Abstract
Objective
Failure to rescue (FTR), defined as in-hospital death following a major complication, has been increasingly studied in patients who undergo cardiothoracic surgery. This study tested the hypothesis that elderly patients undergoing lung cancer resection have greater rates of FTR compared with younger patients.
Methods
Patients who underwent surgery for primary lung cancer between 2011 and 2020 and had at least 1 major postoperative complication were identified using the National Surgical Quality Improvement Program database. Patients who died following complications (FTR) were compared with those who survived in an elderly (80+ years) and younger (<80 years) cohort.
Results
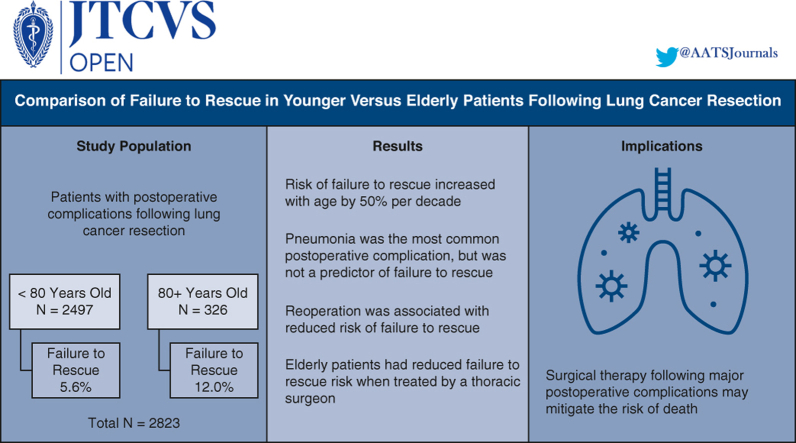
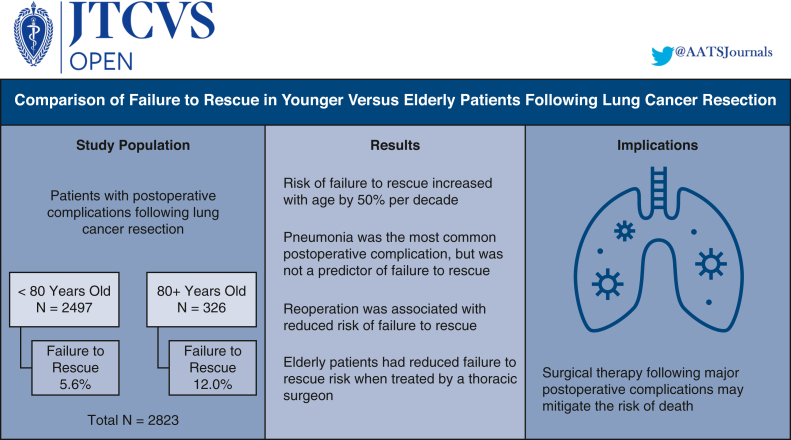
Of the 2823 study patients, the younger cohort comprised 2497 patients (FTR: n = 139 [5.6%]), whereas the elderly cohort comprised 326 patients (FTR: n = 39 [12.0%]). Pneumonia was the most common complication in younger (877/2497, 35.1%) and elderly patients (118/326, 36.2%) but was not associated with FTR on adjusted analysis. Increasing age was associated with FTR (adjusted odds ratio [AOR], 1.55 per decade, P < .001), whereas unplanned reoperation was associated with reduced risk (AOR, 0.55, P = .01). Within the elderly cohort, surgery conducted by a thoracic surgeon was associated with lower FTR risk (AOR, 0.29, P = .028).
Conclusions
FTR following lung cancer resection was more frequent with increasing age. Pneumonia was the most common complication but not a predictor of FTR. Unplanned reoperation was associated with reduced FTR, as was treatment by a thoracic surgeon for elderly patients. Surgical therapy for complications after lung cancer resection and elderly patients managed by a thoracic specialist may mitigate the risk of death following an adverse postoperative event.
Key Words: lung cancer surgery, perioperative outcomes, postoperative complications, failure to rescue, surgeon specialty
Graphical abstract
Factors associated with failure to rescue in patients following lung cancer resection.
Central Message.
Risk of failure to rescue following lung cancer resection increases with age; however, reoperation for complications and treatment by a thoracic surgeon may improve rescue rates.
Perspective.
This study demonstrates that risk of failure to rescue following lung cancer resection increases with age by approximately 50% per decade. Reoperation following complications was associated with reduced risk; however, elderly patients underwent reoperation less frequently despite having similar complications. Elderly patients treated by a thoracic surgeon have lower risk of failure to rescue.
See Discussion on page 873.
Lung cancer continues to be the greatest cause of cancer-related deaths in the United States, with an estimated 130,000 deaths in 2022.1 Although surgical resection remains among the best curative treatment options, particularly for early-stage disease, postoperative complication rates are not insignificant. Recent literature indicates that complication rates continue to range from 25% to 40%,2, 3, 4, 5, 6, 7 with major complications comprising approximately 7% to 10%.4,5 Although postoperative complications pose a serious risk to patients due to significantly worse short- and long-term outcomes,8,9 rates of in-hospital mortality do not always correlate with the frequency of complications but instead relate to the ability to rescue patients following the occurrence of a major complication.10, 11, 12, 13
Failure to rescue (FTR) was first introduced as a quality metric for hospital performance in the 1990s14 and is generally defined as in-hospital death following a major postoperative complication. Research studies examining FTR over the next 2 decades indicated that this phenomenon was predominately associated with hospital factors such as patient volume, teaching status, nursing staff levels, and specialty care services available.15,16 More recently, FTR has been increasingly studied in patients who undergo cardiothoracic surgery.13,17, 18, 19, 20 However, there is limited published data on this outcome measure for patients undergoing lung cancer resection, particularly in relation to patient-related factors that may potentially impact the ability to be rescued following a postoperative complication. The purpose of this study was to examine FTR in a younger and elderly cohort to test the hypothesis that elderly patients have a greater risk of death following postoperative complications from lung cancer resection.
Methods
Data Source
Data from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database was used to perform this retrospective analysis. The ACS NSQIP is a database of risk-adjusted short-term patient outcomes and quality measures collected from more than 700 hospitals in North America from 49 of the 50 states.21
Patient Selection
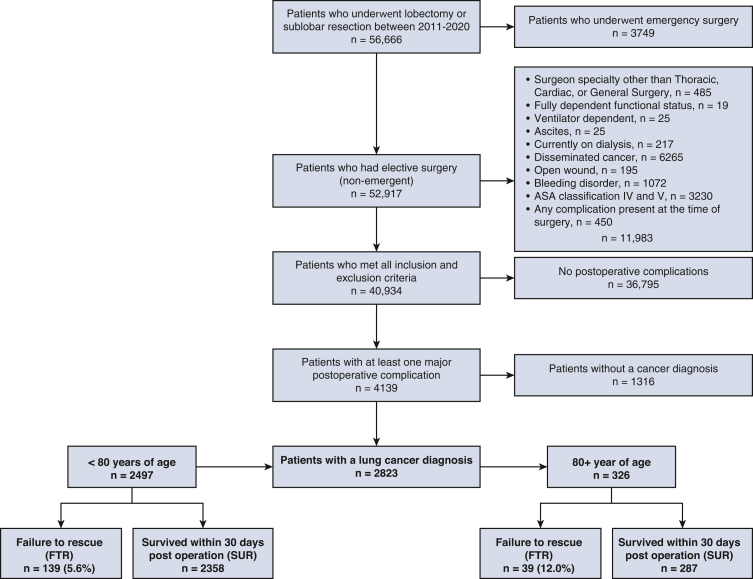
The study cohort was created by identifying patients in the ACS NSQIP who underwent a lobectomy or sublobar resection, defined as either wedge resection or segmentectomy, for primary lung cancer between 2011 and 2020 (Figure 1). Patients were included if they experienced at least 1 major postoperative complication, defined as organ/space surgical site infection, pneumonia, unplanned intubation, pulmonary embolus, deep-vein thrombosis requiring therapy, prolonged mechanical ventilation (defined as >48 hours), renal failure, stroke, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction (MI), bleeding requiring transfusion, sepsis, or unplanned reoperation. Exclusion criteria included emergency surgery, fully dependent functional status, disseminated cancer, ventilator or dialysis dependency, ascites, bleeding disorder, and patients with an American Society of Anesthesiology (ASA) classification of IV or greater. Patients who had any active complications at the time of surgery were also excluded, such as open wounds or sepsis.
Figure 1.
Study cohort diagram depicting inclusion and exclusion criteria.
Statistical Analysis
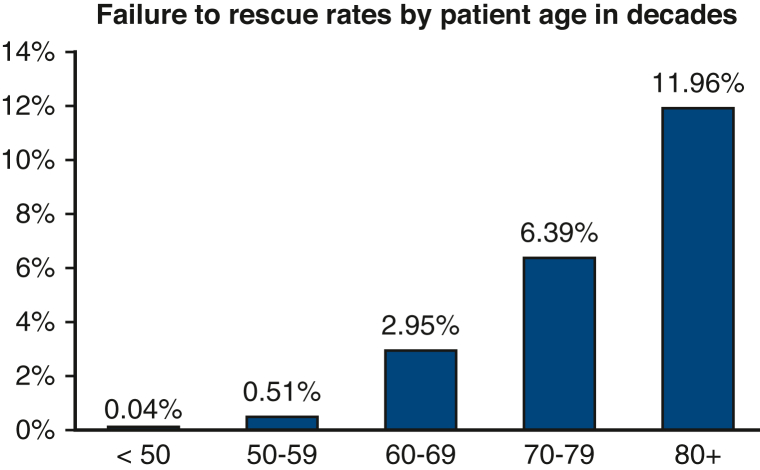
Patients who developed at least 1 major postoperative complication after lung cancer resection were stratified by age into patients younger than 80 years old and patients 80 years or older. For each cohort, patients were grouped according to whether they died after postoperative complications (FTR) or survived to hospital discharge (SUR). An age cutoff of 80 years was used based on the distribution of FTR by decade, as depicted in Figure 2, which demonstrates the largest increase in FTR rates at this decade. Demographics, patient characteristics, and perioperative outcomes were compared between FTR and SUR groups in both the younger and elderly cohort using Wilcoxon rank-sum test for continuous variables and the Fisher exact test and Pearson χ2 test for discrete variables.
Figure 2.
Distribution of failure to rescue rates following lung cancer resection by decade.
Twenty-one variables were tested for significance (P < .05) using logistic regression analysis. Model fit was tested using the goodness-of-fit χ2 and the Hosmer–Lemeshow tests. Variable selection was accomplished using a backward stepwise process. Variables that were statistically significant on univariate analysis or considered clinically relevant were initially included (Tables E1 and E2). The final models were selected based on the lowest Akaike information criterion values. Thus, independent predictors of FTR were estimated using backward stepwise logistic regression modeling, adjusting for age, sex, body mass index (BMI), smoking status, ASA class, surgeon specialty (general thoracic vs nonthoracic), surgical approach (open vs video-assisted thoracoscopic [VATS]), resection type (lobar vs sublobar), history of dyspnea at rest or with moderate exertion, history of chronic obstructive pulmonary disease, and the specific postoperative complications. Pneumonia was the most common complication in both the younger and elderly cohort; therefore, a subgroup analysis was performed comparing FTR and SUR patients who developed pneumonia as their first or only major postoperative complication.
To further address potential confounding variables between the younger and elderly cohort, a 1:1 propensity score matching model was used to create matched cohorts of patients <80 years and 80+ years of age using covariates from baseline characteristics, comorbidities, and operative data. Covariates included sex, race, BMI, smoking status, baseline functional status, ASA class, comorbidities, surgeon specialty, tumor location, extent of resection, and surgical approach (VATS vs open). Perioperative outcomes were compared between the matched cohorts, including rates of FTR.
Categorical variables are presented as a frequency and percentage, and continuous variables are presented as median with interquartile range. A P value of .05 was used to determine statistical significance. All statistical analysis was conducted using R, version 4.2.2 (R Core Team, 2022). This study was approved by the Stanford University institutional review board; individual consent was not required (institutional review board#: 35143, approved March 7, 2017, latest revision April 15, 2021).
Results
Of the 2823 patients who developed a major postoperative complication following lung cancer resection, 2497 (88.4%) were younger than 80 years of age and 326 (11.5%) were 80 years or older. The FTR rate was 5.6% (139/2497) in the younger cohort and 12.0% (39/326) in the elderly cohort. FTR rates demonstrated increased incidence per decade of life (Figure 2). Patient characteristics stratified by age and rescue status are illustrated in Table 1. In the younger cohort, patients with FTR were older (median age 70 vs 68 years, P = .001), had greater rates of baseline dyspnea with moderate exertion (38.1% vs 25.8%, P = .004), and a greater incidence of chronic obstructive pulmonary disease (47.5% vs 37.4%, P = .017). In the elderly cohort, there were no significant differences in baseline demographics between the FTR and SUR groups. With respect to surgery, there were no significant differences in surgeon specialty, tumor location, resection type, surgical approach, operation time, or postoperative length of stay between patients with FTR and SUR in the younger or elderly cohorts (Table 2). Among the entire study cohort, patients with FTR underwent open lung resection more frequently compared with patients in the SUR group (53.9% vs 45.9%, P = .037).
Table 1.
Baseline patient characteristics stratified by age groups
| Characteristic | Younger than 80 years old n = 2497 |
80+ years old n = 326 |
||||
|---|---|---|---|---|---|---|
| FTR, n = 139 | SUR, n = 2358 | P value | FTR, n = 39 | SUR, n = 287 | P value | |
| Median age, y (IQR) | 70.0 (65.0, 75.0) | 68.0 (62.0, 74.0) | .001∗ | 83.0 (81.0, 84.5) | 82.0 (81.0, 84.5) | .148∗ |
| Sex, n (%) | .558† | .225† | ||||
| Female | 65.0 (46.8) | 1163.0 (49.3) | 13.0 (33.3) | 125.0 (43.6) | ||
| Male | 74.0 (53.2) | 1195.0 (50.7) | 26.0 (66.7) | 162.0 (56.4) | ||
| Race, n (%) | .339‡ | .279‡ | ||||
| White | 117.0 (84.2) | 1855.0 (78.7) | 34.0 (87.2) | 223.0 (77.7) | ||
| Asian | 4.0 (2.9) | 69.0 (2.9) | 3.0 (7.7) | 15.0 (5.2) | ||
| Black/African American | 8.0 (5.8) | 147.0 (6.2) | 0.0 (0.0) | 12.0 (4.2) | ||
| Other | 10.0 (7.2) | 287.0 (12.2) | 2.0 (5.1) | 37.0 (12.9) | ||
| Median BMI (IQR) | 27.1 (23.2, 31.0) | 26.6 (22.9, 30.9) | .664∗ | 23.9 (22.3, 29.9) | 26.5 (22.9, 29.6) | .178∗ |
| Active smoker within 1-y, n (%) | 66.0 (47.5) | 1119.0 (47.5) | .995† | 4.0 (10.3) | 37.0 (12.9) | .800‡ |
| Independent functional status, n (%) | 136.0 (97.8) | 2330.0 (98.8) | .329† | 39.0 (100.0) | 280.0 (97.6) | 1.000‡ |
| Comorbidities, n (%) | ||||||
| Diabetes | 22.0 (15.8) | 388.0 (16.5) | .846† | 3.0 (7.7) | 53.0 (18.5) | .094† |
| Dyspnea with moderate exertion | 53.0 (38.1) | 608.0 (25.8) | .004‡ | 10.0 (25.6) | 69.0 (24.0) | .509‡ |
| COPD | 66.0 (47.5) | 882.0 (37.4) | .017† | 10.0 (25.6) | 93.0 (32.4) | .394† |
| Heart failure | 2.0 (1.4) | 19.0 (0.8) | .328‡ | 0.0 (0.0) | 1.0 (0.3) | 1.000‡ |
| Chronic steroid use | 9.0 (6.5) | 142.0 (6.0) | .828† | 3.0 (7.7) | 14.0 (4.9) | .440‡ |
| Weight loss >10% within 6 mo | 4.0 (2.9) | 83.0 (3.5) | 1.000‡ | 3.0 (7.7) | 13.0 (4.5) | .420‡ |
| ASA class, n (%) | .288‡ | .279‡ | ||||
| I—no disturbance | 0.0 (0.0) | 2.0 (0.1) | 0.0 (0.0) | 0.0 (0.0) | ||
| II—mild disturbance | 14.0 (10.1) | 293.0 (12.4) | 3.0 (7.7) | 27.0 (9.4) | ||
| III—severe disturbance | 124.0 (89.2) | 2059 (87.3) | 35.0 (89.7) | 259.0 (90.2) | ||
P values in bold are <.05. FTR, Failure to rescue; SUR, survived within 30 d postoperation; IQR, interquartile range; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists.
Wilcoxon rank sum test.
Pearson χ2 test.
Fisher exact test.
Table 2.
Operative and perioperative outcomes among patients with lung cancer who underwent lobectomy or sublobar resection
| Characteristic | All ages n = 2823 |
Younger than 80 years old n = 2497 |
80+ years old n = 326 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FTR, n = 178 | SUR, n = 2645 | P value | FTR, n = 139 | SUR, n = 2358 | P value | FTR, n = 39 | SUR, n = 287 | P value | |
| Surgeon specialty, n (%) | .181∗ | .185∗ | .182∗ | ||||||
| Cardiac surgery | 1.0 (0.6) | 30.0 (1.1) | 0.0 (0.0) | 28.0 (1.2) | 1.0 (2.6) | 2.0 (0.7) | |||
| General surgery | 25.0 (14.0) | 261.0 (9.9) | 19.0 (13.7) | 232.0 (9.8) | 6.0 (15.4) | 29.0 (10.1) | |||
| Thoracic surgery | 152.0 (85.4) | 2354.0 (89.0) | 120.0 (86.3) | 2098.0 (89.0) | 32.0 (82.1) | 256.0 (89.2) | |||
| Tumor location, n (%) | .801† | .770† | 1.000∗ | ||||||
| Lower lobe | 59.0 (33.1) | 839.0 (31.7) | 44.0 (31.7) | 729.0 (30.9) | 15.0 (38.5) | 110.0 (38.3) | |||
| Middle lobe | 9.0 (5.1) | 114.0 (4.3) | 8.0 (5.8) | 107.0 (4.5) | 1.0 (2.6) | 7.0 (2.4) | |||
| Upper lobe | 110.0 (61.8) | 1692.0 (64.0) | 87.0 (62.6) | 1522.0 (64.5) | 23.0 (59.0) | 170.0 (59.2) | |||
| Operation (CPT code), n (%) | .257† | .453∗ | .601∗ | ||||||
| Open lobectomy (32480) | 86.0 (48.3) | 1053.0 (39.8) | 67.0 (48.2) | 952.0 (40.4) | 19.0 (48.7) | 101.0 (35.2) | |||
| Open segmentectomy (32482) | 7.0 (3.9) | 80.0 (3.0) | 6.0 (4.3) | 75.0 (3.2) | 1.0 (2.6) | 5.0 (1.7) | |||
| Open wedge resection (32505) | 3.0 (1.7) | 81.0 (3.1) | 3.0 (2.2) | 75.0 (3.2) | 0.0 (0.0) | 6.0 (2.1) | |||
| VATS lobectomy (32663) | 66.0 (37.1) | 1142.0 (43.2) | 52.0 (37.4) | 1010.0 (42.8) | 14.0 (35.9) | 132.0 (46.0) | |||
| VATS segmentectomy (32669) | 5.0 (2.8) | 86.0 (3.3) | 4.0 (2.9) | 72.0 (3.1) | 1.0 (2.6) | 14.0 (4.9) | |||
| VATS wedge resection (32666) | 11.0 (6.2) | 203.0 (7.7) | 7.0 (5.0) | 174.0 (7.4) | 4.0 (10.3) | 29.0 (10.1) | |||
| Lobectomy vs sublobar resection, n (%) | .407† | .460† | .604† | ||||||
| Lobectomy | 152.0 (85.4) | 2195.0 (83.0) | 119.0 (85.6) | 1962.0 (83.2) | 33.0 (84.6) | 233.0 (81.2) | |||
| Sublobar resection | 26.0 (14.6) | 450.0 (17.0) | 20.0 (14.4) | 396.0 (16.8) | 6.0 (15.4) | 54.0 (18.8) | |||
| Surgical approach, n (%) | .037† | .068† | .143† | ||||||
| Open | 96.0 (53.9) | 1214.0 (45.9) | 76.0 (54.7) | 1102.0 (46.7) | 20.0 (51.3) | 112.0 (39.0) | |||
| VATS | 82.0 (46.1) | 1431.0 (54.1) | 63.0 (45.3) | 1256.0 (53.3) | 19.0 (48.7) | 175.0 (61.0) | |||
| Median operation time, min (IQR) | 184.0 (132.2, 269.0) | 186.0 (132.0, 257.0) | .687‡ | 190.0 (137.0, 273.5) | 187.0 (133.0, 258.0) | .518‡ | 169.0 (126.0, 245.5) | 167.0 (127.0, 247.5) | .985‡ |
| Median postoperative time, d (IQR) | 8.0 (4.0, 13.8) | 7.0 (5.0, 12.0) | .947‡ | 8.0 (4.0, 14.5) | 7.0 (5.0, 12.0) | .916‡ | 7.0 (5.0, 13.0) | 8.0 (5.0, 13.0) | .562‡ |
| Median days from operation to death (IQR) | 12.0 (6.0, 21.0) | – | – | 12.0 (6.0, 20.5) | – | – | 14.0 (6.0, 22.0) | – | |
FTR, Failure to rescue; SUR, survived within 30 d postoperation; CPT, Current Procedural Terminology; VATS, video-assisted thoracoscopic surgery; IQR, interquartile range.
Fisher exact test.
Pearson χ2 test.
Wilcoxon rank sum test.
Major postoperative complications for each age group are presented in Table 3. Among the entire study cohort, patients with FTR had a greater incidence of the following complications compared with patients in the SUR group: pneumonia, unplanned intubation, pulmonary embolus, prolonged mechanical ventilation, renal failure, stroke, cardiac arrest requiring cardiopulmonary resuscitation or MI, bleeding requiring transfusion, and sepsis or septic shock. Pneumonia was the most common complication in both the younger cohort (877/2497, 35.1%) and elderly cohort (111/326, 36.2%). There were no differences in rates of organ/space surgical-site infection or deep-vein thrombosis requiring therapy between patients in the FTR and SUR groups in either cohort. In the younger cohort, patients with FTR had greater incidence of pulmonary embolus (8.6% vs 4.6%, P = .03) and stroke (11.5% vs 3.0%, P < .001) compared with younger patients in the SUR group, whereas elderly patients had similar rates between patients in the FTR and SUR groups (pulmonary embolus 5.1% vs 4.9%, P = 1.00; stroke 5.1% vs 5.6%, P = 1.00). Notably, younger patients with FTR had a lower incidence of blood transfusion for bleeding (18.0% vs 27.7%, P = .012) and unplanned reoperation (23.0% vs 33.6%, P = .01) compared with younger patients in the SUR group. However, in the elderly cohort, bleeding requiring transfusion and unplanned reoperation were comparable between the FTR and SUR groups.
Table 3.
Major postoperative complications among patients with lung cancer who underwent lobectomy or sublobar resection
| Characteristic | All ages n = 2823 |
Younger than 80 years old n = 2497 |
80+ years old n = 326 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FTR, n = 178, n (%) | SUR, n = 2645, n (%) | P value | FTR, n = 139, n (%) | SUR, n = 2358, n (%) | P value | FTR, n = 39, n (%) | SUR, n = 287, n (%) | P value | |
| Organ/space SSI | 4.0 (2.2) | 141.0 (5.3) | .071∗ | 3.0 (2.2) | 134.0 (5.7) | .076∗ | 1.0 (2.6) | 7.0 (2.4) | 1.000∗ |
| Pneumonia | 88.0 (49.4) | 907.0 (34.3) | <.001∗ | 66.0 (47.5) | 811.0 (34.4) | .002∗ | 22.0 (56.4) | 96.0 (33.4) | .005† |
| Unplanned intubation | 114.0 (64.0) | 423.0 (16.0) | <.001∗ | 92.0 (66.2) | 377.0 (16.0) | <.001∗ | 22.0 (56.4) | 46.0 (16.0) | <.001† |
| Pulmonary embolus | 14.0 (7.9) | 122.0 (4.6) | .050∗ | 12.0 (8.6) | 108.0 (4.6) | .030∗ | 2.0 (5.1) | 14.0 (4.9) | 1.000∗ |
| DVT requiring therapy | 11.0 (6.2) | 118.0 (4.5) | .288∗ | 9.0 (6.5) | 103.0 (4.4) | .244∗ | 2.0 (5.1) | 15.0 (5.2) | 1.000∗ |
| >48 h on ventilator | 78.0 (43.8) | 254.0 (9.6) | <.001∗ | 63.0 (45.3) | 224.0 (9.5) | <.001∗ | 15.0 (38.5) | 30.0 (10.5) | <.001† |
| Progressive RI/ARF | 31.0 (17.4) | 84.0 (3.2) | <.001∗ | 21.0 (15.1) | 76.0 (3.2) | <.001∗ | 10.0 (25.6) | 8.0 (2.8) | <.001∗ |
| Stroke | 18.0 (10.1) | 86.0 (3.3) | <.001∗ | 16.0 (11.5) | 70.0 (3.0) | <.001† | 2.0 (5.1) | 16.0 (5.6) | 1.000∗ |
| Cardiac arrest requiring CPR/MI | 84.0 (47.2) | 131.0 (5.0) | <.001∗ | 69.0 (49.6) | 115.0 (4.9) | <.001∗ | 15.0 (38.5) | 16.0 (5.6) | <.001∗ |
| Bleeding requiring transfusion | 36.0 (20.2) | 737.0 (27.9) | .027∗ | 25.0 (18.0) | 653.0 (27.7) | .012∗ | 11.0 (28.2) | 84.0 (29.3) | .891† |
| Sepsis/septic shock | 47.0 (26.4) | 275.0 (10.4) | <.001∗ | 37.0 (26.6) | 246.0 (10.4) | <.001∗ | 10.0 (25.6) | 29.0 (10.1) | .014∗ |
| Unplanned reoperation | 39 (21.9) | 870 (32.9) | .002∗ | 32.0 (23.0) | 792.0 (33.6) | .010∗ | 7.0 (17.9) | 78.0 (27.2) | .218∗ |
P values in bold are <.05. FTR, Failure to rescue; SUR, survived within 30 d postoperation; SSI, surgical-site infection; DVT, deep-vein thrombosis; RI/ARF, renal insufficiency/acute renal failure; CPR/MI, cardiopulmonary resuscitation/myocardial infarction.
Pearson χ2 test.
Fisher exact test.
Based on multivariable logistic regression, factors associated with FTR in the younger cohort included increasing age (adjusted odds ratio [AOR], 1.03 per decade, P = .021), unplanned intubation (AOR, 4.05, P < .001), prolonged ventilation (AOR, 1.93, P = .022), renal failure (AOR, 2.30, P = .022), stroke (AOR, 4.38, P < .001), and cardiac arrest/MI (AOR, 12.8, P < .001) (Table 4). In the elderly cohort, unplanned intubation (AOR, 4.71, P = .004), renal failure (14.1, P < .001), and cardiac arrest/MI (AOR 11.5, P < .001) were predictors of FTR. Unplanned reoperation was independently associated with reduced risk of death in the younger cohort (AOR, 0.52, P = .014) but not the elderly cohort (AOR, 0.77, P = .631). In contrast, elderly patients had reduced risk of death when surgery was performed by a general thoracic surgeon compared with a nonthoracic surgeon (AOR, 0.29, P = .028). Pneumonia was not included in the final adjustment set after variable selection in neither the younger (AOR, 0.89, P = .627) nor elderly cohort models (AOR, 2.02, P = .169) (Table E1).
Table 4.
Multivariable predictors of failure to rescue after lobectomy or sublobar resection for lung cancer stratified by age
| Characteristic | Model 1: All |
Model 2: <80 years old |
Model 3: 80+ years old |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (in decades) | 1.55 | 1.27-1.92 | <.001 | 1.03 | 1.01-1.06 | .021 | 1.16 | 1.00-1.35 | .055 |
| Surgery by thoracic surgeon vs nonthoracic surgeon | 0.68 | 0.41-1.15 | .132 | 0.81 | 0.46-1.50 | .489 | 0.29 | 0.10-0.93 | .028 |
| Open resection vs VATS | 1.18 | 0.82-1.69 | .383 | 1.18 | 0.78-1.78 | .435 | – | ||
| Lobectomy vs sublobar resection | 1.12 | 0.70-1.87 | .640 | 1.29 | 0.75-2.33 | .374 | – | ||
| Unplanned intubation vs no complication | 4.10 | 2.60-6.43 | <.001 | 4.05 | 2.41-6.77 | <.001 | 4.71 | 1.64-13.7 | .004 |
| Pulmonary embolus vs no complication | 1.43 | 0.64-2.91 | .355 | 1.53 | 0.64-3.35 | .311 | – | ||
| >48 h on ventilator vs no complication | 1.70 | 1.03-2.79 | .037 | 1.93 | 1.10-3.38 | .022 | 0.85 | 0.25-2.79 | .796 |
| Progressive RI/ARF vs no complication | 3.33 | 1.81, 5.97 | <.001 | 2.30 | 1.10-4.60 | .022 | 14.1 | 4.06-51.1 | <.001 |
| Stroke vs no complication | 3.69 | 1.82-7.08 | <.001 | 4.38 | 2.02-8.93 | <.001 | – | ||
| Cardiac arrest requiring CPR/MI vs no complication | 11.4 | 7.70-16.9 | <.001 | 12.8 | 8.28-19.7 | <.001 | 11.5 | 4.32-32.1 | <.001 |
| Sepsis and septic shock vs no complication | 1.49 | 0.92-2.38 | .100 | 1.48 | 0.86-2.49 | .152 | – | ||
| Unplanned reoperation vs no complication | 0.55 | 0.34-0.85 | .010 | 0.52 | 0.31-0.86 | .014 | 0.77 | 0.25-2.14 | .631 |
| No. of observations | 2823 | 2497 | 326 | ||||||
| No. of FTR events | 178 | 139 | 39 | ||||||
P values in bold are <.05. OR, Odds ratio; CI, confidence interval; VATS, video-assisted thoracoscopic surgery; RI/ARF, renal insufficiency/acute renal failure; CPR/MI, cardiopulmonary resuscitation/myocardial infarction; FTR, failure to rescue.
Subgroup analysis of patients who developed pneumonia as their first or only postoperative complication is highlighted in Table 5 and Table E3. In patients with pneumonia, the FTR rate was 7.4% (59/789) in younger patients and 19.2% (20/104) in elderly patients. There were no differences in baseline characteristics between elderly patients with FTR and elderly patients in the SUR group who experienced postoperative pneumonia. However, in the younger pneumonia cohort, patients with FTR were older (median age 70 vs 68 years P = .011) and had a greater incidence of ASA class III (94.9% vs 87.8%, P = .033) compared with younger patients in the SUR group. Interestingly, active smoking rates were greater in the younger SUR group compared with the younger FTR group (55.3% vs 40.7%, P = .030). Similar to the primary analysis, rates of unplanned intubation, prolonged mechanical ventilation, renal failure, cardiac arrest, and sepsis were greater among younger and elderly patients in the FTR group compared with younger and elderly patients in the SUR group, respectively (Table E3). In the younger pneumonia cohort, incidence of stroke was greater in patients with FTR compared with patients in the SUR group (8.5% vs 1.2%, P = .002). In the elderly pneumonia cohort, incidence of bleeding requiring transfusion was greater in patients in the FTR group compared with patients in the SUR group (30.0% vs 7.1%, P = .011). Multivariable analysis examining patients who developed postoperative pneumonia demonstrated that increasing age (AOR, 1.63 per decade, P = .003), unplanned intubation (AOR, 7.06, P < .001), renal failure (AOR, 3.27, P = .003), and cardiac arrest/MI (AOR, 5.35, P < .001) were predictors of FTR, whereas unplanned reoperation was associated with reduced odds of death (AOR, 0.44, P = .026) (Table 5).
Table 5.
Multivariable predictors of failure to rescue for patients with lung cancer who underwent lobectomy or sublobar resection with pneumonia as the first or only postoperative complication, n = 893
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Age (in decades) | 1.63 | 1.19-2.26 | .003 |
| Male vs female | 1.46 | 0.82-2.63 | .201 |
| Surgery by thoracic surgeon vs nonthoracic surgeon | 1.39 | 0.59-3.71 | .483 |
| Unplanned intubation vs no complication | 7.06 | 3.11-16.4 | <.001 |
| Pulmonary embolus vs no complication | 1.25 | 0.28-4.43 | .752 |
| >48 h on ventilator vs no complication | 1.30 | 0.65-2.67 | .468 |
| Progressive RI/ARF vs no complication | 3.27 | 1.50-7.07 | .003 |
| Cardiac arrest requiring CPR/MI vs no complication | 5.35 | 2.73-10.6 | <.001 |
| Sepsis and septic shock vs no complication | 1.74 | 0.94-3.21 | .074 |
| Unplanned reoperation vs no complication | 0.44 | 0.21-0.88 | .026 |
| No. of observations | 893 | ||
| No. of events | 79 | ||
P values in bold are <.05. OR, Odds ratio; CI, confidence interval; RI/ARF, renal insufficiency/acute renal failure; CPR/MI, cardiopulmonary resuscitation/myocardial infarction.
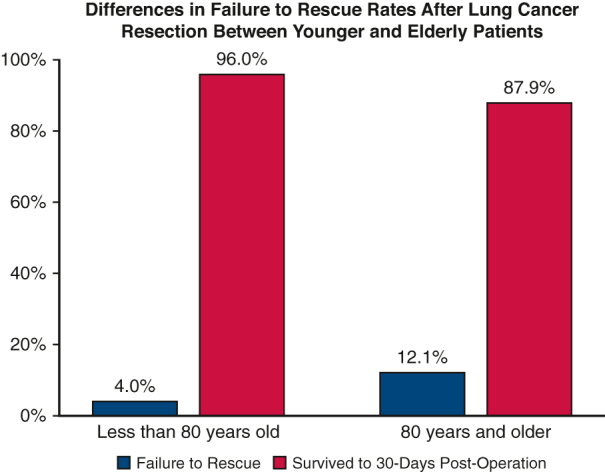
A matched analysis based on propensity scores was conducted to create a cohort of patients <80 year old and 80+ years old with matched baseline and operative characteristics, resulting in 2 groups with 323 patients each. There were no statistically significant differences between these 2 groups with respect to sex, race, BMI, smoking status, baseline functional status, comorbidities, ASA class, surgeon specialty, tumor location, extent of resection, and surgical approach (Table E4). When we compared postoperative outcomes, the younger cohort had a greater incidence of surgical-site infection (7.1% vs 2.2%, P = .003) and unplanned reoperation (36.2% vs 26.0%, P = .005) compared with the elderly cohort. Rates of all other complications were comparable between the matched younger and elderly cohorts (Table 6). Similar to the primary analysis, incidence of FTR was significantly greater in elderly patients who underwent lung cancer resection compared with the younger matched cohort (12.1% vs 4.0%, P < .001). The standardized mean differences for the propensity-matched analyses are highlighted in Table E5. See Figure 3 for a graphical abstract of the study.
Table 6.
Postoperative outcomes of propensity score–matched patients with lung cancer undergoing lobectomy or sublobar resection
| Characteristic | <80 years old, N = 323, n (%) | 80+ years old, N = 323, n (%) | P value |
|---|---|---|---|
| Outcome within 30 d postoperation | <.001∗ | ||
| Failure to rescue | 13.0 (4.0) | 39.0 (12.1) | |
| Survived | 310.0 (96.0) | 284.0 (87.9) | |
| Organ/space SSI | 23.0 (7.1) | 7.0 (2.2) | .003∗ |
| Pneumonia | 116.0 (35.9) | 117.0 (36.2) | .935∗ |
| Unplanned intubation | 61.0 (18.9) | 68.0 (21.1) | .491∗ |
| Pulmonary embolism | 17.0 (5.3) | 15.0 (4.6) | .717∗ |
| DVT requiring therapy | 14.0 (4.3) | 17.0 (5.3) | .581∗ |
| On Ventilator greater than 48 h | 33.0 (10.2) | 45.0 (13.9) | .147∗ |
| Progressive RI/ARF | 16.0 (5.0) | 18.0 (5.6) | .725∗ |
| Stroke | 14.0 (4.3) | 17.0 (5.3) | .581∗ |
| Cardiac arrest requiring CPR/MI | 25.0 (7.7) | 31.0 (9.6) | .401∗ |
| Bleeding requiring transfusion | 81.0 (25.1) | 94.0 (29.1) | .250∗ |
| Sepsis/septic shock | 41.0 (12.7) | 38.0 (11.8) | .719∗ |
| Unplanned reoperation | 117.0 (36.2) | 84.0 (26.0) | .005∗ |
P values in bold are <.05. SSI, Surgical-site infection; DVT, deep-vein thrombosis; RI/ARF, renal insufficiency/acute renal failure; CPR/MI, cardiopulmonary resuscitation/myocardial infarction.
Pearson χ2 test.
Figure 3.
Graphical abstract depicting factors associated with failure to rescue in younger and elderly lung cancer resection patients.
Discussion
With the implementation of health care payment models that use penalties and reimbursements to incentivize hospitals and providers to provide quality care, there is a growing interest in quality measures such as FTR. This is likely related to numerous publications indicating FTR is more closely associated with hospital characteristics, such as hospital ratings or volume and the presence of subspecialty services,4,10,15,22,23 that may potentially be modified to improve rescue rates. In contrast, traditional quality metrics such as postoperative complication rates may be more reflective of patient factors that are difficult to improve upon, such as baseline comorbid conditions or functional status.14,24 Nonetheless, understanding patient-related factors associated with FTR is important, as these data can potentially inform preoperative risk stratification and provide guidance during difficult family conversations about whether a patient is likely to survive following specific postoperative complications. There is published literature pertaining to patient-related factors associated with FTR after cardiac surgery25,26 and esophagectomy.12,27 However, to date there are limited data on patient factors associated with FTR following lung cancer resection.
In the current study, FTR rates were nearly twice as high in elderly patients who underwent lung cancer resection compared with younger patients. Aside from age, no other patient factors were associated with FTR in this analysis, including sex, race, BMI, smoking status, functional status, comorbid conditions, or ASA class. The incidence of baseline pulmonary conditions was greater in younger patients with FTR compared with younger patients in the SUR group; however, this association was not seen after adjusting for confounding variables. Age, however, remained significant on adjusted analysis, demonstrating roughly 50% increased risk of FTR per decade (AOR, 1.55, P < .001). Furthermore, when we examined patients who experienced postoperative pneumonia, increasing age was the only patient-related factor independently associated with increased risk of FTR (AOR, 1.63 per decade, P = .003). Interestingly, there was no difference between FTR and SUR rates when we compared surgical approach, tumor location, resection type, or operating time in either age cohort, suggesting that intraoperative variables may not have a significant impact on the ability to rescue patients following postoperative complications. Patients in the FTR group did undergo open surgery more frequently that patients in the SUR group when we examined the entire study cohort (53.9% vs 45.9%, P = .037); however, this variable was not significant on adjusted analysis (open resection vs VATS; AOR, 1.18, P = .383).
It is notable that despite pneumonia being the most common complication in both younger (35.1%) and elderly (36.2%) patients who underwent lung cancer resection, this complication was not an independent predictor of FTR in either cohort on multivariable analysis. This finding implies that patients who undergo lung cancer resection and contract postoperative pneumonia can potentially be rescued; therefore, early and aggressive measures should be considered when there is suspicion for this complication. Although pneumonia was not independently associated with mortality, other major complications related to organ failure predicted FTR. With the risk of death ranging from 2-fold to more than 14-fold depending on the specific complication and age cohort, this information may help inform family of prognosis and guide decision-making for critically ill patients.
There were 2 instances in the current analysis where risk of death was mitigated following a postoperative complication. Unplanned reoperation was independently associated with approximately 50% reduction in FTR for all groups analyzed aside from the elderly cohort (total study cohort, AOR, 0.55, P = .01; younger cohort AOR, 0.55, P = .014; any patient with pneumonia AOR, 0.44, P = .026). This suggests that surgical therapy in the appropriate setting is important to consider and may improve chances of rescue following an adverse postoperative event. Based on the current analysis, we were not able to identify associations between specific complications and reoperation procedures. A summary of reoperation procedures is presented in Table E6. Nonetheless, our data indicate that reoperation should be considered if a complication arises where surgical therapy is a treatment option.
Importantly, rates of unplanned reoperation were significantly lower in the elderly cohort compared with younger patients, both in the unmatched (26.1% vs 33.0%, P = .012) and matched analyses (26.0% vs 36.3%, P = .005) (Table E7). It could be speculated that perhaps elderly patients experience different complications compared with younger patients, resulting in differences in the need for reoperation. However, following propensity score matching, all baseline characteristics, operative variables, and rates of postoperative complications were similar between the younger and elderly matched cohorts aside from organ/space surgical-site infection, unplanned reoperation, and FTR. This matched analysis indicates that elderly patients are undergoing unplanned reoperation less frequently compared with younger patients despite having similar types and rates of postoperative complications, which is potentially impacting the opportunity to rescue elderly patients. Data pertaining to the reasons why reoperation was or was not conducted are not captured in the NSQIP database; therefore, this aspect of our analysis could not be explored further.
The second factor that was shown to mitigate the risk of FTR in our analysis relates to surgeon specialty. In elderly patients who underwent lung cancer resection and experienced postoperative complications, surgery performed by a general thoracic surgeon was associated with 70% reduction in risk of death compared with elderly patients who had surgery performed by a nonthoracic surgeon (AOR. 0.29, P = .028). Although the details of this association cannot be determined based on this retrospective analysis, we speculate that dedicated general thoracic surgeons may be more experienced and skilled at managing postoperative complications related to lung resection in a tenuous elderly patient. There are previously published data on the associations between surgeon specialty and outcomes after lung cancer resection, indicating that general thoracic surgeons overall have improved short- and long-term outcomes compared with other surgical specialists.28, 29, 30, 31 However, an important limitation for this analysis relates to the fact that NSQIP does not capture hospital-level data. Since potential confounding variables related to hospital volume and capabilities could not be adjusted for, it is possible that this finding serves as a surrogate marker for hospital, rather than provider, quality. Nonetheless, to our knowledge this association has not been previously studied specifically in the elderly population and in the context of rescuing patients following postoperative complications.
Aside from the limitations noted previously, the study results should be considered in light of several additional limitations. This study is retrospective in nature, and causality cannot be determined. Granular data pertaining to other patient-related factors that may impact FTR rates are not tabulated in NSQIP, such as frailty, preoperative pulmonary function, parameters related to severity of underlying lung disease such as the Global Initiative for Chronic Obstructive Lung Disease system, and cancer staging. As a result, our analyses are unable to account for patient-centered factors that are traditionally linked to rates of FTR, such as frailty and preoperative pulmonary function status. Specific details related to sublobar resections, including the decision to perform a wedge resection over segmentectomy and the number of segments removed, are also not captured in NSQIP. Regarding postoperative pneumonia, it is not specified in the NSQIP database as to whether this diagnosis was reached by clinical assessment, radiographic findings, positive cultures, or other means. There are no specific data on hospital status or volume, which are important variables that pertain to FTR based on previous publications. Despite this limitation, this database was used due to the fact that NSQIP specifically captures preoperative risk factors, variables pertaining to surgeon specialty and the operation, and details regarding postoperative adverse events while providing the ability to select for patients with lung cancer. Given that the aim of this study was to examine patient-related factors associated with FTR, we felt this database was most appropriate, even though hospital-level data are lacking. NSQIP also does not capture socioeconomic status, insurance type, education level, and income. This limits our ability to study the underserved and underrepresented, who historically have worse postoperative outcomes as well as greater rates of FTR.7 Finally, the elderly FTR subgroup had small numbers relative to the overall sample size.
Conclusions
In conclusion, risk of death from postoperative complications following lung cancer resection increases with age by approximately 50% per decade. Although pneumonia was the most common complication in both younger and elderly patients who underwent lung cancer resection, pneumonia was not independently associated with FTR. Unplanned reoperation was associated with reduced odds of death; however, elderly patients underwent reoperation less frequently despite having similar postoperative complications as younger patients. Elderly patients treated by a general thoracic surgeon had a greater chance to survive postoperative complications. Surgical therapy for complications after lung cancer resection and elderly patients managed by a thoracic specialist may mitigate the risk of death following a postoperative adverse event.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/comparison-of-failure-to-rescue-in-younger-versus-elderly-patients-following-lung-cancer-resection.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
Table E1.
Multivariable predictors of failure to rescue after lobectomy or sublobar resection for lung cancer including all clinically relevant variables (full model)
| Characteristic | Model 1: All ages |
Model 2: <80 years old |
Model 3: 80+ years old |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Age in decades | 1.59 | 1.28-1.99 | <.001 | 1.03 | 1.01-1.07 | .019 | 1.13 | 0.96-1.34 | .151 |
| Male vs female | 1.06 | 0.73-1.54 | .756 | 0.94 | 0.62-1.44 | .791 | 1.43 | 0.58-3.67 | .441 |
| Underweight: BMI <18.5 vs BMI >18.5 | 1.34 | 0.51-3.06 | .515 | 1.51 | 0.51-3.75 | .414 | 1.46 | 0.07-10.9 | .749 |
| Obese: BMI >30 vs BMI <30 | 0.91 | 0.60-1.37 | .658 | 0.98 | 0.62- 1.55 | .948 | 0.83 | 0.27-2.27 | .722 |
| Active smoker within 1 y vs nonsmoker within 1 y | 1.08 | 0.72-1.61 | .721 | 1.08 | 0.70-1.67 | .735 | 1.48 | 0.33-5.38 | .574 |
| ASA class III vs class I/II | 0.83 | 0.48-1.50 | .522 | 0.87 | 0.47-1.67 | .650 | 1.06 | 0.26-5.76 | .943 |
| Surgery by thoracic surgeon vs nonthoracic surgeon | 0.67 | 0.41-1.15 | .131 | 0.81 | 0.46-1.50 | .482 | 0.30 | 0.10-1.02 | .045 |
| Open resection vs VATS | 1.15 | 0.79-1.67 | .463 | 1.20 | 0.79-1.84 | .393 | 0.89 | 0.35-2.16 | .792 |
| Lobectomy vs sub-lobar | 1.17 | 0.72-1.97 | .530 | 1.39 | 0.80-2.53 | .268 | 0.99 | 0.32-3.41 | .986 |
| Dyspnea at rest or with moderate exertion vs no dyspnea | 1.40 | 0.94-2.07 | .096 | 1.37 | 0.88-2.12 | .159 | 1.88 | 0.65-5.44 | .238 |
| COPD vs no COPD | 0.91 | 0.62-1.34 | .648 | 1.03 | 0.67-1.58 | .897 | 0.45 | 0.15-1.25 | .138 |
| Pneumonia vs no complication | 1.01 | 0.67-1.51 | .965 | 0.89 | 0.56-1.41 | .627 | 2.02 | 0.74-5.62 | .169 |
| Unplanned intubation vs no complication | 3.93 | 2.47-6.21 | <.001 | 3.77 | 2.22-6.35 | <.001 | 4.43 | 1.43-13.8 | .009 |
| Pulmonary embolus vs no complication | 1.47 | 0.66-3.07 | .323 | 1.65 | 0.68-3.66 | .242 | 0.98 | 0.11-5.88 | .982 |
| DVT requiring therapy vs no complication | 0.88 | 0.38-1.84 | .747 | 0.76 | 0.29-1.76 | .545 | 2.81 | 0.34-15.7 | .273 |
| >48 h on ventilator vs no complication | 1.78 | 1.06-2.98 | .029 | 2.10 | 1.18-3.75 | .012 | 0.78 | 0.18-3.01 | .723 |
| Progressive RI/ARF vs no complication | 3.36 | 1.80-6.10 | <.001 | 2.39 | 1.13-4.83 | .019 | 14.9 | 3.66-67.3 | <.001 |
| Stroke vs No complication | 3.60 | 1.76-6.97 | <.001 | 4.13 | 1.89-8.53 | <.001 | 1.76 | 0.15-11.4 | .596 |
| Cardiac arrest requiring CPR/MI vs no complication | 11.5 | 7.77-17.2 | <.001 | 12.7 | 8.21-19.7 | <.001 | 16.9 | 5.43-56.7 | <.001 |
| Bleeding requiring transfusion vs no complication | 0.81 | 0.51-1.28 | .380 | 0.68 | 0.39-1.16 | .170 | 1.54 | 0.55-4.25 | .403 |
| Sepsis and septic shock vs no complication | 1.47 | 0.89-2.38 | .122 | 1.50 | 0.85-2.58 | .150 | 1.22 | 0.34-4.06 | .751 |
| Unplanned reoperation vs no complication | 0.55 | 0.34-0.86 | .011 | 0.53 | 0.31-0.87 | .015 | 0.94 | 0.28-2.81 | .921 |
| No. of observations | 2823 | 2497 | 326 | ||||||
| No. of FTR events | 178 | 139 | 39 | ||||||
P values in bold are <.05. OR, Odds ratio; CI, confidence interval; BMI, body mass index; ASA, American Society of Anesthesiologists; VATS, video-assisted thoracoscopic surgery; COPD, chronic obstructive pulmonary disease; DVT, deep-vein thrombosis; RI/ARF, renal insufficiency/acute renal failure; CPR/MI, cardiopulmonary resuscitation/myocardial infarction; FTR, failure to rescue.
Table E2.
Multivariable predictors of failure to rescue for patients with lung cancer who underwent lobectomy or sublobar resection with pneumonia as the first or only postoperative complication, n = 893 (full model)
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Age in decades | 1.52 | 1.09-2.16 | .015 |
| Male vs female | 1.53 | 0.83-2.87 | .175 |
| Underweight: BMI <18.5 vs BMI >18.5 | 0.49 | 0.02-3.03 | .527 |
| Obese: BMI >30 vs BMI <30 | 1.12 | 0.57-2.12 | .742 |
| Active smoker within 1 y vs nonsmoker within 1 y | 0.69 | 0.36-1.31 | .255 |
| ASA class III vs class I/II | 1.00 | 0.39-3.01 | .999 |
| Surgery by thoracic surgeon vs nonthoracic surgeon | 1.37 | 0.56-3.76 | .511 |
| Open resection vs VATS | 0.97 | 0.54-1.74 | .922 |
| Lobectomy vs sublobar | 0.91 | 0.42-2.18 | .831 |
| Dyspnea at rest or with moderate exertion vs no dyspnea | 0.96 | 0.51-1.78 | .899 |
| COPD vs no COPD | 1.06 | 0.58-1.93 | .850 |
| Unplanned intubation vs no complication | 7.95 | 3.45-18.8 | <.001 |
| Pulmonary embolus vs no complication | 1.34 | 0.31-4.64 | .666 |
| DVT requiring therapy vs no complication | 0.87 | 0.26-2.42 | .798 |
| >48 h on ventilator vs no complication | 1.36 | 0.65-2.90 | .414 |
| Progressive RI/ARF vs no complication | 3.30 | 1.48-7.33 | .003 |
| Stroke vs no complication | 1.00 | 0.23-3.83 | .995 |
| Cardiac arrest requiring CPR/MI vs no complication | 5.35 | 2.66-10.8 | <.001 |
| Bleeding requiring transfusion vs no complication | 0.57 | 0.24-1.28 | .187 |
| Sepsis and septic shock vs no complication | 1.75 | 0.93-3.27 | .080 |
| Unplanned reoperation vs no complication | 0.43 | 0.20-0.88 | .026 |
| No. of observations | 893 | ||
| No. of events | 79 | ||
P values in bold are <.05. OR, Odds ratio; CI, confidence interval; BMI, body mass index; ASA, American Society of Anesthesiologists; VATS, video-assisted thoracoscopic surgery; COPD, chronic obstructive pulmonary disease; DVT, deep-vein thrombosis; RI/ARF, renal insufficiency/acute renal failure; CPR/MI, cardiopulmonary resuscitation/myocardial infarction.
Table E3.
Characteristics of lung cancer patients who underwent lobectomy or sublobar resection with pneumonia as the first or only postoperative complication
| Characteristic | All ages n = 893 | Younger than 80 years old n = 789 | 80+ years old n = 104 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FTR, n = 79 | SUR, n = 814 | P value | FTR, n = 59 | SUR, n = 730 | P value | FTR, n = 20 | SUR, n = 84 | P value | ||
| Median age, y (IQR) | 74 (67.0, 79.5) | 69.0 (63.0, 75.0) | <.001 | 70.0 (65.0, 74.5) | 68.0 (62.0, 73.0) | .011 | 83.0 (81.0, 84.5) | 82.0 (80.8, 85.0) | .42 | |
| Age category | <.001 | |||||||||
| <80 y old | 59 (74.7%) | 730 (89.7%) | – | – | – | – | – | – | ||
| 80+ y old | 20 (25.3%) | 84 (10.3%) | ||||||||
| Sex | .018 | .13 | .087 | |||||||
| Female | 26 (32.9%) | 381 (46.8%) | 22 (37.3%) | 347 (47.5%) | 4 (20.0%) | 34 (40.5%) | ||||
| Male | 53 (67.1%) | 433 (53.2%) | 37 (62.7%) | 383 (52.5%) | 16 (80.0%) | 50 (59.5%) | ||||
| Race | .10 | .47 | .14 | |||||||
| Asian | 1 (1.3%) | 23 (2.8%) | 1 (1.7%) | 19 (2.6%) | 0 (0%) | 4 (4.8%) | ||||
| Black/African American | 4 (5.1%) | 48 (5.9%) | 4 (6.8%) | 43 (5.9%) | 0 (0%) | 5 (6.0%) | ||||
| Other | 4 (5.1%) | 111 (13.6%) | 4 (6.8%) | 99 (13.6%) | 0 (0%) | 12 (14.3%) | ||||
| White | 70 (88.6%) | 632 (77.6%) | 50 (84.7%) | 569 (77.9%) | 20 (100.0%) | 63 (75.0%) | ||||
| Median BMI (IQR) | 25.6 (23.0, 30.9) | 26.7 (23.1, 30.4) | .57 | 26.6 (23.1, 30.9) | 26.7 (23.1, 30.5) | .86 | 24.0 (22.0, 30.5) | 26.6 (23.3, 29.6) | .44 | |
| Active smoker within 1 y | 26 (32.9%) | 412.0 (50.6%) | .003 | 24 (40.7%) | 404 (55.3%) | .030 | 2 (10.0%) | 8 (9.5%) | 1.00 | |
| Independent functional status | 77 (97.5%) | 802 (98.5%) | .46 | 57 (96.6%) | 719 (98.5%) | .25 | 20 (100.0%) | 83 (98.8%) | 1.00 | |
| Comorbidities | .54 | .92 | .29 | |||||||
| Diabetes | 11 (13.9%) | 135 (16.6%) | 10 (16.9%) | 120 (16.4%) | 1 (5.0%) | 15 (17.9%) | ||||
| Dyspnea with moderate exertion | 27 (34.2%) | 233 (28.6%) | .51 | 23 (39.0%) | 211 (28.9%) | .17 | 4 (20.0%) | 22 (26.2%) | .81 | |
| COPD | 38 (48.1%) | 359 (44.1%) | .50 | 32 (54.2%) | 328 (44.9%) | .17 | 6 (30.0%) | 31 (36.9%) | .56 | |
| Heart failure | 0 (0.0%) | 7 (0.9%) | 1.00 | 0 (0%) | 7 (1.0%) | 1.00 | 0 (0%) | 0 (0%) | – | |
| Chronic steroid use | 7 (8.9%) | 54 (6.6%) | .45 | 4 (6.8%) | 48 (6.6%) | 1.00 | 3 (15.0%) | 6 (7.1%) | .37 | |
| Weight loss >10% within 6 mo | 5.0 (6.3%) | 31 (3.8%) | .24 | 2 (3.4%) | 28 (3.8%) | 1.00 | 3 (15.0%) | 3 (3.6%) | .084 | |
| ASA class | .12 | .033 | .13 | |||||||
| 2—mild disturbance | 5.0 (6.3%) | 91 (11.2%) | 2 (3.4%) | 87 (11.9%) | 3 (15.0%) | 4 (4.8%) | ||||
| 3—severe disturbance | 73.0 (92.4%) | 721 (88.6%) | 56 (94.9%) | 641 (87.8%) | 17 (85.0%) | 80 (95.2%) | ||||
| None assigned | 1.0 (1.3%) | 2 (0.2%) | 1 (1.7%) | 2 (0.3%) | ||||||
| Surgeon specialty | 1.00 | .75 | .60 | |||||||
| Cardiac surgery | 0.0 (0.0%) | 10 (1.2%) | 0 (0%) | 9 (1.2%) | 0 (0.0%) | 1 (1.2%) | ||||
| General surgery | 8.0 (10.1%) | 87 (10.7%) | 4 (6.8%) | 75 (10.3%) | 4 (20.0%) | 12 (14.3%) | ||||
| Thoracic Surgery | 71.0 (89.9%) | 717 (88.1%) | 55 (93.2%) | 646 (88.5%) | 16 (80.0%) | 71 (84.5%) | ||||
| Tumor location | .42 | .32 | .31 | |||||||
| Lower lobe | 32.0 (40.5%) | 279 (34.3%) | 25 (42.4%) | 242 (33.2%) | 7 (35.0%) | 37 (44.0%) | ||||
| Middle lobe | 4.0 (5.1%) | 34.0 (4.2%) | 3.0 (5.1%) | 33.0 (4.5%) | 1.0 (5.0%) | 1.0 (1.2%) | ||||
| Upper lobe | 43 (54.4%) | 501 (61.5%) | 31 (52.5%) | 455 (62.3%) | 12 (60.0%) | 46 (54.8%) | ||||
| Operation | .57 | .84 | .60 | |||||||
| Open lobectomy | 38 (48.1%) | 318 (39.1%) | 29 (49.2%) | 290 (39.7%) | 9 (45.0%) | 28 (33.3%) | ||||
| Open segmentectomy | 3 (3.8%) | 23 (2.8%) | 2 (3.4%) | 22 (3.0%) | 1 (5.0%) | 1 (1.2%) | ||||
| Open wedge resection | 1 (1.3%) | 26 (3.2%) | 1 (1.7%) | 24 (3.3%) | 0 (0.0%) | 2 (2.4%) | ||||
| VATS lobectomy | 31 (39.2%) | 365 (44.8%) | 23 (39.0%) | 323 (44.2%) | 8 (40.0%) | 42 (50.0%) | ||||
| VATS segmentectomy | 1 (1.3%) | 30 (3.7%) | 1 (1.7%) | 26 (3.6%) | 0 (0.0%) | 4 (4.8%) | ||||
| VATS wedge resection | 5 (6.3%) | 52 (6.4%) | 3 (5.1%) | 45 (6.2%) | 2 (10.0%) | 7 (8.3%) | ||||
| Lobectomy vs sublobar resection | .42 | .40 | 1.00 | |||||||
| Lobectomy | 69 (87.3%) | 683 (83.9%) | 52 (88.1%) | 613 (84.0%) | 17 (85.0%) | 70 (83.3%) | ||||
| Sublobar resection | 10 (12.7%) | 131 (16.1%) | 7 (11.9%) | 117 (16.0%) | 3 (15.0%) | 14 (16.7%) | ||||
| Surgical approach | .17 | .22 | .28 | |||||||
| Open | 42 (53.2%) | 367 (45.1%) | 32 (54.2%) | 336 (46.0%) | 10 (50.0%) | 31 (36.9%) | ||||
| VATS | 37 (46.8%) | 447 (54.9%) | 27 (45.8%) | 394 (54.0%) | 10 (50.0%) | 53 (63.1%) | ||||
| Median operation time, min (IQR) | 197.0 (144.0, 264.5) | 189.0 (133.0, 255.0) | .33 | 202.0 (163.5, 264.5) | 189.0 (133.0, 255.0) | .13 | 160.5 (114.2, 258.0) | 189.0 (135.0, 264.2) | .54 | |
| Median postoperative length of stay, d (IQR) | 11.0 (5.5, 19.0) | 9.0 (6.0, 15.0) | .24 | 11.0 (5.0, 19.5) | 9.0 (6.0, 15.0) | .11 | 10.5 (6.0, 13.8) | 10.0 (7.0, 20.0) | .29 | |
| Median days from operation to death (IQR) | 16.0 (9.0, 22.0) | – | 18.0 (10.0, 22.0) | – | 14.0 (6.8, 22.2) | – | ||||
| Organ/space SSI | 2 (2.5%) | 39 (4.8%) | .57 | 2 (3.4%) | 39 (5.3%) | .76 | – | – | – | |
| Unplanned intubation | 66 (83.5%) | 192 (23.6%) | <.001 | 50 (84.7%) | 167 (22.9%) | <.001 | 16 (80.0%) | 25 (29.8%) | <.001 | |
| Pulmonary embolus | 4 (5.1%) | 26 (3.2%) | .33 | 4 (6.8%) | 23 (3.2%) | .14 | 0 (0%) | 3 (3.6%) | 1.000 | |
| DVT requiring therapy | 5 (6.3%) | 35 (4.3%) | .39 | 5 (8.5%) | 32 (4.4%) | .19 | 0 (0%) | 3 (3.6%) | 1.000 | |
| >48 h on ventilator | 50 (63.3%) | 144 (17.7%) | <.001 | 38 (64.4%) | 127 (17.4%) | <.001 | 12 (60%) | 17 (20.2%) | <.001 | |
| Renal failure | 22 (27.8%) | 28 (3.4%) | <.001 | 14 (23.7%) | 27 (3.7%) | <.001 | 8.0 (40.0%) | 1 (1.2%) | <.001 | |
| Stroke | 5 (6.3%) | 11 (1.4%) | .010 | 5 (8.5%) | 9 (1.2%) | .002 | 0 (0%) | 2 (2.4%) | 1.00 | |
| Cardiac arrest requiring transfusion | 32 (40.5%) | 33 (4.1%) | <.001 | 27 (45.8%) | 29 (4.0%) | <.001 | 5 (25.0%) | 4 (4.8%) | .012 | |
| Bleeding requiring transfusion | 13 (16.5%) | 52 (6.4%) | .001 | 7 (11.9%) | 46 (6.3%) | .11 | 6 (30.0%) | 6 (7.1%) | .011 | |
| Sepsis/septic shock | 36 (45.6%) | 136 (16.7%) | <.001 | 27 (45.8%) | 123 (16.8%) | <.001 | 9 (45.0%) | 13 (15.5%) | .012 | |
| Unplanned reoperation | 18 (22.8%) | 144 (17.7%) | .26 | 14 (23.7%) | 136 (18.6%) | .34 | 4 (20.0%) | 8 (9.5%) | .24 | |
P values in bold are <.05. FTR, Failure to rescue; SUR, survived within 30 d postoperation; IQR, interquartile range; BMI, body mass index, COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists; VATS, video-assisted thoracoscopic surgery; SSI, surgical-site infection; DVT, deep-vein thrombosis.
Table E4.
Baseline and operative characteristics of the matched groups
| Characteristic | <80 years old, N = 323, n (%) | 80+ years old, N = 323, n (%) | P value |
|---|---|---|---|
| Sex | .873∗ | ||
| Female | 138.0 (42.7) | 136.0 (42.1) | |
| Male | 185.0 (57.3) | 187.0 (57.9) | |
| Race | .922∗ | ||
| White | 254.0 (78.6) | 256.0 (79.3) | |
| Asian | 21.0 (6.5) | 17.0 (5.3) | |
| Black/African American | 11.0 (3.4) | 12.0 (3.7) | |
| Other | 37.0 (11.5) | 38.0 (11.8) | |
| Median BMI (IQR) | 25.9 (22.7, 29.8) | 26.1 (22.9, 29.6) | .742† |
| Active smoker within 1 y | 38.0 (11.8) | 41.0 (12.7) | .719∗ |
| Independent functional status | 320.0 (99.1) | 316.0 (97.8) | .340‡ |
| Comorbidities | |||
| Diabetes | 53.0 (16.4) | 55.0 (17.0) | .833∗ |
| Dyspnea with moderate exertion | 80.0 (24.8) | 79.0 (24.5) | .762∗ |
| COPD | 99.0 (30.7) | 103.0 (31.9) | .734∗ |
| Heart failure | 0.0 (0.0) | 1.0 (0.3) | 1.000‡ |
| Chronic steroid use | 20.0 (6.2) | 17.0 (5.3) | .611∗ |
| Weight loss >10% within 6 mo | 14.0 (4.3) | 16.0 (5.0) | .708∗ |
| ASA class | .921‡ | ||
| 2—mild disturbance | 33.0 (10.2) | 30.0 (9.3) | |
| 3—severe disturbance | 288.0 (89.2) | 291.0 (90.1) | |
| None assigned | 2.0 (0.6) | 2.0 (0.6) | |
| Surgeon specialty | 1.000‡ | ||
| Cardiac surgery | 2.0 (0.6) | 3.0 (0.9) | |
| General surgery | 36.0 (11.1) | 35.0 (10.8) | |
| Thoracic surgery | 285.0 (88.2) | 285.0 (88.2) | |
| Tumor location | .771∗ | ||
| Lower lobe | 126.0 (39.0) | 125.0 (38.7) | |
| Middle lobe | 11.0 (3.4) | 8.0 (2.5) | |
| Upper lobe | 186.0 (57.6) | 190.0 (58.8) | |
| Lobectomy vs sublobar resection | .430∗ | ||
| Lobectomy | 255.0 (78.9) | 263.0 (81.4) | |
| Sublobar resection | 68.0 (21.1) | 60.0 (18.6) | |
| Surgical approach | .810∗ | ||
| Open | 134.0 (41.5) | 131.0 (40.6) | |
| VATS | 189.0 (58.5) | 192.0 (59.4) |
BMI, Body mass index; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists; VATS, video-assisted thoracoscopic surgery.
Pearson χ2 test.
Wilcoxon rank sum test.
Fisher exact test.
Table E5.
Summary of balance for age-matched data
| Characteristic | <80 years old, N = 323, n (%) | 80+ years old, N = 323, n (%) | Absolute standard mean differences |
|---|---|---|---|
| Sex | |||
| Female | 138 (43) | 136 (42) | 0.0125 |
| Male | 185 (57) | 187 (58) | 0.0125 |
| Race | |||
| White | 254 (79) | 256 (79) | 0.0153 |
| Asian | 21 (6.5) | 17 (5.3) | 0.0555 |
| Black/African American | 11 (3.4) | 12 (3.7) | 0.0164 |
| Other | 37 (11) | 38 (12) | 0.0096 |
| BMI, median (IQR) | 25.9 (22.7, 29.8) | 26.1 (22.9, 29.6) | 0.0402 |
| Active smoker within 1 y | 38 (12) | 41 (13) | 0.0279 |
| Functional status | |||
| Independent | 320 (99) | 316 (98) | 0.0850 |
| Partially dependent | 3 (0.9) | 6 (1.9) | 0.0688 |
| Diabetes | |||
| Diabetes | 53 (16) | 55 (17) | 0.0165 |
| No diabetes | 270 (84) | 268 (83) | 0.0165 |
| Dyspnea | |||
| At rest | 7 (2.2) | 10 (3.1) | 0.0536 |
| Moderate exertion | 80 (25) | 79 (24) | 0.0072 |
| No | 236 (73) | 234 (72) | 0.0139 |
| COPD | 99 (31) | 103 (32) | 0.0266 |
| Heart failure | 0 (0) | 1 (0.3) | 0.0557 |
| Steroid | 20 (6.2) | 17 (5.3) | 0.0416 |
| Weight loss >10% within 6 mo | 14 (4.3) | 16 (5.0) | 0.0285 |
| ASA class | |||
| 2—mild disturbance | 33 (10) | 30 (9.3) | 0.0320 |
| 3—severe disturbance | 288 (89) | 291 (90) | 0.0311 |
| Surgeon specialty | |||
| Cardiac surgery | 2 (0.6) | 3 (0.9) | 0.0323 |
| General surgery | 36 (11) | 35 (11) | 0.0100 |
| Thoracic surgery | 285 (88) | 285 (88) | 0.0000 |
| Tumor location | |||
| Lower lobe | 126 (39) | 125 (39) | 0.0064 |
| Middle lobe | 11 (3.4) | 8 (2.5) | 0.0598 |
| Upper lobe | 186 (58) | 190 (59) | 0.0252 |
| Initial surgical approach | |||
| Open | 134 (41) | 131 (41) | 0.0189 |
| VATS | 189 (59) | 192 (59) | 0.0189 |
| Operation type | |||
| Lobectomy | 255 (79) | 263 (81) | 0.0637 |
| Sublobar resection | 68 (21) | 60 (19) | 0.0637 |
BMI, Body mass index; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists; VATS, video-assisted thoracoscopic surgery.
Table E6.
Reoperation interventions after postoperative complication
| Reoperation | n = 909, n (%) |
|---|---|
| Bronchoscopy | 173 (19.0) |
| Chest reoperation | 371 (40.8) |
| Tracheostomy | 44 (4.8) |
| Other scopes | 38 (4.2) |
| Abdominal surgery | 42 (4.6) |
| Other | 94 (10.3) |
| Unspecified procedure | 147 (16.2) |
Table E7.
Distribution of reoperations by age group
| Reoperation cases by age group | ||||
|---|---|---|---|---|
| Characteristic | N | No reoperation, N = 1914, n (%) | Reoperation, N = 909, n (%) | P value |
| Age in decades | 2823 | .042∗ | ||
| <50 y | 40 (2.1) | 30 (3.3) | ||
| 50-59 y | 244 (13) | 112 (12) | ||
| 60-69 y | 629 (33) | 312 (34) | ||
| 70-79 y | 760 (40) | 370 (41) | ||
| 80+ y | 241 (13) | 85 (9.4) | ||
Pearson χ2 test.
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program Cancer stat facts: lung and bronchus cancer. 2022. https://seer.cancer.gov/statfacts/html/lungb.html
- 2.Boffa D.J., Allen M.S., Grab J.D., Gaissert H.A., Harpole D.H., Wright C.D. Data from the Society of Thoracic Surgeons General Thoracic Surgery Database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 3.Yao L., Luo J., Liu L., Wu Q., Zhou R., Li L., et al. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery: a retrospective study. Medicine (Baltimore) 2021;100:e25295. doi: 10.1097/MD.0000000000025295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farjah F., Backhus L., Cheng A., Englum B., Kim S., Saha-Chaudhuri P., et al. Failure to rescue and pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2015;149:1365–1371. doi: 10.1016/j.jtcvs.2015.01.063. discussion 1371-3.e3. [DOI] [PubMed] [Google Scholar]
- 5.Farjah F., Grau-Sepulveda M.V., Gaissert H., Block M., Grogan E., Brown L.M., et al. Volume pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518–3527. doi: 10.1200/JCO.20.00329. [DOI] [PubMed] [Google Scholar]
- 6.Grenda T.R., Revels S.L., Yin H., Birkmeyer J.D., Wong S.L. Lung cancer resection at hospitals with high versus low mortality rates. JAMA Surg. 2015;150:1034–1040. doi: 10.1001/jamasurg.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reames B.N., Birkmeyer N.J., Dimick J.B., Ghaferi A.A. Socioeconomic disparities in mortality after cancer surgery: failure to rescue. JAMA Surg. 2014;149:475–481. doi: 10.1001/jamasurg.2013.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andalib A., Ramana-Kumar A.V., Bartlett G., Franco E.L., Ferri L.E. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol. 2013;8:554–561. doi: 10.1097/JTO.0b013e3182862e7e. [DOI] [PubMed] [Google Scholar]
- 9.Nathan H., Yin H., Wong S.L. Postoperative complications and long-term survival after complex cancer resection. Ann Surg Oncol. 2017;24:638–644. doi: 10.1245/s10434-016-5569-5. [DOI] [PubMed] [Google Scholar]
- 10.Ghaferi A.A., Birkmeyer J.D., Dimick J.B. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 11.Ghaferi A.A., Birkmeyer J.D., Dimick J.B. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 12.Liou D.Z., Serna-Gallegos D., Mirocha J., Bairamian V., Alban R.F., Soukiasian H.J. Predictors of failure to rescue after esophagectomy. Ann Thorac Surg. 2018;105:871–878. doi: 10.1016/j.athoracsur.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Milojevic M., Bond C., He C., Shannon F.L., Clark M., Theurer P.F., et al. Failure to rescue: variation in mortality after cardiac surgery. Interact Cardiovasc Thorac Surg. 2021;33:848–856. doi: 10.1093/icvts/ivab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silber J.H., Williams S.V., Krakauer H., Schwartz J.S. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ghaferi A.A., Birkmeyer J.D., Dimick J.B. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 16.Hollenbeck B.K., Daignault S., Dunn R.L., Gilbert S., Weizer A.Z., Miller D.C. Getting under the hood of the volume–outcome relationship for radical cystectomy. J Urol. 2007;177:2095–2099. doi: 10.1016/j.juro.2007.01.153. discussion 2099. [DOI] [PubMed] [Google Scholar]
- 17.Alabbadi S., Roach A., Chikwe J., Egorova N.N. National trend in failure to rescue after cardiac surgeries. J Thorac Cardiovasc Surg. 2023:1157–1165.e6. doi: 10.1016/j.jtcvs.2022.02.037. [DOI] [PubMed] [Google Scholar]
- 18.Gross C.R., Adams D.H., Patel P., Varghese R. Failure to rescue: a quality metric for cardiac surgery and cardiovascular critical care. Can J Cardiol. 2023;39:487–496. doi: 10.1016/j.cjca.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Kurlansky P.A., O'Brien S.M., Vassileva C.M., Lobdell K.W., Edwards F.H., Jacobs J.P., et al. Failure to rescue: a new Society of Thoracic Surgeons quality metric for cardiac surgery. Ann Thorac Surg. 2022;113:1935–1942. doi: 10.1016/j.athoracsur.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Strobel R.J., Kaplan E.F., Young A.M., Rotar E.P., Mehaffey J.H., Hawkins R.B., et al. Socioeconomic distress is associated with failure to rescue in cardiac surgery. J Thorac Cardiovasc Surg. 20 July 2022 doi: 10.1016/j.jtcvs.2022.07.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Surgeons National Surgical Quality Improvement Program. 2023. https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/
- 22.Mehta R., Paredes A.Z., Tsilimigras D.I., Farooq A., Sahara K., Merath K., et al. CMS hospital compare system of star ratings and surgical outcomes among patients undergoing surgery for cancer: do the ratings matter? Ann Surg Oncol. 2020;27:3138–3146. doi: 10.1245/s10434-019-08088-y. [DOI] [PubMed] [Google Scholar]
- 23.Tran Z., Verma A., Williamson C., Hadaya J., Sanaiha Y., Gandjian M., et al. Failure to rescue after surgical re-exploration in lung resection. Surgery. 2021;170:257–262. doi: 10.1016/j.surg.2021.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Farjah F. Failure-to-rescue in thoracic surgery. Thorac Surg Clin. 2017;27:257–266. doi: 10.1016/j.thorsurg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Dewan K.C., Navale S.M., Hirji S.A., Koroukian S.M., Dewan K.S., Svensson L.G., et al. The role of frailty in failure to rescue after cardiovascular surgery. Ann Thorac Surg. 2021;111:472–478. doi: 10.1016/j.athoracsur.2020.06.065. [DOI] [PubMed] [Google Scholar]
- 26.Shah R., Attwood K., Arya S., Hall D.E., Johanning J.M., Gabriel E., et al. Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surg. 2018;153:e180214. doi: 10.1001/jamasurg.2018.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelsattar Z.M., Habermann E., Borah B.J., Moriarty J.P., Rojas R.L., Blackmon S.H. Understanding failure to rescue after esophagectomy in the United States. Ann Thorac Surg. 2020;109:865–871. doi: 10.1016/j.athoracsur.2019.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Ellis M.C., Diggs B.S., Vetto J.T., Schipper P.H. Intraoperative oncologic staging and outcomes for lung cancer resection vary by surgeon specialty. Ann Thorac Surg. 2011;92:1958–1963. doi: 10.1016/j.athoracsur.2011.05.120. discussion 1963-54. [DOI] [PubMed] [Google Scholar]
- 29.Farjah F., Flum D.R., Varghese T.K., Jr., Symons R.G., Wood D.E. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg. 2009;87:995–1004. doi: 10.1016/j.athoracsur.2008.12.030. discussion 1005-6. [DOI] [PubMed] [Google Scholar]
- 30.Goodney P.P., Lucas F.L., Stukel T.A., Birkmeyer J.D. Surgeon specialty and operative mortality with lung resection. Ann Surg. 2005;241:179–184. doi: 10.1097/01.sla.0000149428.17238.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Meyenfeldt E.M., Gooiker G.A., van Gijn W., Post P.N., van de Velde C.J., Tollenaar R.A., et al. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2012;7:1170–1178. doi: 10.1097/JTO.0b013e318257cc45. [DOI] [PubMed] [Google Scholar]