Abstract
The incorporation of HPV DNA testing into cervical screening programs has shown that many HPV-positive women are cytologically normal, with HPV-positivity fluctuating throughout life. Such results suggest that papillomaviruses may persist in a latent state after disease clearance, with sporadic recurrence. It appears that virus latency represents a narrow slot in a wider spectrum of subclinical and possibly productive infections. Clinical studies, and animal model infection studies, suggested a key role for host immune surveillance in maintaining such asymptomatic infections, and although infections may also be cleared, most studies have used the term ‘clearance’ to describe a situation where the presence of HPV DNA falls below the clinical detection level. Given our knowledge of papillomavirus immune evasion strategies and the restricted pattern of viral gene expression required for ‘basal cell’ persistence, the term ‘apparent clearance’ and ‘subclinical persistence’ of infection may better summarise our understanding. Subclinical infection also encompasses the lag phase, which occurs between infection and lesion development. This is dependent on infection titre, with multifocal infections developing more rapidly to disease. These concepts can usefully influence patient management where HPV-positivity occurs sometime after the onset of sexual activity, and where vertical transmission is suspected despite a lag period.
1. Chronic viral infections typically require a ‘reservoir of infection’ to facilitate persistence
Virus infections are generally classified as ‘acute’ or ‘chronic’, according to their infection strategies and the disease biology that they cause. Acute infections are typically characterised by the rapid development of symptomatic disease - followed by the initiation of an adaptive immune response, which over a period of days or weeks leads to virus clearance and the resolution of disease symptoms [1,2]. Many respiratory infections, including those caused by Influenza and Coronavirus follow this route, and once the initial infection is brought under control and disease symptoms subside, the protective immune response will gradually decline, allowing subsequent reinfection and disease recurrence with milder systems. Viruses which cause acute infections are typically associated with cycles of infection and clearance through life, and for many RNA viruses, this is facilitated by error prone viral replication which contributes to virus diversity [1,2].
An alternative life cycle strategy that is also commonly used by viruses, including Human Immunodeficiency virus (HIV), Human T-cell leukaemia virus (HTLV), Polyomaviruses and several members of the Herpes virus family, is to establish a chronic infection in a cell type that can act as a reservoir of infection, but which is not lysed by the virus. Typically, such ‘cellular reservoirs of infection’ are able to persist in the body over an extended period of time without being recognised by the immune system - a situation which facilitates the production of infectious virus particles as these cells divide to give rise to ‘daughter cells’ which go on to differentiate [[3], [4], [5]]. This is the strategy used by Epstein Barr virus and Cytomegalovirus for instance, which persist in B cell and monocytes reservoirs respectively, but is also the strategy used by papillomaviruses, who’s cellular reservoir of infection is contained amongst the basal cells that make up the epithelial basal layer [6,7]. Viruses which establish chronic infections in this way are often controlled by the host’s immune system, which can regulate the extent of productive infection, but which cannot clear the cellular reservoir of infection where viral gene expression is maintained at very low levels. Indeed, the so-called ‘latently infected cells’ that have been characterised by Herpes virus biologists, have tightly regulated latency-associated virus transcription patterns, and represent a cellular reservoir from which productive infection can be initiated when the immune environment allows it [8]. The separation of the HPV life cycle into an immunologically quiet basal cell ‘reservoir compartment’, and a ‘productive/genome amplification compartment’ which is derived from this reservoir following differentiation [9,10], we can draw analogies between papillomaviruses and other viruses which cause chronic infections and which have well established subclinical and/or latent states.
2. Human Papillomavirus persistence at the population level; virus shedding & transmission strategies
Papillomaviruses are a diverse group of small DNA viruses that have genomes of around 8 thousand base pairs, and which infect a wide range of epithelial sites in a host and tissue-specific manner [11]. Their evolutionary divergence, which has led to the emergence of more than 200 human types, has been linked to the evolution of epithelial structures in their various hosts, which includes reptiles, birds, marsupials and mammals [12]. Cutaneous epithelial hair follicles are a rich source of Beta and Gamma HPV types in humans, while in the mouse and other animal species, papillomavirus types from a range of genera have been identified, with characteristics and tropisms similar to human Beta types. Members of the human Mu genus are thought to have a tropism for eccrine glands, while the human Alpha papillomavirus types complete their life cycle at a range of interfollicular epithelial sites, and have diverged into distinct species groups with tropism preferences for oral or genital epithelial sites, or for the cutaneous epithelium where members of the Mu, Gamma and Beta papillomaviruses are also found [13]. Interestingly, the vast majority of these viruses cause only benign self-limiting papillomas or subclinical/inapparent infections in immunocompetent hosts, but can cause problematic papillomatosis in immune-compromised individuals, and in individual with genetic susceptibilities which affect the ability of the host to control infection. These genetic predispositions include Epidermodysplasia Verruciformis (EV), Tree Man syndrome and Recurrent Respiratory Papillomatosis, which are associated with cutaneous Beta/Gamma, cutaneous Gamma/Alpha, and low risk ‘mucosal’ Alpha HPV types respectively [14]. The appearance of problematic disease in such individuals, and the widespread distribution of human papillomaviruses in the general population, point to an important role for the immune system in limiting HPV associated disease in the general population. Indeed, the vast majority of Beta and Gamma HPV types have no known disease associations in the general population, even though the vast majority of children become infected before the age of five [15,16]. The conclusion from this is that papillomaviruses from the Beta and Gamma genera have the ability to persist as subclinical productive infections. Furthermore, it appears that their activity is controlled by the normal immunesurveillance mechanisms that protect the skin from pathogens, and that this life cycle strategy is a result of papillomaviruses long term coevolution with their human hosts. Extrapolation of these basic principles to the medically important Alpha HPV types, provides us with a baseline hypothesis from which to understand how sporadic HPV positivity can occur at the cervix throughout life and how subclinical persistence occurs at penile epithelial sites, and also offers a conceptual understanding of the 5% high-risk HPV positivity in mouthwash samples collected from the general population [17,18]. It appears that papillomaviruses produce infectious virions from conspicuous lesions such as warts, as well as from inconspicuous subclinical infections.
3. Defining latency and immune control as components of the Papillomavirus infectious cycle
Viral latency is well recognised as a phase in the life cycle of some viruses, where after initial infection, the production of new virus particles ceases, without the eradication of the virus from the body. In these situations, latency is linked to the possibility of future reactivation and renewed virus particle production as the host immune response changes over time. One of the best characterised example of this is Shingles, which is a re-emergence of a productive Varicella Zoster infection of the skin, which can occur many years after the resolution of chicken pox [1,2]. Our current concepts of papillomavirus latency and reactivation have to a large extent been derived from studies carried out in animal infection models, and appear to fit this definition of ‘viral latency’ [19]. Furthermore, these studies suggest that host immunity may act to suppress the productive stages of the life cycle, but not necessarily clear viral genomes from the infected basal cells, where the expression of viral antigens are below detectable levels. In general, these models have focused on immune responses occurring soon after disease resolution, or have used low-titres of virus or nucleic acid scarification-approaches to generate subclinical infections [20,21]. In human studies, the time since infection is more difficult to precisely assess, and although we may visualise ‘true’ viral latency as a particular type of subclinical infection, it appears more appropriate to regard HPV latency (Fig. 1) as a special state, with clearance of infection, and persistence as subclinical productive infection representing alternative end stages when immune-mediated disease resolution occurs (Fig. 1). Indeed, the subclinical productive infections associated with the Beta and Gamma HPV types appear to represent the successful control of infection by the host, and the suppression of HPV gene expression by immunesurveillance to prevent problematic disease. Our basic understanding of how these stages in the HPV infectious cycle are modulated is relatively uncontroversial, and is drawn from observations made using other virus systems, and also from the use of both immune compromised and immune competent animal models of papillomavirus infection and disease clearance, as outlined in Fig. 2 and described below.
-
A
The Post-Infection Incubation Period or Lag Phase
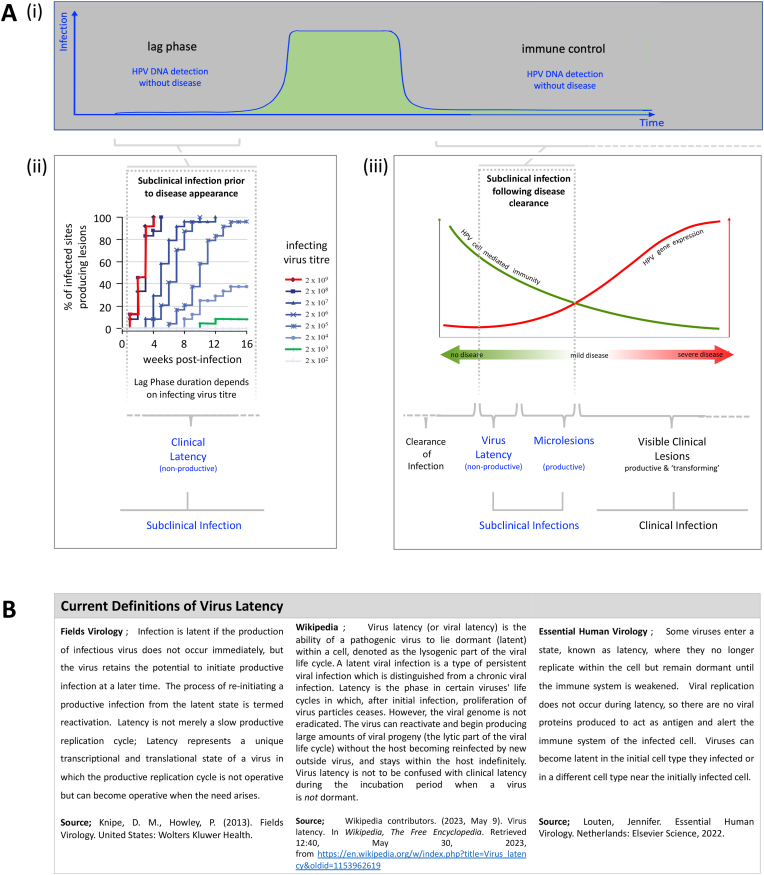
Fig. 1.
Virus Latency as a Specific Category of Subclinical Infection. A(i) Although papillomaviruses cause chronic clinically-apparent infections that may persist for months or years, these are preceded by subclinical infection stages known as the lag phase (or incubation period), where infected cells are dividing as part of the lesion-formation process, and a period of immune control, where viral gene expression is suppressed by the host immune system. A(ii) The duration of the lag phase is dependent on the infectious virus titre, and can extend to at least 3 months in experimental systems [24]. The term ‘clinical latency’ is sometimes used to distinguish this phase from ‘virus latency’. A(iii) Subclinical infections occurring after disease clearance include those that are productive, and those that fit the established definition of ‘virus latency’ which requires maintenance of a reservoir of infection without virus synthesis, but with the possibility of reactivation. B) Virus latency is a recognised stage in the life cycle of many viruses, and is defined in a similar way by different sources [1,2,55].
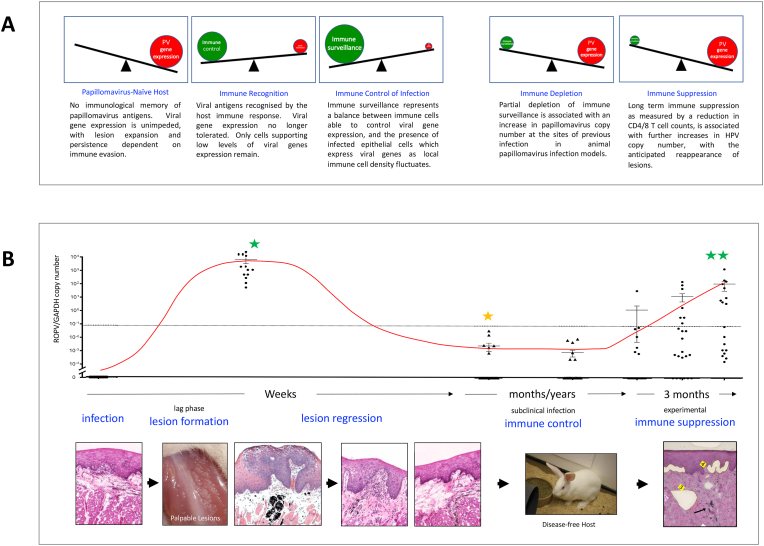
Fig. 2.
Immune Control of Papillomavirus Infections. A) The balance between viral gene expression and the immune response to infection is shown in a see-saw format at the different stages of the papillomavirus infection cycle. In a naïve host with no immunological memory of the infecting papillomavirus types, lesion formation proceeds unchecked. As the immune response develops, viral gene expression (and cells expressing viral genes) declines. Transient immune depletion or more prolonged immune suppression can change immune surveillance status to allow the redetection of papillomavirus DNA. B) Summary of infection and regression data obtained using the rabbit model of mucosal epithelial infection [19,22]. In this system, experimental infection leads to the rapid appearance of palpable lesions over the course of 5 weeks with papillomavirus DNA readily detectable in surface swabs (single green star). Following immune regression, detectable copies decline by approximately seven orders of magnitude (orange star; note log scale), but increase following sustained immune suppression (double green star). The images beneath show tissue histology and disease pathology during the course of lesion formation and regression (following H&E staining), as well as the appearance of the experimentally-induced palpable lesions on the tongue. Following lesions regression, the animals showed no symptoms. The right-most histology image shows the sites of tissue fragments that have been removed following laser-capture microdissection for the quantitation of papillomavirus DNA as described in Refs. [19,22]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Following the infection of an immunologically naïve (new) host, where immunity to the infecting papillomavirus type has not yet developed, lesion formation can occur quite rapidly (Fig. 2), with visible lesions apparent by week 4 or 5 weeks post infection [22,23]. The timing of lesion formation can however take longer than this, and in experimental systems, the process takes over three months when infectious titres are low (Fig. 2, [24]) with early studies in humans proposing a range of between 3 and 18 months [25]. The explanation for this appears straightforward. Low infectious titres result in small numbers of infected basal cells, and because these infected cells must expand in competition with their uninfected neighbours to produce a lesion, the time before palpable lesions are seen can be lengthy. By contrast, high titre inoculation gives rise to multiple foci of infection and the appearance of lesions in weeks, a situation which may explain why genital warts transmitted by close physical contact have been reported to be polyclonal [26], while indirectly transmitted plantar warts can be clonal [27]. In virological terms, the lag phase is distinct from the latent phase, and indeed, the idea that the lag phase may extend for months or even years in some cell environments, has significant implications when considering vertical transmission of HPV from mother to child, and the extended time frame of lesion appearance that has sometimes been reported.
-
B
Mechanisms of Lesion Persistence in Immune Competent Individuals
HPV infection of cells in the epithelial basal layer, is followed by the expansion of the basal cell population, and eventually to the differentiation of cells from this infection reservoir. The immune status of the host influences these downstream events, and in mouse PV infection models can restrict lesion expansion within days [28]. Lesion expansion requires the expression of viral gene products involved in cell proliferation and the recognition of cell density, and requires PV-mediated modulation of the cellular pathways that normally control epithelial homeostasis [10]. Clearly, the viral E6 protein is an important epithelial homeostasis regulator, with E7 providing additional regulatory functions during cell cycle progression and cell differentiation. In addition, PVs require the expression of E2, and possibly also E1 to ensure episomal HPV genome maintenance and genome partitioning in the basal cell reservoir. Importantly, the evasion of immune detection is also critically important for lesion persistence, and as is typical amongst viruses, papillomaviruses use a plethora of approaches to extend the duration of infection and to optimise virus production and transmission [29]. The most basic of these strategies involves the tight control of viral gene expression in the basal and parabasal epithelial layers where immune detection is most likely, with late gene expression and the assembly of virus particles restricted to the upper epithelial layers [29]. In addition, papillomaviruses use active methods to limit immune detection, including the expression of E5, which restricts MHC class I presentation on the cell surface, and the expression of E7, which suppresses the TAP 1-mediated processing and display of foreign antigens. Indeed, the E6 and E7 gene products, which are integral in driving basal cell expansion and in modulating epithelial homeostasis, have key immune evasion functions, and can dampen the response to a range of cytokines, inhibit pathogen-associated molecular pattern (PAMPS) responses (e.g. to double stranded cytoplasmic DNA), and restrict the local density of immune cells, including the Langerhans cells (and other epithelial dendritic cells) that are a first line of defence in the activation of the adaptive immune system [29,30]. Persistence may also be facilitated in particular cell compartments, and indeed, in the epithelial basal layer a hierarch of cells is thought to exist, with the long-lived stem cell like compartment having distinct survival characteristics [31]. Stem cells may have lower HLA abundance and have other anti-apoptotic mechanisms to resist immune-mediated destruction, with stem cell compartments often considered as immune privileged sites [32]. The bulge region of the hair follicle, and the uterus are sites that can become conditionally immune privileged, and it is though that local immune privileged niches may allow stem cell persistence at other sites [33]. Using these strategies, productive papillomavirus infections are able to evade effective immune control, to persist in the body, and to ensure adequate levels of transmission in the human population.
-
C
Immune Detection, Disease Regression and Immune Surveillance
Despite the evolution of immune evasion functions, the usual end consequence of infection is lesion regression and disease clearance following the activation of a HPV-specific cell-mediated immune response. Our working model suggests that although viral immune evasion functions act to extend lesion-duration, in most individuals a successful antiviral T-cell response to the infecting HPV type can be mounted, and that this occurs with variable timing during the months or years that follow initial infection (Fig. 3). Treatments that increase the chance of immune detection, such as the use of Immiquimod or even partial lesional excision, increase the probability of lesion regression by increasing local immune cell density or by enhancing the access of immune cells to viral antigens. It is clear however, that in some situations, the genetic background of the host affects the course of disease following HPV infection, with defects in the EVER genes [34] or CD28 (amongst others [14,35]) predisposing to the proliferation of cutaneous lesions in EV and Tree man syndrome patients, or CXCR4 mutation which contributes to HPV susceptibility in WHIMS individuals [36]. Although not all HPV susceptibilities are so well defined (e.g. RRP), our general understanding of virus infections [37] tells us that different HPV types will be seen differently in individuals with different HLA backgrounds, and that is likely to influence persistence. Indeed, we can go further and suggest that the host immune response has played an important role in HPV diversification during evolution, with different HPV types having inherently different persistence probabilities that are linked to the genetics and immune status of the host. Unfortunately, our understanding of this has been hampered by the use of DNA-based HPV typing approaches, which cannot precisely establish causality when multiple HPV types are present, and also because of the diversity of environmental and genetic risk factors which influence infection risk and the risk of progression to cancer in the host. Even so, several studies have noted associations between HLA haplotype and the development cervical cancer [38,39], with further analytical clarity requiring consideration of the infecting HPV type and the duration of infection, and the likelyhood of deregulated viral gene expression occurring, which is influenced by the site of infection within the cervix. In situations where disease clearance has been monitored, observational studies suggest that T-cell infiltration may lead to the cytokine-mediated suppression of viral gene expression rather than to extensive cytotoxic T-cell mediated killing of infected cells within the lesion (Fig. 3, [22,40,41]). Although this model fits with observations from both animals and humans [[42], [43], [44]], our mechanistic understanding of the disease regression process is startlingly incomplete, especially given the importance of such information for the design of immune therapeutics. Similarly, we lack a detailed insight of how persistent subclinical infections, such as those associated with the ubiquitous Beta and Gamma types are regulated by the host’s immune system to allow long term virus shedding. Immune control of infection, and long-term immune surveillance is clearly however part of the papillomavirus strategy.
-
D
Immune Control, Latency and the Concept of Clearance or Reactivation
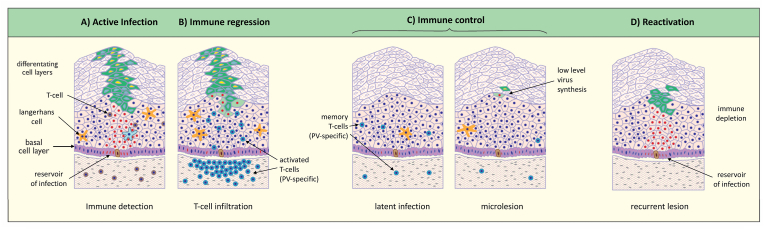
Fig. 3.
Disease Regression and the Immune Control of Papillomavirus Infections. Current thinking suggests that immune detection involves dendritic cells that despite papillomavirus immune-evasion strategies, will eventually become primed to PV-specific antigens (A) and present these to naïve T-cells in the local lymph node. Subsequent T-cell activation and T-cell infiltration is thought to suppress viral gene expression, leaving infected basal cells which already express viral gene products at low levels (B). Immune control and long-term immune surveillance is dependent on memory T-cells and on the balance of ongoing stimulation by viral antigens, and the suppression of viral gene expression following T-cell recognition (C). It is anticipated that this balance of immune control and viral gene expression may support low level virus particle production. (D) Removal of immunesurveillance allows viral gene expression to go unregulated, leading to the redetection of infection as viral copy number increases. Images based on data outlined in Refs. [19,22,56,57].
The sensitivity of HPV DNA detection in cytology samples has been set to maximise clinical utility, and aims to detect the majority HSIL/CIN2+ lesions, in order to allow colposcopy and subsequent treatment. Although HPV DNA testing also detects LSIL/CIN1, it’s sensitivity cut off limits the detection of subclinical or asymptomatic infections which are not associated with a significant cancer risk. At first glance, it is surprising therefore, that a significant fraction of women without cytological abnormalities are identified as HPV positive during routine screening [[45], [46], [47]]. In addition, several studies have shown that HPV positivity can fluctuate over a woman’s lifetime and that the re-occurrence, often after repeated negative HPV tests, shows no apparent correlation with the acquisition of a new sex partner, but can occur in women who are not sexually active at the time of HPV testing, or who are in a stable partner relationship [46]. Given our knowledge of chronic viral infections, and our understanding of the basal cell reservoir where HPV genomes can persist, the simplest explanation is that HPV infections can persist subclinically, and that the levels of HPV DNA at the cervix can sometimes rise above the clinical detection threshold, and can in some individuals, oscillate above and below this threshold throughout life. Indeed, it is well known that immune status can affect the extent of viral gene expression and disease recurrence in individual harbouring cutaneous HPV, and in infection models using immunocompetent animals, immunosuppression led to a dramatic increase in viral copy number at sites of previous disease. In the latter case, where the sites of productive infection had been precisely marked using tattoo ink, papillomavirus copy number rises were found to be restricted to the tattoo site, with tissue sites not previously infected showing only baseline levels of PV DNA. Given these observations, and many others linking host immunity to the control of papillomavirus infection, it appears that there may be a continuum of clinical and subclinical HPV infections at different sites across the body, that are controlled by immunsurveillance. Indeed, the Beta and Gamma HPV types, appear to depend on this balance to ensure the long-term and possibly lifelong shedding of infectious HPV particles from cutaneous skin sites. This description of papillomaviruses as being subject to immune control, but still maintaining an ability to shed infectious virions, does not however fit with the classical definition of viral latency, which precludes vegetative virus synthesis except during periods of reactivation. While subclinical or asymptomatic persistence may be relatively common following disease clearance, the term ‘virological latency’ carries with it a level of inferred precision which remains speculative. Although ‘clinical latency’ is an alternative term that could be used to describe subclinical or asymptomatic infections, it seems that ‘immune control of infection’, more specifically and clearly describes the situation that appears to ensue following disease regression. This brings the question of whether HPV infections are ever completely cleared by the immune system, or whether they are always brought under ‘immune control’. As infected basal cells express viral genes at only very low levels, even during productive infection, we can speculate that complete clearance (by an immune response that must detect the intracellular expression of foreign antigens in the cell) is not a certainty. However, some degree of viral gene expression does appear to be required to maintain the infected cell in the basal layer and to reduce the chance of inadvertent delamination, and we can invoke a latency-associated transcription pattern which would possibly include E6, E2 and E1, in a similar way to the minimal transcript patterns proposed for other viruses. Establishing just how often immune-mediated ‘disease clearance’ gives rise to a persistent but subclinical immune controlled infection, true viral latency or to the absolute clearance of all infected cells from the body remains unresolved. Furthermore, although many clinical ‘natural history’ studies report intermittent patterns of HPV-positivity and negativity, and may also detect recurrence of the same HPV type [48], it is clear that some individuals have a pattern of HPV positivity followed by consistent HPV-negativity. Although this is sometimes reported as clearance of both infection and disease, in most cases the sensitivity of the detection process, which is often carried out on exfoliated cell collected by cytobrush or lavage is not sufficiently rigorous for us to reach such definitive conclusions. Interestingly, our own work using RNAscope approaches has revealed evidence of basal and suprabasal HPV gene expression in biopsies that were negative following HPV typing on the cervical smear but positive in the whole tissue section. It is interesting that other studies using targeted research-based approaches and/or more sensitive tissue-based HPV typing methodologies have suggested that subclinical, immune controlled or latent infections may be more common than at first anticipated, particularly in women with a past history of HPV-associated cervical neoplasia [49,50].
4. Extrapolating our understanding to human disease management, screening & treatment
Although papillomaviruses are often described as infecting epithelial basal cells in order to drive the formation of an epithelial lesion which can support the production and release of new infectious particles, the biology of these viruses is significantly more complex than this. The epithelial sites where the high-risk HPV types cause neoplasia and cancer are not in general the conventional stratified epithelial sites that are modelled in organotypic raft culture, but specific epithelial niches with discrete mechanisms of homeostasis control, that are influenced by local immune and growth factor microenvironments. These niches contain columnar epithelial cells such as at the cervical TZ, as well as epithelial cells with specialised epithelial organisations. The general conclusions drawn from the analysis of animal papillomaviruses need to be considered alongside clinical observations in order generate a working model of how papillomaviruses may behave at these various sites. Clearly the idea that HPV infected cells that remain after surgical excision and which express E6 & E7, may eventually repopulate the surrounding epithelium and reform the lesion is straightforward, and the identification of ‘clear margins’ following the excision of cervical neoplasia attempts to limit this possibility. The stimulation of a cell mediated immune response able to suppress viral gene expression in these infected cells is an important additional control which is likely to contribute to clearance in some individuals (Fig. 4). As cervical cancer typically occurs in women who are unable to effectively resolve their infection, and who do not appear to generate an effective immune response, an additional layer of caution would seem appropriate to limit the possibility of reinfection such as might occur from neighbouring productive LSIL during cervical excision, particularly given our understanding of HPV infection, lesion formation and the HPV titre-dependent lag phase that precedes the appearance of clinical disease. Current vaccines are prophylactic rather than therapeutic, but would appear to have a rationale for use here. Although the lag or incubation period has been known about for decades, the long duration between infection and lesion appearance seen in animal models, may explain some of the long and unexplained time frames reported for vertical transmission that have been reported in the literature, and which should perhaps be taken into account when considering the acquisition of genital warts in young children, especially in cases of possible sexual abuse where correctly assigning the source of infection is important. Interestingly, these animal model transmission studies also highlighted the remarkable stability of papillomavirus particles in the environment and their ability to persist for at least a year without significant loss of titre.
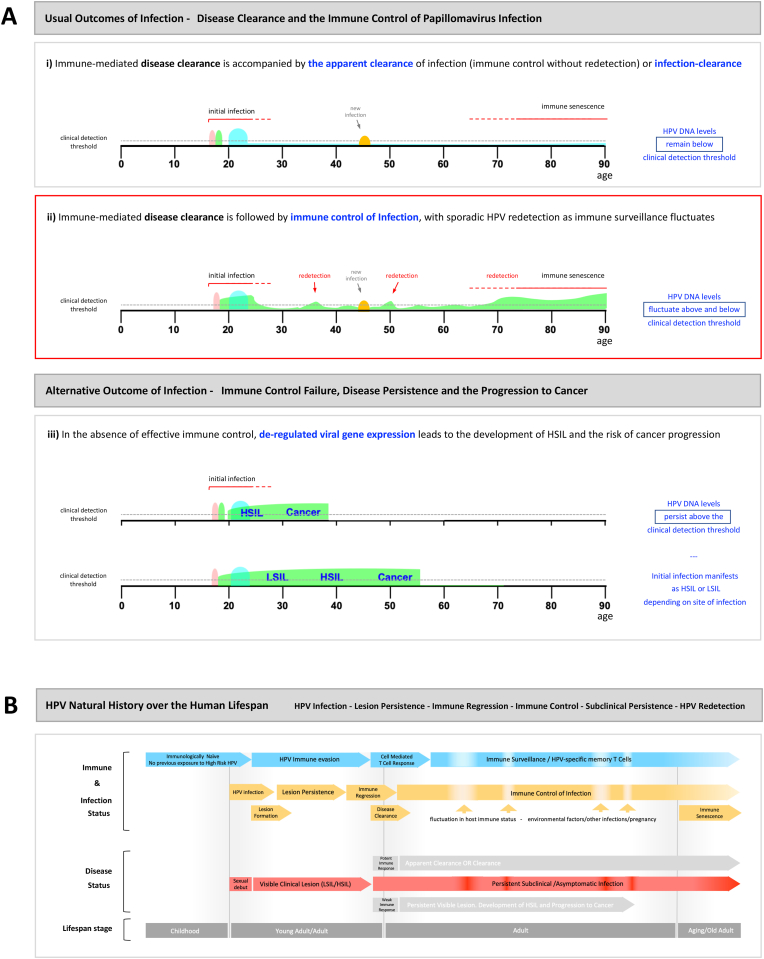
Fig. 4.
Human Papillomavirus Regulation and De-regulation over the Human Lifespan. Extrapolation of our current concepts of papillomavirus infection and immune control to infection by high-risk HPV types over the human lifespan can explains current clinical observations. A(i) Initial infection and disease formation occurs in early adulthood, followed by immune resolution leading to ‘clearance’ or ‘apparent clearance’. Recurrence of the initial infection to levels where DNA levels rise above the clinical detection threshold does not occur in this case and the individual remains HPV-negative. New detection is the result of new infection. A(ii) Initial infection and disease formation occurs in early adulthood, but immune control suppresses viral gene expression without driving clearance of infection. HPV DNA levels rise above the clinical detection threshold sporadically throughout life. New HPV detection may result from reactivation of an existing controlled infection or the acquisition of a new infection. A(iii) The outcomes described in A(i) and (ii) may occur, but one HPV type persists as an active persistent infection with deregulated viral gene expression. At some epithelial sites, such as the cervical transformation zone or the tonsillar crypts, HSIL may not necessarily be preceded by LSIL, which will shorten the cancer progression time. B) Approximate timeframe of key events in the high risk HPV infectious cycle over the human life span. The different outcomes of immune regression are outlined in the ‘disease status’ row, with subclinical asymptomatic infection highlighted in red. Lifespan events such as pregnancy, stress, other illnesses and immune senescence during aging are known or anticipated to affect ability to detect HPV DNA. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The lag phase or incubation period, differs from our understanding of latency, which has a standard virology definition (Fig. 1). Indeed this definition is quite tight, and differs from the relatively loose way in which the term latency is currently used by the cross-disciplinary papillomavirus community, which includes practicing clinicians, healthcare professional, and researchers in a wide range of disciplines including virologists. Latency, which is a state in which productive infection is not supported, seems to be positioned at one end of an ‘immunological’ sliding scale, which includes subclinical productive infections to the right, and clearance of infection to the left. Although we have no clear data to help us distinguish between cleared, latent and immune controlled infections, the realisation that HPV infections can persist in the absence of disease symptoms, clearly explains why some women who are HPV positive have normal cytology (Fig. 4). What matters here is their risk of developing neoplasia and cancer, which although higher than those who consistently test negative, is considerably lower than the HSIL group [45,47,51]. Having a scientific basis for the HPV positivity is also a benefit in patient counselling and can avoid patient concern regarding a new unexplained sexually transmitted infection. Although HPV testing has value in highlighting the ‘at risk’ group as part of the cervical screening programme, the approach has limited value amongst young women where HPV infection is more common, and plays a primary role in detecting HPV persistence in those over the age of 30. The detection of HPV throughout life in the absence of disease has been an emerging reality since the introduction of routine testing over the last decade, and emphasises the important of triage approaches that can distinguish those women have identifiable HSIL. Although this is an inconvenience of using HPV testing for primary screening, it is clear that a better understanding of how immune control is mediated, can lead us towards the development of better intervention strategies in the future.
5. Conclusions
There are many examples of subclinical or asymptomatic HPV infections, including those caused by the Beta and Gamma HPV types, but also many caused by Alpha HPV types such as HPV3 and 10 which cause inconspicuous flat lesions, and even the high risk Alpha HPV types, which cause inapparent penile lesions in men [52] as well as being detected in up to 7% or so of mouthwash samples collected from the general population depending on region [17,18,53]. Such infections pose a challenge for HPV-based cervical screening programmes, and most likely explain the sporadic HPV-positivity that can occur following disease clearance in some women. For virologists, the term ‘virus latency’ defines a particular type of subclinical infection that does not support virus synthesis, but has the capacity to do so upon reactivation, with members of the Herpes virus family such as HSV, EBV and CMV leading the way in developing this understanding. Curiously, recent work has challenged this conventional idea, and has suggested that Herpes Simplex virus latency is ‘noisier the closer we look’, and may be associated with sporadic low-level virus shedding [54]. The description of HPV infections as subclinical, asymptomatic or inapparent would appear a more straightforward term when the precise nature of the subclinical infection remains undefined. These states appear to be broadly equivalent to what is also sometimes referred to as ‘clinical latency’ … i.e. the detection of viral DNA and thus the inference of virus infection, in the absence of apparent clinical disease. In this context, many of the clinical observations that centre around recurrence and sporadic HPV-positivity in women who lack cytological abnormalities becomes relatively straightforward to interpret as infections can be subclinical or clinically-apparent. A clearer understanding of the molecular processes that drive lesion regression and the processes of immune surveillance, will hopefully in future help us to understand how subclinical HPV infections are maintained.
Author statement
I am happy for this article to be published in TVR. I am the sole author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the Medical Research Council (MC-PC-13050 and MR/S024409/1). The author thanks the international community of papillomavirus biologists who have contributed to discussions on papillomavirus latency over the years, including Betty Steinberg, Patti Gravitt, Robbie Burk, Mark Schiffman, Nacho Bravo, Anna-Barbara Moscicki, Anne Hammer, Merilyn Hibma, Peter Stern, Margaret Stanley … and many many others.
Data availability
No data was used for the research described in the article.
References
- 1.Knipe D.M., Howley P. Wolters Kluwer Health; 2013. Fields Virology. [Google Scholar]
- 2.Louten J. Elsevier Science; 2022. Essential Human Virology. [Google Scholar]
- 3.Pfaender S., Brown R.J., Pietschmann T., Steinmann E. Natural reservoirs for homologs of hepatitis C virus. Emerg. Microb. Infect. 2014;3:1–9. doi: 10.1038/emi.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- 5.Chun T.-W., Fauci A.S. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. Aids. 2012;26:1261–1268. doi: 10.1097/QAD.0b013e328353f3f1. [DOI] [PubMed] [Google Scholar]
- 6.Shannon-Lowe C., Rickinson A. The global landscape of EBV-associated tumors. Frontiers in oncology. 2019;9:713. doi: 10.3389/fonc.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills M.R., Poole E., Lau B., Krishna B., Sinclair J.H. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell. Mol. Immunol. 2015;12:128–138. doi: 10.1038/cmi.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoll M.P., Proença J.T., Efstathiou S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012;36:684–705. doi: 10.1111/j.1574-6976.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorbar J., Quint W., Banks L., Bravo I.G., Stoler M., Broker T.R., Stanley M.A. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 10.Doorbar J., Zheng K., Aiyenuro A., Yin W., Walker C.M., Chen Y., Egawa N., Griffin H.M. Principles of epithelial homeostasis control during persistent human papillomavirus infection and its deregulation at the cervical transformation zone. Curr Opin Virol. 2021;51:96–105. doi: 10.1016/j.coviro.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Doorbar J., Egawa N., Griffin H., Kranjec C., Murakami I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015;25(Suppl 1):2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egawa N., Egawa K., Griffin H., Doorbar J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottschling M., Stamatakis A., Nindl I., Stockfleth E., Alonso A., Bravo I.G. Multiple evolutionary mechanisms drive papillomavirus diversification. Mol. Biol. Evol. 2007;24:1242–1258. doi: 10.1093/molbev/msm039. [DOI] [PubMed] [Google Scholar]
- 14.Uitto J., Saeidian A.H., Youssefian L., Saffarian Z., Casanova J.L., Beziat V., Jouanguy E., Vahidnezhad H. Recalcitrant warts, epidermodysplasia verruciformis, and the tree-man syndrome: phenotypic spectrum of cutaneous human papillomavirus infections at the intersection of genetic variability of viral and human genomes. J. Invest. Dermatol. 2022;142:1265–1269. doi: 10.1016/j.jid.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonsson A., Forslund O., Ekberg H., Sterner G., Hansson B.G. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 2000;74:11636–11641. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonsson A., Karanfilovska S., Lindqvist P.G., Hansson B.G. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 2003;41:2509–2514. doi: 10.1128/JCM.41.6.2509-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gheit T., Muwonge R., Lucas E., Galati L., Anantharaman D., McKay-Chopin S., Malvi S.G., Jayant K., Joshi S., Esmy P.O., Pillai M.R., Basu P., Sankaranarayanan R., Tommasino M. Impact of HPV vaccination on HPV-related oral infections. Oral Oncol. 2023;136 doi: 10.1016/j.oraloncology.2022.106244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillison M.L., Broutian T., Pickard R.K., Tong Z.Y., Xiao W., Kahle L., Graubard B.I., Chaturvedi A.K. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maglennon G.A., McIntosh P.B., Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J. Virol. 2014;88:710–716. doi: 10.1128/JVI.02589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amella C.A., Lofgren L.A., Ronn A.M., Nouri M., Shikowitz M.J., Steinberg B.M. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency. Am. J. Pathol. 1994;144:1167–1171. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P., Nouri M., Brandsma J.L., Iftner T., Steinberg B.M. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation. Virology. 1999;263:388–394. doi: 10.1006/viro.1999.9950. [DOI] [PubMed] [Google Scholar]
- 22.Maglennon G.A., McIntosh P., Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–163. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y. vol. 2. 1975. (Papilloma-Myxoma Viruses). [Google Scholar]
- 24.Egawa N., Shiraz A., Crawford R., Saunders-Wood T., Yarwood J., Rogers M., Sharma A., Eichenbaum G., Doorbar J. Dynamics of papillomavirus in vivo disease formation & susceptibility to high-level disinfection-Implications for transmission in clinical settings. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowson K.E., Mahy B.W. Human papova (wart) virus. Bacteriol. Rev. 1967;31:110–131. doi: 10.1128/br.31.2.110-131.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman J.M., Fialkow P.J. Viral tumorigenesis in man: cell markers in condylomata accuminata. Int. J. Cancer. 1976;17 doi: 10.1002/ijc.2910170109. [DOI] [PubMed] [Google Scholar]
- 27.Murray R.F., Hobbs J., Payne B. Possible clonal origin of common warts (Verruca vulgaris) Nature. 1971;232:51–52. doi: 10.1038/232051a0. [DOI] [PubMed] [Google Scholar]
- 28.Saunders-Wood T., Egawa N., Zheng K., Giaretta A., Griffin H.M., Doorbar J. Role of E6 in maintaining the basal cell reservoir during productive papillomavirus infection. J. Virol. 2022;96 doi: 10.1128/jvi.01181-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doorbar J. Host control of human papillomavirus infection and disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:27–41. doi: 10.1016/j.bpobgyn.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Hibma M.H. The immune response to papillomavirus during infection persistence and regression. Open Virol. J. 2012;6:241–248. doi: 10.2174/1874357901206010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doupe D.P., Jones P.H. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. Bioessays. 2013;35:443–451. doi: 10.1002/bies.201200166. [DOI] [PubMed] [Google Scholar]
- 32.Kot M., Baj-Krzyworzeka M., Szatanek R., Musial-Wysocka A., Suda-Szczurek M., Majka M. The importance of HLA assessment in "Off-the-Shelf" allogeneic mesenchymal stem cells based-therapies. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20225680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agudo J. Immune privilege of skin stem cells: what do we know and what can we learn? Exp. Dermatol. 2021;30:522–528. doi: 10.1111/exd.14221. [DOI] [PubMed] [Google Scholar]
- 34.Ramoz N., Rueda L.A., Bouadjar B., Montoya L.S., Orth G., Favre M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 2002;32:579–581. doi: 10.1038/ng1044. [DOI] [PubMed] [Google Scholar]
- 35.Beziat V., Rapaport F., Hu J., Titeux M., Bonnet des Claustres M., Bourgey M., Griffin H., Bandet E., Ma C.S., Sherkat R., Rokni-Zadeh H., Louis D.M., Changi-Ashtiani M., Delmonte O.M., Fukushima T., Habib T., Guennoun A., Khan T., Bender N., Rahman M., About F., Yang R., Rao G., Rouzaud C., Li J., Shearer D., Balogh K., Al Ali F., Ata M., Dabiri S., Momenilandi M., Nammour J., Alyanakian M.A., Leruez-Ville M., Guenat D., Materna M., Marcot L., Vladikine N., Soret C., Vahidnezhad H., Youssefian L., Saeidian A.H., Uitto J., Catherinot E., Navabi S.S., Zarhrate M., Woodley D.T., Jeljeli M., Abraham T., Belkaya S., et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell. 2021;184:3812–3828 e30. doi: 10.1016/j.cell.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow KY, Brotin E, Ben Khalifa Y, Carthagena L, Teissier S, Danckaert A, Galzi JL, Arenzana-Seisdedos F, Thierry F, Bachelerie F. A pivotal role for CXCL12 signaling in HPV-mediated transformation of keratinocytes: clues to understanding HPV-pathogenesis in WHIM syndrome. Cell Host Microbe 8:523-533. [DOI] [PubMed]
- 37.McLaren P.J., Fellay J. HIV-1 and human genetic variation. Nat. Rev. Genet. 2021;22:645–657. doi: 10.1038/s41576-021-00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowden S.J., Bodinier B., Kalliala I., Zuber V., Vuckovic D., Doulgeraki T., Whitaker M.D., Wielscher M., Cartwright R., Tsilidis K.K., Bennett P., Jarvelin M.R., Flanagan J.M., Chadeau-Hyam M., Kyrgiou M., FinnGen c. Genetic variation in cervical preinvasive and invasive disease: a genome-wide association study. Lancet Oncol. 2021;22:548–557. doi: 10.1016/S1470-2045(21)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D., Cui T., Ek W.E., Liu H., Wang H., Gyllensten U. Analysis of the genetic architecture of susceptibility to cervical cancer indicates that common SNPs explain a large proportion of the heritability. Carcinogenesis. 2015;36:992–998. doi: 10.1093/carcin/bgv083. [DOI] [PubMed] [Google Scholar]
- 40.Wilgenburg B.J., Budgeon L.R., Lang C.M., Griffith J.W., Christensen N.D. Characterization of immune responses during regression of rabbit oral papillomavirus infections. Comp. Med. 2005;55:431–439. [PubMed] [Google Scholar]
- 41.Nicholls P.K., Moore P.F., Anderson D.M., Moore R.A., Parry N.R., Gough G.W., Stanley M.A. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology. 2001;283:31–39. doi: 10.1006/viro.2000.0789. [DOI] [PubMed] [Google Scholar]
- 42.Knowles G., O'Neil B.W., Campo M.S. Phenotypical characterization of lymphocytes infiltrating regressing papillomas. J. Virol. 1996;70:8451–8458. doi: 10.1128/jvi.70.12.8451-8458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman N., Birley H.D.L., Renton A.M., Hanna N.F., Ryait B.K., Byrne M., al e. Immunological events in regressing genital warts. Am. J. Clin. Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 44.Nobbenhuis M.A., Helmerhorst T.J., van den Brule A.J., Rozendaal L., Voorhorst F.J., Bezemer P.D., Verheijen R.H., Meijer C.J. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet. 2001;358:1782–1783. doi: 10.1016/S0140-6736(01)06809-X. [DOI] [PubMed] [Google Scholar]
- 45.Malagon T., Volesky K.D., Bouten S., Laprise C., El-Zein M., Franco E.L. Cumulative risk of cervical intraepithelial neoplasia for women with normal cytology but positive for human papillomavirus: systematic review and meta-analysis. Int. J. Cancer. 2020;147:2695–2707. doi: 10.1002/ijc.33035. [DOI] [PubMed] [Google Scholar]
- 46.Paul P., Hammer A., Rositch A.F., Burke A.E., Viscidi R.P., Silver M.I., Campos N., Youk A.O., Gravitt P.E. Rates of new human papillomavirus detection and loss of detection in middle-aged women by recent and past sexual behavior. J. Infect. Dis. 2021;223:1423–1432. doi: 10.1093/infdis/jiaa557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polman N.J., Veldhuijzen N.J., Heideman D.A.M., Snijders P.J.F., Meijer C., Berkhof J. HPV-positive women with normal cytology remain at increased risk of CIN3 after a negative repeat HPV test. Br. J. Cancer. 2017;117:1557–1561. doi: 10.1038/bjc.2017.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Souza G., Clemens G., Strickler H.D., Wiley D.J., Troy T., Struijk L., Gillison M., Fakhry C. Long-term persistence of oral HPV over 7 Years of follow-up. JNCI Cancer Spectr. 2020;4 doi: 10.1093/jncics/pkaa047. pkaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammer A., de Koning M.N., Blaakaer J., Steiniche T., Doorbar J., Griffin H., Mejlgaard E., Svanholm H., Quint W.G., Gravitt P.E. Whole tissue cervical mapping of HPV infection: molecular evidence for focal latent HPV infection in humans. Papillomavirus Res. 2019;7:82–87. doi: 10.1016/j.pvr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammer A., Blaakaer J., de Koning M.N.C., Steiniche T., Mejlgaard E., Svanholm H., Roensbo M.T., Fuglsang K., Doorbar J., Andersen R.H., Quint W.G.V., Gravitt P.E. Evidence of latent HPV infection in older Danish women with a previous history of cervical dysplasia. Acta Obstet. Gynecol. Scand. 2022;101:608–615. doi: 10.1111/aogs.14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veldhuijzen N.J., Polman N.J., Snijders P.J.F., Meijer C., Berkhof J. Stratifying HPV-positive women for CIN3+ risk after one and two rounds of HPV-based screening. Int. J. Cancer. 2017;141:1551–1560. doi: 10.1002/ijc.30865. [DOI] [PubMed] [Google Scholar]
- 52.Bleeker M.C., Snijders P.F., Voorhorst F.J., Meijer C.J. Flat penile lesions: the infectious "invisible" link in the transmission of human papillomavirus. Int. J. Cancer. 2006;119:2505–2512. doi: 10.1002/ijc.22209. [DOI] [PubMed] [Google Scholar]
- 53.Bettampadi D., Villa L.L., Ponce E.L., Salmeron J., Sirak B.A., Abrahamsen M., Rathwell J.A., Reich R.R., Giuliano A.R. Oral human papillomavirus prevalence and type distribution by country (Brazil, Mexico and the United States) and age among HPV infection in men study participants. Int. J. Cancer. 2020;146:3026–3033. doi: 10.1002/ijc.32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh N., Tscharke D.C. Herpes simplex virus latency is noisier the closer we look. J. Virol. 2020;94 doi: 10.1128/JVI.01701-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.contributors W. 9 May 2023 11:32 UTC 9 May 2023 11:32 UTC. Virus latency, on Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/w/index.php?title=Virus_latency&oldid=1153962619. Accessed 30 May 2023 12:40 UTC.
- 56.Maglennon G.A., Doorbar J. The biology of papillomavirus latency. Open Virol. J. 2012;6:190–197. doi: 10.2174/1874357901206010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doorbar J. Latent papillomavirus infections and their regulation. Curr Opin Virol. 2013;3:416–421. doi: 10.1016/j.coviro.2013.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.