAHT) with exogenous testosterone use. Of these 11 patients, fertility treatment types included in vitro fertilization (IVF), co-IVF, embryo cryopreservation, oocyte cryopreservation, and intrauterine insemination (IUI). Cohort 1 included patients undergoing IVF, co-IVF, and embryo cryopreservation (n = 4); cohort 2 included patients undergoing oocyte cryopreservation (n = 4); and cohort 3 included patients undergoing IUI (n = 3).
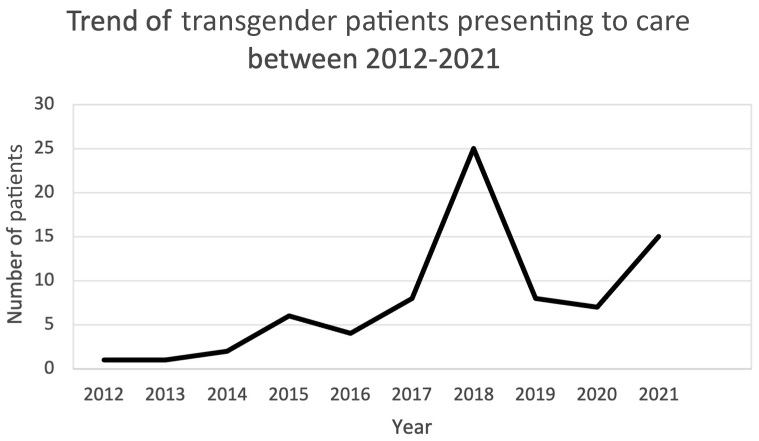
Figure 2 illustrates the trend of TG male patients who presented for consultation over the study period. There was an overall increase in TG males presenting for care over the 8 years of the study. Notably, 82% (n = 63) of patients who presented for care over the study period did so after the establishment of the Mount Sinai Center for Transgender Medicine and Surgery in 2016. In 2019, the number of patients presenting for care decreased by 68%, then began to rise again in 2021.
Figure 2.
The trend of transgender patients presenting for care illustrates the trend of transgender male patients who presented for consultation over the study period. There was an overall increase in transgender males presenting to care over the 8 years of the study. Notably, 82% (n = 63) of patients who presented for care over the study period did so after the establishment of the Mount Sinai Center for Transgender Medicine and Surgery in 2016. In 2019, the number of patients presenting for care decreased by 68%, then began to rise again in 2021.
Demographics
Eleven of the 16 (68.8%) patients who underwent treatment had previously taken exogenous testosterone for GAHT. The average age for TG men with prior testosterone use was 26.8 ± 4.6 years. The average body mass index for TG men with prior testosterone use was 24.3 ± 3.7. Of the patients who proceeded to cycle, 56.3% presented with a partner.
Gender-Affirming Care History
None of the TG men who underwent fertility treatment had a history of genital surgery. All TG men who underwent exogenous testosterone therapy for GAHT temporarily discontinued its use before proceeding to treatment. Nine of the 11 (81.1%) patients who had prior testosterone use did not resume menses.
On average, the testosterone dose before discontinuation was 45.8 ± 19.1 mg/week (range 25–62.5 mg/week). The average testosterone level at intake was 383.2 ± 421.1 ng/dL, with a wide range of 38–968 ng/dL. The time on and off of testosterone therapy also varied widely. Total time on testosterone ranged from 3 weeks to 120 months, and time off testosterone ranged from 2 weeks to 24 months.
Ovarian reserve characteristics
Antimüllerian hormone levels, day 3 FSH levels, and BAFC were all within reason for reproductive-age people with ovaries. The average AMH level was 4.5 ± 3.8 ng/mL, the average day 3 FSH level was 6.4 ± 2.2 IU/mL, and the average BAFC was 20.3 ± 8.3.
Cohort 1: IVF, Co-IVF, and Embryo Cryopreservation Cycle Outcomes
The ART outcomes for cohort 1 are summarized in Table 1. Within cohort 1, the average amount of total gonadotropin used throughout the cycle was 3,930.8 ± 1,859 IU (range of 1,573–4,350 IU). The average peak E2 level was 2,579.5 ± 809.5 pg/mL (range 1,883–3,614 pg/mL). The average maturity rate of oocytes retrieved was 78.1 ± 20.7% (range 50%–100%), and the fertilization rates were all >80%. The average embryo grade was 4AA for all patients. All embryo cryopreservation cycles froze three or more embryos. Both the patients who underwent IVF and the patients who underwent co-IVF achieved live births (LBs).
Table 1.
In vitro fertilization (IVF), co-IVF, and embryo cryopreservation cycle outcomes in transgender men with a history of receiving testosterone therapy.
| Patient | Age | Cycle type | Ovarian stimulation characteristics |
Ovarian stimulation cycle outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length of cycle (days) | Total gonadotropin used (IU) | Peak E2 (pg/mL) | Number of oocytes retrieved | Number of MIIs | Maturity rate (%) | Number of MII oocytes successfully fertilized | Fertilization Rate (%) | Average Embryo Grade | Outcome | |||
| 1 | 24 | IVF | 7 | 1573 | 2831 | 34 | 28 | 82.4 | 25 | 89.3 | 3BA | Live birth |
| 2 | 33 | Co-IVF | 9 | 3725 | 3614 | 20 | 16 | 80 | 16 | 100 | 4AC | Live birth |
| 3 | 31 | Embryo cryopreservation | 12 | 6075 | 1883 | 6 | 3 | 50 | 3 | 100 | 4AA | Freeze all |
| 4 | 26 | Embryo cryopreservation | 12 | 4350 | 1990 | 5 | 5 | 100 | 4 | 80 | 5AA | Freeze all |
| Mean | 10.0 ± 2.4 | 3930.8 ± 1859 | 2579.5 ± 809.5 | 16.3 ± 13.7 | 13.0 ± 11.5 | 78.1 ± 20.7 | 12 ± 10.5 | 92.3 ± 9.6 | 4AA | |||
| Range | 7-12 | 1573-4350 | 1883-3614 | 5-34 | 3-28 | 50-100 | 3-25 | 80-100 | ||||
MII = metaphase II oocyte.
Cohort 2: Oocyte Cryopreservation Cycle Outcomes
Within cohort 2, the average length of the cycle was 9.3 ± 1.5 days (range 7–10). The average amount of total gonadotropin used was 2,265.5 ± 2,028.6 IU (range 1,212–3,900 IU), and the average peak E2 level was 3,227.8 ± 17.4 pg/dL (range 1,239–6,062 pg/dL). The average number of oocytes retrieved was 24.3 ± 17.4 (range 8–49), and the average number of mature oocytes frozen was 19.3 ± 16.2 (range 6–43).
Cohort 3: IUI Cycle Outcomes
All patients undergoing IUI underwent 1–2 cycles. Four of the five IUI cycles used clomiphene citrate (Clomid) as the ovulatory stimulating agent, although the remaining cycle was a natural, unmedicated cycle. Each cycle had one mature follicle at the time of trigger, and all patients within this group achieved LB.
Discussion
Because access to reproductive healthcare expands across the United States, the TGD community will continue to have increased utilization of fertility care services. Therefore, it is essential for providers to gain a better understanding of the specific needs of the TGD population. It is also imperative to gather more comprehensive data regarding treatment outcomes for this population and the challenges they face when undergoing treatment.
This study has one of the most robust sample sizes of studies evaluating ART outcomes in TG men who had used testosterone therapy previously. All patients who were receiving GAHT with exogenous testosterone (n = 16) discontinued its use before initiating fertility cycles in this study. It was found that six patients completed oocyte or embryo cryopreservation, whereas two completed fresh or frozen transfers with LBs. All patients who completed IVF or embryo cryopreservation produced high-quality blastocytes. To our knowledge, this is also the first to evaluate IUI cycles in patients with a history of GAHT testosterone use.
Our study builds on prior work and adds to the growing body of data focused on the impact of GAHT testosterone therapy on fertility care. Prior small cohort studies have described fertility outcomes in TG men. Several of these focused on TG men who chose to complete oocyte cryopreservation before initiating testosterone therapy (20, 21, 22). Other studies evaluated outcomes for TG patients with prior use of exogenous testosterone therapy (23, 24, 25, 26, 27). In particular, a 2019 study by Leung et al. (25) and a 2020 study by Amir et al. (26) compared outcomes from oocyte cryopreservation, IVF with embryo cryopreservation, and IVF with embryo transfer in TG men with a history of testosterone use to fertile cisgender women. In our study, the total amount of gonadotropin used and peak E2 level within our patient population were consistent with those used in prior studies (25, 26). Additionally, we found no correlation between patient age, time on or off testosterone therapy, total gonadotropin used, and the number of oocytes retrieved, as previously reported (28). Notably, 100% of the IUI cycles achieved pregnancy using clomiphene citrate. Although we do not have explicit documentation regarding the reason providers chose to stimulate with certain dosages, the results are reassuring that a history of testosterone therapy does not require higher doses of gonadotropin, which, in turn, minimizes the risk of ovarian hyperstimulation syndrome in patients, although supporting their ability to achieve good results. It is also important to mention several case reports describing TG men who elected to continue testosterone therapy despite undergoing controlled ovarian stimulation (29, 30, 31). Although these studies report feasibility in ovarian stimulation and oocyte cryopreservation without testosterone cessation, the impact of active testosterone therapy on reproductive potential has yet to be elucidated in prospective or higher volume studies.
Within our study population, we identified patterns in patient demographics that may be useful in counseling TG patients with a history of testosterone use. It was found that 56.3% of TG men who proceeded to cycle presented with a partner. Almost all patients engaging in active family building had partners. although 66.7% of patients who underwent fertility preservation did not present to treatment with a partner. All patients who attempted pregnancy were married or in a domestic partnership, aside from one single patient who underwent IUI with donor sperm. All patients completing fertility preservation cycles were single, aside from one married patient who underwent embryo freezing with their partner before the planned GAS. This information may aid providers in anticipating which types of fertility treatment their TG patients may inquire about, depending on their marital or partner status, although a thorough discussion with patients regarding all of their fertility treatment options is still recommended.
A strength of this study is that it is the first of its kind to capture the entire breadth of fertility consultation and care for TG men. Our patient population underwent consultation, IVF, co-IVF, IUI, egg cryopreservation, and embryo cryopreservation. Because we were able to capture all patients who contacted the practice to access care, we were able to gain a better understanding of the significant attrition rate within this patient population.
It is important to note the significant attrition rate (65.2%) from referral to treatment within our study. Although the data are limited, this is consistent with other studies that have had a high attrition rate ranging from 50.9%–68.4% (25, 26). Because this study was retrospective in nature, we were limited in our ability to assess why patients did not proceed to care or reasons for discontinuation after prior cycle(s). Prior studies have found prohibiting factors ranging from cost, to reluctance toward postponing gender-affirming care to concerns about gender dysphoria (32). Despite the attrition rate, it is important to point out the increased access to comprehensive gender-affirming care that is provided in a specialized center for TG medicine. As mentioned previously, 82% of patients who presented to care over the study period did so after the establishment of the Mount Sinai Center for Transgender Medicine and Surgery. The establishment of such centers helps patients navigate an incredibly challenging healthcare landscape and find crucial TGD resources. Therefore, as the number of TG patients using fertility services increases, the high attrition rate for TG patients is likely not explained by a lack of visibility or a lack of providers to care for this group. Instead, patient attrition is more likely explained by other barriers described previously. Although there is no clear explanation for the decrease in patients presenting in 2019, the persistent decline in 2020 can likely be attributed to the coronavirus disease of the 2019 pandemic. This high attrition rate will be a worthwhile focus of future investigations to better understand the varied reasons why this particular patient population defers or declines fertility care. Additionally, disparities in care are an important part of the comprehensive understanding of this patient population that we should seek to develop as clinicians. Disparities can be found within access to care, ability to complete treatment, and patient demographics. With more knowledge regarding these factors, providers can build actionable counters to improve access to care. The creation of normative data sets mapping access to care across several points in time for the TGD population will help create pathways that cater to the needs of the community.
Similar to many prior studies on fertility care for TG men, the main weakness of this study is the limited sample size. The challenges faced in a study with a small patient population prompt further areas for growth in future research. Although the number of TG men presenting to individual fertility centers for consultation is small, multicenter collaborative studies may be a means by which a more substantial patient population can be gathered to draw conclusions that are more definitive.
Another area for growth in working with this patient population is consistency in documentation, regarding gender-affirming care. By establishing an intake protocol for these patients, providers can consistently document key characteristics of gender-affirming care, including prior or planned surgeries, history of GAHT, route of administration, dosing, and time on and off treatment.
To our knowledge, the patients in this study tolerated stimulation, retrievals, and transfers well. However, another limitation of our study related to documentation was the lack of explicit follow-up on complaints or complications from fertility treatment, particularly pertaining to triggers of gender dysphoria. Future studies should include built-in models and providers to assess TG patient experience within the comprehensive care model (e.g., social work, nutrition, psychology). A model such as this would be a means by which providers may be able to better identify patients who were triggered, experienced gender dysphoria, or had other related issues. This information could then be used to identify areas for improvement in the patient experience.
Conclusions
This study demonstrates that TG men who have used GAHT previously can successfully undergo fertility treatments to attain oocyte and embryo cryopreservation, pregnancy, and LBs. Although all patients in this study discontinued testosterone therapy before completing cycles, future multicenter studies with large sample sizes are needed to evaluate ART outcomes in patients who remain receiving testosterone therapy. A large-scale study of this nature may ultimately facilitate improved outcomes for these individuals by mitigating concerns about the physical and psychological effects of suspending GAHT. Because trends at this particular fertility center have demonstrated, that more TG males have pursued fertility care and ART over time, and it is evident that this community seeks a wide array of services. In conclusion, this study provides reassurance that, through evidence-based counseling, we can support patients in balancing their desires for family building or fertility preservation while simultaneously transitioning to or maintaining their gender identity.
Declaration of interests
A.G. has nothing to disclose. S.L.E. has nothing to disclose. C.G. has nothing to disclose. D.G. has nothing to disclose. J.A.L. has nothing to disclose. K.T. has nothing to disclose. A.B.C. reports stock options and leadership roles for Progyny and Sema4 outside the submitted work.
References
- 1.Herman J.L., Flores A.R., O’Neill K.K. How many adults and youth identify as transgender in the United States? https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/ Available at:
- 2.Pirtea P., Ayoubi J.M., Desmedt S., T’Sjoen G. Ovarian, breast, and metabolic changes induced by androgen treatment in transgender men. Fertil Steril. 2021;116:936–942. doi: 10.1016/j.fertnstert.2021.07.1206. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K., Baba T., Noguchi H., Nagasawa K., Endo T., Kiya T., et al. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28:453–461. doi: 10.1093/humrep/des385. [DOI] [PubMed] [Google Scholar]
- 4.De Roo C., Lierman S., Tilleman K., Peynshaert K., Braeckmans K., Caanen M., et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online. 2017;34:557–566. doi: 10.1016/j.rbmo.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Ghofranian A., Aharon D., Friedenthal J., Hanley W.J., Lee J.A., Daneyko M., et al. Family building in transgender patients: modern strategies with assisted reproductive technology treatment. Transgend Health. 2022 doi: 10.1089/trgh.2021.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch M.B., Feldman J.L. Updated recommendations from the world professional association for transgender health standards of care. Am Fam Phys. 2013;87:89–93. [PubMed] [Google Scholar]
- 7.Ethics Committee of the American Society for Reproductive Medicine Access to fertility services by transgender and nonbinary persons: an Ethics Committee opinion. Fertil Steril. 2021;115:874–878. doi: 10.1016/j.fertnstert.2021.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Hembree W.C., Cohen-Kettenis P.T., Gooren L., Hannema S.E., Meyer W.J., Murad M.H., et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 9.Lai T.C., McDougall R., Feldman D., Elder C.V., Pang K.C. Fertility counseling for transgender adolescents: a review. J Adolesc Health. 2020;66:658–665. doi: 10.1016/j.jadohealth.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Armuand G., Dhejne C., Olofsson J.I., Rodriguez-Wallberg K.A. Transgender men’s experiences of fertility preservation: A qualitative study. Hum Reprod. 2017;32:383–390. doi: 10.1093/humrep/dew323. [DOI] [PubMed] [Google Scholar]
- 11.Yeshua A., Lee J.A., Witkin G., Copperman A.B. Female couples undergoing IVF with partner eggs (Co-IVF): pathways to parenthood. LGBT Health. 2015;2:135–139. doi: 10.1089/lgbt.2014.0126. [DOI] [PubMed] [Google Scholar]
- 12.Sekhon L., Lee J.A., Flisser E., Copperman A.B., Stein D. Blastocyst vitrification, cryostorage and warming does not affect live birth rate, infant birth weight or timing of delivery. Reprod Biomed Online. 2018;37:33–42. doi: 10.1016/j.rbmo.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Purata J., Lee J., Whitehouse M., Duke M., Grunfeld L., Sandler B., et al. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet. 2016;33:401–412. doi: 10.1007/s10815-016-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazem T.G., Sekhon L., Lee J.A., Overbey J., Pan S., Duke M., et al. The correlation between morphology and implantation of euploid human blastocysts. Reprod Biomed Online. 2019;38:169–176. doi: 10.1016/j.rbmo.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Nieto C., Lee J.A., Slifkin R., Sandler B., Copperman A.B., Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34:1697–1706. doi: 10.1093/humrep/dez129. [DOI] [PubMed] [Google Scholar]
- 16.Practice Committee of the American Society for Reproductive Medicine and the Practice Committee for the Society for Assisted Reproductive Technologies Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2021;116:651–654. doi: 10.1016/j.fertnstert.2021.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Practice Committees of the American Society for Reproductive Medicine and Society of Reproductive Biologists and Technologists A review of best practices of rapid-cooling vitrification for oocytes and embryos: a committee opinion. Fertil Steril. 2021;115:305–310. doi: 10.1016/j.fertnstert.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Purata J., Lee J., Whitehouse M., Sandler B., Copperman A., Mukherjee T. Comparision of letrozole with timed intercourse versus clomiphene citrate with intrauterine insemination in patients with unexplained infertility. J Reprod Med. 2016;61:425–430. [PubMed] [Google Scholar]
- 19.Nazem T.G., Chang S., Lee J.A., Briton-Jones C., Copperman A.B., McAvey B. Understanding the reproductive experience and pregnancy outcomes of lesbian women undergoing donor intrauterine insemination. LGBT Health. 2019;6:62–67. doi: 10.1089/lgbt.2018.0151. [DOI] [PubMed] [Google Scholar]
- 20.Amir H., Oren A., Klochendler Frishman E., Sapir O., Shufaro Y., Segev Becker A., et al. Oocyte retrieval outcomes among adolescent transgender males. J Assist Reprod Genet. 2020;37:1737–1744. doi: 10.1007/s10815-020-01815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D., Bernardi L.A., Pavone M.E., Feinberg E.C., Moravek M.B. Oocyte cryopreservation among transmasculine youth: a case series. J Assist Reprod Genet. 2018;35:2057–2061. doi: 10.1007/s10815-018-1292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell S., Noyes N., Keefe D., Berkeley A.S., Goldman K.N. Pregnancy outcomes after fertility preservation in transgender men. Obstet Gynecol. 2017;129:1031–1034. doi: 10.1097/AOG.0000000000002036. [DOI] [PubMed] [Google Scholar]
- 23.Insogna I.G., Ginsburg E., Srouji S. Fertility preservation for adolescent transgender male patients: a case series. J Adolesc Health. 2020;66:750–753. doi: 10.1016/j.jadohealth.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Barrett F., Shaw J., Blakemore J.K., Fino M.E. Fertility preservation for adolescent and young adult transmen: a case series and insights on oocyte cryopreservation. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.873508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung A., Sakkas D., Pang S., Thornton K., Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril. 2019;112:858–865. doi: 10.1016/j.fertnstert.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Amir H., Yaish I., Samara N., Hasson J., Groutz A., Azem F. Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. J Assist Reprod Genet. 2020;37:2463–2472. doi: 10.1007/s10815-020-01902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeleye A.J., Cedars M.I., Smith J., Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet. 2019;36:2155–2161. doi: 10.1007/s10815-019-01558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albar M., Koziarz A., McMahon E., Chan C., Liu K. Timing of testosterone discontinuation and assisted reproductive technology outcomes in transgender patients: a cohort study. F S Rep. 2023;4:55–60. doi: 10.1016/j.xfre.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho K., Harjee R., Roberts J., Dunne C. Fertility preservation in a transgender man without prolonged discontinuation of testosterone: a case report and literature review. F S Rep. 2020;1:43–47. doi: 10.1016/j.xfre.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gale J., Magee B., Forsyth-Greig A., Visram H., Jackson A. Oocyte cryopreservation in a transgender man on long-term testosterone therapy: a case report. F S Rep. 2021;2:249–251. doi: 10.1016/j.xfre.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark B.A., Mok-Lin E. Fertility preservation in transgender men without discontinuation of testosterone. F S Rep. 2022;3:153–156. doi: 10.1016/j.xfre.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpern S., Yaish I., Wagner-Kolasko G., Greenman Y., Sofer Y., Paltiel Lifshitz D., et al. Why fertility preservation rates of transgender men are much lower than those of transgender women. Reprod Biomed Online. 2022;44:943–950. doi: 10.1016/j.rbmo.2022.01.003. [DOI] [PubMed] [Google Scholar]