Abstract
Background
Molecular informed therapy changed treatment patterns of metastatic colorectal cancer (mCRC). Recently KRAS G12, the most prevalent RAS mutation in mCRC, was investigated to be a negative predictive marker for the efficacy of trifluridine/tipiracil (FTD/TPI). Whether this proposed selectivity remains when FTD/TPI is combined with bevacizumab remains elusive. We aimed to describe the efficacy of FTD/TPI + bevacizumab depending on the RAS mutational status in a real-world population.
Patients and methods
Patients from five different cancer centers in Austria who received FTD/TPI + bevacizumab in any treatment line having available information on their molecular profile were eligible. Data were retrospectively collected by chart review. Survival data were compared using log-rank test. Multivariate Cox regression models included several established covariates.
Results
One hundred and twenty-three patients with mCRC were included in this study. Median overall survival (OS) was highly similar in the RAS wild type (WT) [9.63 months (95% confidence interval [CI] 8.055-13.775 months)] and the RAS mutant cohorts [8.78 months (95% CI 8.055-11.014 months)], which was confirmed in a multivariable model adjusting for potential confounders; hazard ratio (HR): 1.05 (95% CI 0.618-1.785; P = 0.857). In addition, no effect of KRAS G12 status on patient outcome was observed. In detail, OS was 8.88 months (95% CI 7.332-12.921 months) in patients with KRAS G12 mutation, compared to 9.47 months (95% CI 8.088-11.375 months) in patients with RAS WT/no-KRAS G12 disease [HR: 0.822 (95% CI 0.527-1.282; P = 0.387)].
Conclusion
This real-world study indicates that the efficacy of FTD/TPI + bevacizumab is independent of RAS mutational status and that bevacizumab may therefore mitigate the potentially limited efficacy of FTD/TPI monotherapy in the KRAS G12-mutated population.
Key words: mCRC, FTD/TPI, bevacizumab, KRAS G12, real-world data
Highlights
-
•
Efficacy of FTD/TPI and bevacizumab was confirmed in the largest European real-world cohort.
-
•
The combination of FTD/TPI and bevacizumab showed therapeutic benefits irrespective of RAS mutational status.
-
•
KRAS G12 mutations were not significantly associated with poorer survival benefit.
Introduction
The application of precision oncology in metastatic colorectal cancer (mCRC) management, including the use of biomarkers such as microsatellite instability (MSI), RAS, HER2neu, BRAF V600E and considerations of the primary tumor location, has revolutionized the treatment landscape for this patient population.1, 2, 3, 4
(K)RAS mutations, which are present in ∼40% of mCRC cases, pioneered precision medicine in mCRC. It is well known that only RAS wild type (WT) patients are recommended for epidermal growth factor receptor antibody treatment combined with chemotherapy.5
Precision oncology is so far largely used to select antibodies, selective pathway inhibitors or immunotherapy for treatment. Chemotherapy, which is still the cornerstone of most therapeutic treatments in mCRC, is considered to be unselective and therefore lacking biological predictors.4
Trifluridine/tipiracil (FTD/TPI), a composite formulation encompassing trifluridine (FTD), a nucleoside analog, and tipiracil (TPI), an inhibitor of thymidine phosphorylase, represents a standard therapeutic option for later lines of treatment, as evidenced by the RECOURSE trial. This compound marks the most recent advancement in the field of chemotherapeutic approaches for mCRC.6, 7, 8
The role of the TAS-102 drug (FTD/TPI), particularly in relation to (K)RAS mutant (MT) subgroups, has been an upcoming issue. Retrospective analyses of the RECOURSE trial and real-world data from European cohorts suggest that patients with mutations in KRAS exon 2 codon 12, which are by far the most prevalent alterations and constitute about one-third of all mCRC cases, seem to derive less benefit from FTD/TPI compared to those with KRAS WT or no-KRAS G12-altered mCRC.9
The advent of the SUNLIGHT trial later this year, which demonstrated a clinically meaningful advantage of combining bevacizumab with FTD/TPI over FTD/TPI monotherapy, presents new treatment possibilities and takes over the standard of care in further lines.10 A key question that arises is whether the addition of bevacizumab to FTD/TPI might mitigate the less favorable response to FTD/TPI in patients with KRAS G12 mutations.11
Our planned multicenter real-world analysis aiming to investigate the effectiveness of FTD/TPI + bevacizumab treatment in patients with mCRC can provide crucial insights into this matter and will undeniably contribute significantly to the ongoing discussions surrounding the optimization of mCRC treatment in the context of precision oncology.
Patients and methods
Patient selection
We retrospectively collected data of 123 patients with mCRC treated with FTD/TPI as part of their standard of care between 2016 and 2023 at five academic centers or teaching hospitals across Austria. Patients aged >18 years with a histologically confirmed diagnosis of CRC who received FTD/TPI + bevacizumab in any treatment line in the metastatic setting, having available information on their molecular profile, were eligible. Tumor mutational profiling (at least KRAS, NRAS and BRAF status) was conducted according to local recommendations. We excluded patients with missing RAS status, incomplete medical records or patients receiving previous FTD/TPI monotherapy or FTD/TPI in combination with antiangiogenic agents other than bevacizumab. The FTD/TPI + bevacizumab regimen consisted of FTD/TPI administered orally at a dose of 35 mg/m2 of body surface area, administered twice daily on days 1-5 and 8-12 in a 28-day cycle, and bevacizumab administered intravenously at a dose of 5 mg/kg of bodyweight, delivered every 2 weeks in a 28-day cycle. Dose reductions were at the discretion of the treating physicians.
Data cut-off was July 2023. The study was approved by the Medical Ethical Committee of the Kepler University Linz (1205/2023) and was conducted in accordance with the Declaration of Helsinki (fourth edition). All patients provided written informed consent for the analysis of tumor genomic data during routine care. Written consent for participation in this study was not required due to the retrospective analysis of routine data.
Outcome measurement
The principal objective was to elucidate the correlation between KRAS G12 mutation status and overall survival (OS), while the secondary objective focused on its association with progression-free survival (PFS) and response rates. These associations were analyzed across the entire cohort as well as within specific subgroups defined by RAS/RAF mutations. All endpoints considered in this real-world assessment were determined from the commencement of FTD/TPI therapy and were monitored throughout the treatment duration at collaborating institutions, adhering to local clinical standards. Survival data derived from statistical analysis are expressed as median values.
Statistics
Median OS and median PFS were calculated using the Kaplan–Meier (KM) method. Estimations of follow-up rates were constructed by the reversing KM method. Differences in OS and PFS were determined using the log-rank test. Differences in baseline characteristics and response rate were calculated using Fisher’s exact test. The two-sided significance level for exploratory endpoint was set to 0.05. The proportional hazards assumption was based on Schoenfeld residuals with a significance threshold of P = 0.05. Unadjusted Cox regression analyses were carried out in a univariate manner [hazard ratio (HR) with 95% confidence interval (CI) were indicated]. For the multivariate Cox regression analyses 16 variables were selected. This selection included factors prespecified in the RECOURSE or SUNLIGHT trials and analysis derived from the publication of van de Haar et al.6,10,11 These included: age (<65 versus ≥65 years); sex; primary site of the disease (left versus right origin); previous surgery in curative intention (yes versus no); number of previous regimens in a metastatic setting (0-1, 2 or ≥3); disease refractory to fluoropyrimidine as part of the last previous regimen (yes versus no); prior exposure to bevacizumab or other vascular endothelial growth factor (VEGF) inhibitors (yes versus no); time since diagnosis of metastatic disease to initiation of FTD/TPI + bevacizumab (<18 versus ≥18 months); Eastern Cooperative Oncology Group (ECOG) performance status (0-1 versus 2); number of metastatic sites (1-2 versus ≥3); peritoneal disease at the start of FTD/TPI + bevacizumab treatment (yes versus no); lung-only disease (yes versus no); local therapy after initiation of FTD/TPI + bevacizumab (yes versus no); further systemic therapy after FTD/TPI + bevacizumab (yes versus no); prior exposure to irinotecan (yes versus no); and prior exposure to oxaliplatin (yes versus no). All statistical analyses were done using STATA version 18.0 (StataCorp, College Station, TX).
Results
Characterization of the study cohort
One hundred and twenty-three patients were included in our analysis (Table 1). The median age of the overall cohort was 65.2 years (95% CI 59.48-69.33 years). ECOG performance status was 0 or 1 in 87.8% of the cases. In 59.3% of the included patients, prior surgery was carried out in a curative intention. The majority of patients (74%) had left-sided origin of the tumor. In 79.7% of patients, FTD/TPI + bevacizumab was initiated in the third or fourth line of treatment sequence. Median follow-up was 20.45 months (95% CI 14.827-23.836 months).
Table 1.
Patients included in the study
|
RAS WT |
RAS MT |
KRAS G12 |
KRAS G13 |
RAS other | Total (overall cohort) | KRAS WT versus KRASMT (P value) | Overall cohort:G12 versus noG12 (P value) | RAS MT cohort:G12 versus noG12 (P value) | KRAS exon 2 cohort:G12 versus noG12 (P value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 47 (38.2%) | 76 (61.8%) | 51 (41.5%) | 8 (6.5%) | 25 (20.3%) | 123 (100.0%) | ||||
| Age, years [95% IQR] | 64.3 [60.49-68.92] | 65.5 [62.64-68.64] | 66.1 [59.45-71.08] | 64.8 [58.78-72.31] | 64.8 [59.48-69.33] | 65.2 [62.73-68.05] | 0.909 | 0.796 | 0.807 | 0.959 |
| <65 | 24 (51.1%) | 38 (50.0%) | 25 (49.0%) | 4 (50.0%) | 13 (52.0%) | 59 (50.4%) | ||||
| ≥65 | 23 (48.9%) | 38 (50.0%) | 26 (51.0%) | 4 (50.0%) | 12 (48.0%) | 58 (49.6%) | ||||
| Sex | ||||||||||
| M | 30 (63.8%) | 45 (59.2%) | 28 (54.9%) | 6 (75.0%) | 17 (68.0%) | 71 (60.7%) | 0.610 | 0.245 | 0.275 | 0.285 |
| F | 17 (36.2%) | 31 (40.8%) | 23 (45.1%) | 2 (25.0%) | 8 (32.0%) | 46 (39.3%) | ||||
| Left primary site | 36 (76.6%) | 55 (72.4%) | 37 (72.5%) | 5 (62.5%) | 18 (72.0%) | 91 (74.0%) | 0.604 | 0.760 | 0.960 | 0.560 |
| Surgery in curative intention | 30 (63.8%) | 43 (56.6%) | 32 (62.7%) | 1 (12.5%) | 11 (44.0%) | 73 (59.3%) | 0.426 | 0.519 | 0.121 | 0.008 |
| Prior lines | ||||||||||
| 0-1 | 5 (10.6%) | 20 (26.3%) | 14 (27.5%) | 2 (25.0%) | 6 (24.0%) | 25 (20.3%) | 0.036 | 0.098 | 0.748 | 0.885 |
| 2 | 24 (51.1%) | 42 (55.3%) | 28 (54.9%) | 6 (75.0%) | 14 (56.0%) | 66 (53.7%) | 0.650 | 0.816 | 0.928 | 0.285 |
| 3 or more | 18 (38.3%) | 14 (18.4%) | 9 (17.6%) | 0 (0.0%) | 5 (20.0%) | 32 (26.0%) | 0.015 | 0.075 | 0.804 | 0.197 |
| Refractory to 5-FU | 25 (53.2%) | 45 (59.2%) | 31 (60.8%) | 5 (62.5%) | 14 (56.0%) | 70 (56.9%) | 0.512 | 0.465 | 0.690 | 0.926 |
| Prior bevacizumab | 34 (72.3%) | 68 (89.5%) | 47 (92.2%) | 8 (100.0%) | 21 (84.0%) | 102 (82.9%) | 0.014 | 0.022 | 0.276 | 0.412 |
| Meta-time <18 month | 17 (36.2%) | 35 (46.1%) | 22 (43.1%) | 4 (50.0%) | 13 (52.0%) | 52 (42.3%) | 0.281 | 0.871 | 0.466 | 0.716 |
| ECOG | ||||||||||
| 0-1 | 40 (85.1%) | 68 (89.5%) | 47 (92.2%) | 7 (87.5%) | 21 (84.0%) | 108 (87.8%) | 0.472 | 0.214 | 0.276 | 0.660 |
| Number of metastatic sites | ||||||||||
| 1-2 | 38 (80.9%) | 51 (67.1%) | 33 (64.7%) | 4 (50.0%) | 18 (72.0%) | 89 (72.4%) | 0.098 | 0.110 | 0.525 | 0.424 |
| Peritoneal involvement | 11 (23.4%) | 21 (27.6%) | 11 (21.6%) | 4 (50.0%) | 10 (40.0%) | 32 (26.0%) | 0.604 | 0.344 | 0.091 | 0.086 |
| Lung only disease | 4 (8.5%) | 8 (10.5%) | 7 (13.7%) | 1 (12.5%) | 1 (4.0%) | 12 (9.8%) | 0.714 | 0.212 | 0.194 | 0.925 |
| Local therapy | 10 (21.3%) | 9 (11.8%) | 4 (7.8%) | 0 (0.0%) | 5 (20.0%) | 19 (15.4%) | 0.159 | 0.050 | 0.123 | 0.412 |
| Further systemic therapy | 18 (38.3%) | 30 (39.5%) | 22 (43.1%) | 2 (25.0%) | 8 (32.0%) | 48 (39.0%) | 0.897 | 0.431 | 0.351 | 0.332 |
| Prior irinotecan | 43 (91.5%) | 59 (77.6%) | 40 (78.4%) | 6 (75.0%) | 19 (76.0%) | 102 (82.9%) | 0.047 | 0.265 | 0.811 | 0.828 |
| Prior oxaliplatin | 44 (93.6%) | 58 (76.3%) | 40 (78.4%) | 6 (75.0%) | 18 (72.0%) | 102 (82.9%) | 0.013 | 0.265 | 0.536 | 0.828 |
| Best overall response | ||||||||||
| CR | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.515 | 0.99 | 0.851 | 0.99 |
| PR | 2 (4.5%) | 5 (7.1%) | 3 (6.4%) | 0 (0%) | 2 (8.7%) | 7 (6.1%) | ||||
| SD | 16 (36.4%) | 28 (40%) | 18 (38.3%) | 3 (37.5%) | 10 (43.5%) | 44 (38.6%) | ||||
| DCR | 18 (40.9%) | 33 (47.1%) | 21 (44.7%) | 3 (37.5%) | 12 (52.2%) | 51 (44.7%) | ||||
| PD | 26 (59.1%) | 37 (52.9%) | 26 (55.3%) | 5 (62.5%) | 11 (47.8%) | 63 (55.3%) | ||||
| NA | 3 (6.4%) | 6 (7.9%) | 4 (7.8%) | 0 (0%) | 2 (8%) | 9 (7.3%) |
5-FU, 5-fluorouracil; CR, complete response; DCR (CR+PR+SD), disease control rate; ECOG, Eastern Cooperative Oncology Group; meta-time, time since diagnosis of first metastases; MT, mutant; NA, not available; PD, progressive disease; PR, partial response; SD, stable disease; WT, wild type.
Most patients harbored a KRAS mutation (61.8%). Among these KRAS, exon 2 codon 12 alterations were most prevalent (41.5% of the overall cohort or 67.1% of the RAS MT cohort). Among the other RAS mutations, nine were NRAS mutations, eight were KRAS exon 2 codon 13–altered tumor and eight had very rare KRAS mutations (A146T, Q61L or K117N). The molecular subgroups (KRAS WT, KRAS MT, KRAS G12, other KRAS mutations and KRAS G13) were well balanced regarding the major patient characteristics depicted in Table 1. Significantly more RAS MT patients received FTD/TPI + bevacizumab in the first or second palliative line compared to RAS WT (26.3% versus 10.6%, P = 0.036). On the other hand, FTD/TPI + bevacizumab was more often initiated in the fourth line and beyond in RAS WT compared to RAS MT patients (38.3% versus 18.4%, P = 0.014). Details are provided in Table 1.
Efficacy of FTD/TPI + bevacizumab in the overall cohort
Disease control rate (DCR) in the overall cohort was 44.7%, with 6.1% of the treated patients achieving partial remission as best radiographic response. PFS was 3.95 months (95% CI 3.255-5.326 months) and OS was 9.37 months (95% CI 8.252-11.014 months).
Treatment efficacy and RAS mutational status
DCRs between the RAS WT and the RAS MT group were comparable, with 40.9% versus 47.1%, respectively (P = 0.515). Numerically higher response rates were observed in the RAS MT group (4.5% versus 7.1%; P = 0.707) (Table 1).
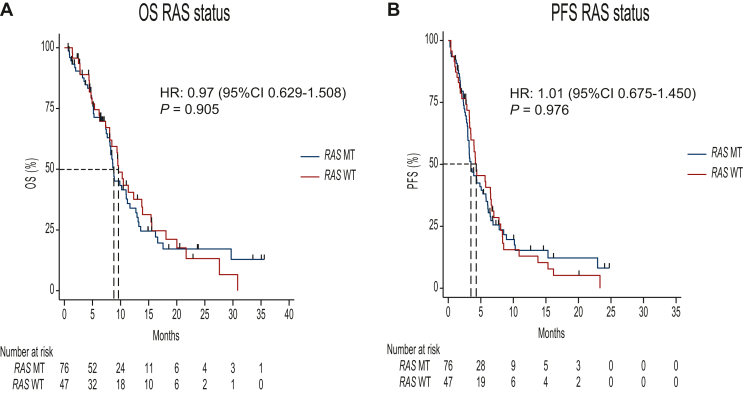
We observed no statistically significant difference in PFS or OS according to RAS mutational status. PFS was 4.31 months (95% CI 3.288-6.608 months) in the RAS WT group versus 3.49 months (95% CI 2.992-5.326 months) in the RAS MT group in the unadjusted univariate analysis [HR 1.01 (95% CI 0.675-1.450; P = 0.976)]. After adjusting for potential cofounders, similar results were observed [HR 0.91 (95% CI 0.560-1.468; P = 0.690)] (Figure 1B).
Figure 1.
Kaplan–Meier plots depicting OS (A, left) and PFS (B, right) curves of RAS WT compared to the RAS MT population. CI, confidence interval; HR, hazard ratio; MT, mutant; OS, overall survival; PFS, progression-free survival; WT, wild type.
OS was 9.63 months (95% CI 8.055-13.775 months) for the RAS WT cohort versus 8.78 months (95% CI 8.055-11.014 months) for the RAS MT group, which resulted in a HR of 0.97 (95% CI 0.629-1.508; P = 0.905) in the univariate analysis, and a HR of 1.05 (95% CI 0.618-1.785; P = 0.857) in the multivariate analysis (Figure 1A). To assess whether there is an interaction between RAS mutational status and bevacizumab pre-treatment in terms of association with outcome (OS), we included an interaction term between these two variables in our multivariate model; the estimated interaction was not significant (P = 0.243).
Efficacy dependent on KRAS codon 12 mutational status
We next examined a potential (K)RAS codon-specific impact on survival and first compared survival rates according to the KRAS G12 mutation status.
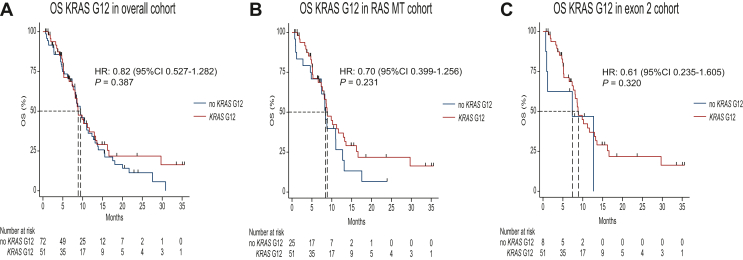
OS in patients with KRAS G12–mutant disease was 8.88 months (95% CI 7.332-12.921 months) and 9.47 months (95% CI 8.088-11.375 months) in the residual cohort (RAS WT and no KRAS G12) resulting in a HR of 0.82 (95% CI 0.527-1.282; P = 0.387) in the univariate analysis and 0.87 (95% CI 0.488-1.537; P = 0.624) in the multivariate analysis (Figure 2A). For the largest subgroup (n = 66) of strictly third-line patients, OS was 8.09 months (95% CI 4.932-9.501 months) for the KRAS G12 cohort versus 8.05 months (95% CI 5.162-11.671 months) for no-KRAS G12 with a HR of 1.25 (95% CI 0.575-2.705; P = 0.58). Furthermore, we could not detect a significant prognostic impact of KRAS G12 status in the smaller subsets of patients receiving FTD/TPI + bevacizumab in the first and second line [n = 25; HR 0.21 (95% CI 0.011-3.789; P = 0.29)] or fourth line and beyond [n = 32; HR 0.25 (95% CI 0.024-2.505; P = 0.24)].
Figure 2.
Kaplan–Meier plots depicting OS curves of the KRAS G12-altered cohort versus RAS WT and no KRAS G12-altered cohort (A, left), KRAS G12-harboring cohort versus no KRAS G12-mutated group (B, middle) and the KRAS G12-harboring cohort versus KRAS G13 variants (C, right). CI, confidence interval; HR, hazard ratio; OS, overall survival; MT, mutant; WT, wild type.
PFS was also highly comparable between KRAS G12 and the RAS WT/no KRAS G12 cohort: 3.32 months (95% CI 2.762-5.063 months) for the KRAS G12 cohort versus 4.18 months (95% CI 3.255-6.477 months) in the RAS WT/no KRAS G12 cohort. The HR was 1.03 (95% CI 0.691-1.545; P = 0.874) in the univariate analysis and 1.21 (95% CI 0.730-2.00; P = 0.461) in the multivariate analysis.
Efficacy dependent on KRAS codon 12 mutational status compared to other RAS mutations
Among the 76 RAS-mutated tumors, OS in the no-KRAS G12 groups was 8.45 months (95% CI 4.833-11.014 months) with a HR of 0.699 (95% CI 0.399-1.256; P = 0.231) in the univariate analysis and a HR of 0.681 (95% CI 0.290-1.599; P = 0.378) in the multivariate analysis (Figure 2B).
PFS for no-KRAS G12 patients was 3.91 months (95% CI 2.926-6.871 months). A HR of 1.104 (95% CI 0.635-1.918; P = 0.726) in the univariate or a HR of 1.384 (95% CI 0.658-2.912; P = 0.392) in the multivariate analysis revealed no difference between the two cohorts.
In the group of KRAS exon 2–mutated patients, no difference was found between KRAS G12 and KRAS G13 variants. OS for KRAS G13 was 7.364 months [95% CI 0.690 months-not reached (NR)], and PFS was 2.992 months (95% CI 0.263 months-NR) (Figure 2C).
HR compared to KRAS G12 was for OS in the univariate analysis [HR 0.614 (95% CI 0.235-1.605; P = 0.320)] and remained not significant in the multivariate analysis [HR 0.632 (95% CI 0.188-2.126; P = 0.459)].
Results for PFS were similar [HR 1.096 (95% CI 0.429-2.80; P = 0.848) and HR 1.232 (95% CI 0.401-3.783; P = 0.716)] in the multivariate model.
Survival outcome in relation to clinical and molecular factors
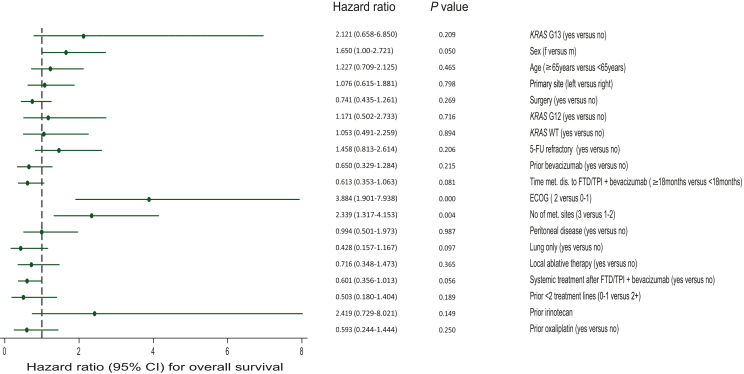
In a multivariate model including RAS mutational status, the ECOG performance status (ECOG 2 versus 0/1) [HR 3.88 (95% CI 1.901-7.938; P = <0.0001)] and the number of metastatic sites at treatment start (3 or more versus 2 or less) [HR 2.34 (95% CI 1.317-4.153; P = 0.004)] emerged to be the only significant predictors of OS under treatment with FTD/TPI + bevacizumab. We further found a trend for significance for gender (F versus M) [HR 1.650 (95% CI 1-2.721; P = 0.05)], systemic treatment after FTD/TPI + bevacizumab (yes versus no) [HR 0.601 (95% CI 80.356-1.013; P = 0.056)] and time of metastatic disease to initiation of FTD/TPI + bevacizumab (≥18 months versus <18 months) [HR 0.613 (95% CI 0.353-1.063; P = 0.081)] (Figure 3).
Figure 3.
Multivariate Cox regression model including, besides (K)RAS mutational subgroups, 16 covariates. No predictive value for KRAS WT, KRAS G12 or KRAS G13 could be detected. 5-FU, 5-fluorouracil; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FTD, trifluridine; MT, mutant; TPI, tipiracil; WT, wild type.
RAS mutational status was not prognostic in terms of benefit for FTD/TPI + bevacizumab in this model. HR for KRAS G13 (yes versus no) was 2.12 (95% CI 0.658-6.850; P = 0.209), HR for KRAS WT (yes versus no) was 1.05 (95% CI 0.491-2.259; P = 0.894), and HR for KRAS G12 (yes versus no) was 1.171 (95% CI 0.502-2.733; P = 0.716) (Figure 3).
Discussion
This study holds significant relevance for physicians in routine clinical practice for several reasons: (i) it represents the largest real-world dataset of patients treated with FTD/TPI + bevacizumab in routine practice in the Western world,12, 13, 14, 15 and (ii) it places a particular focus on distinct molecular subgroups.
Earlier this year, discussions emerged in the gastrointestinal cancer scientific community following a retrospective analysis of FTD/TPI in real-world cohorts.9 These analyses, alongside the retrospective analysis of the RECOURSE prospective third-line trial, suggested that KRAS G12–altered mCRC may not derive benefit from FTD/TPI treatment. This proposition holds substantial implications for clinical decision making in routine practice, as KRAS G12–altered mCRC is the most prevalent alteration in mCRC. This scenario would potentially necessitate retesting through liquid biopsy or repeated tumor biopsy before the application of FTD/TPI. It remains so far uncertain whether KRAS G12 cases would be shifted towards multikinase inhibitors rather than chemotherapeutic approaches with standard third-line treatments.
The SUNLIGHT trial, however, has established a new standard of care for later lines in mCRC. In this prospective phase III trial, the addition of a VEGF inhibitor, bevacizumab, to FTD/TPI demonstrated a 3.3-month OS advantage compared to FTD/TPI alone.10
In our investigation, OS was observed to be decreased by over 1 month (9.37 months versus 10.8 months) in comparison to the results from the SUNLIGHT study. This variation can be predominantly attributed to our cohort including 12.2% of subjects with an ECOG performance status of 2, as well as a notably older cohort, with 49.6% being 65 years or older, in contrast to 40.7% in the SUNLIGHT study. Moreover, within our cohort, 26% of subjects were administered FTD/TPI in conjunction with bevacizumab as a fourth-line treatment or subsequent lines of therapy. Despite these variances, our findings resonate with other real-world cohorts.12
The SUNLIGHT trial did not detect a significant difference in the efficacy of FTD/TPI + bevacizumab between RAS WT and the overall RAS MT populations, findings corroborated by a smaller phase II trial in an Asian population.16 Our data align with these previous results.
Our trial notably focused on the KRAS G12–mutated subgroup, which comprised 41.5% of the study population, consistent with other previously published populations. Our analysis, compared KRAS G12–altered mCRC to three different groups. We detected no significant difference in terms of response rates, PFS or OS compared to the overall population (RAS WT + no KRAS G12 alterations), the RAS-mutated population or the KRAS exon 2–altered group. These findings suggest that FTD/TPI + bevacizumab maintains efficacy regardless of the molecular subgroup. Importantly, this could indicate that addition of bevacizumab to FTD/TPI may overcome the limitation of FTD/TPI on the KRAS G12 subgroup. Basic science results suggest that KRAS mutations may render tumors particularly sensitive to VEGF inhibition, offering a potential explanation for our observations.17, 18, 19
Limitations
We recognize the limitations inherent in providing a retrospective analysis. Specifically, our trial did not exclusively represent a true third-line population, as characterized in the SUNLIGHT trial. Moreover, the subgroup of no KRAS G12–mutated mCRC in our study was too small to draw definitive conclusions regarding the differential efficacy on rare and ultra-rare (K)RAS variants. Furthermore, we actually lack mechanistic support from cell lines or organoid models.
Conclusion
Our retrospective analysis of real-world data on FTD/TPI + bevacizumab in mCRC contributes to the ongoing debate concerning the need to consider RAS mutational status, particularly the most prevalent KRAS G12 mutation, when applying FTD/TPI-based regimens in later lines of mCRC therapy. Our results suggest that the new standard of care in third-line treatment with FTD/TPI + bevacizumab, as defined by the SUNLIGHT trial, is not contingent on RAS mutational status, and that KRAS G12C in particular is as sensitive to this treatment as the KRAS WT population or other (K)RAS mutational subgroups.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Xie Y.-H., Chen Y.-X., Fang J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 3.Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 4.Nicolantonio F.D., Vitiello P.P., Marsoni S., et al. Precision oncology in metastatic colorectal cancer—from biology to medicine. Nat Rev Clin Oncol. 2021;18:506–525. doi: 10.1038/s41571-021-00495-z. [DOI] [PubMed] [Google Scholar]
- 5.Doleschal B., Petzer A., Rumpold H. Current concepts of anti-EGFR targeting in metastatic colorectal cancer. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1048166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer R.J., Cutsem E.V., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima M., Suzuki N., Emura T., et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol. 2000;59:1227–1236. doi: 10.1016/s0006-2952(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 8.Emura T., Suzuki N., Yamaguchi M., Ohshimo H., Fukushima M. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol. 2004;25:571–578. [PubMed] [Google Scholar]
- 9.Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer. Nat Med. 2023;29:605–614. doi: 10.1038/s41591-023-02240-8. Haar J van de, Ma X, Ooft SN, et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prager G.W., Taieb J., Fakih M., et al. Trifluridine–tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388:1657–1667. doi: 10.1056/NEJMoa2214963. [DOI] [PubMed] [Google Scholar]
- 11.van de Haar J., Valeri N., Voest E.E. Third-line therapy in metastatic colorectal cancer. N Engl J Med. 2023;389:190–191. doi: 10.1056/NEJMc2306486. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Lago N., Chucla T.C., Castro B.A.D., et al. Efficacy, safety and prognostic factors in patients with refractory metastatic colorectal cancer treated with trifluridine/tipiracil plus bevacizumab in a real-world setting. Sci Rep. 2022;12 doi: 10.1038/s41598-022-18871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang C.S., Low Q.J., Ho C.L., et al. Real-world experience on the efficacy and tolerability of biweekly trifluridine/tipiracil with or without bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2022;40:3584. [Google Scholar]
- 14.Kotani D., Kuboki Y., Horasawa S., et al. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer. 2019;19:1253. doi: 10.1186/s12885-019-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nevala-Plagemann C.D., Ying J., Sama S., Florou V., Haaland B., Garrido-Laguna I. A real-world comparison of trifluridine/tipiracil and regorafenib in refractory metastatic colorectal cancer in the United States. J Clin Oncol. 2022;40:39. doi: 10.6004/jnccn.2022.7082. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T., Yamazaki K., Oki E., et al. Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meadows K.N., Bryant P., Pumiglia K. Vascular endothelial growth factor induction of the angiogenic phenotype requires ras activation. J Biol Chem. 2001;276:49289–49298. doi: 10.1074/jbc.M108069200. [DOI] [PubMed] [Google Scholar]
- 18.Stintzing S., Miller-Phillips L., Modest D.P., et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50–60. doi: 10.1016/j.ejca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz H.I., Yi J., Ince W., Novotny W.F., Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]