Abstract
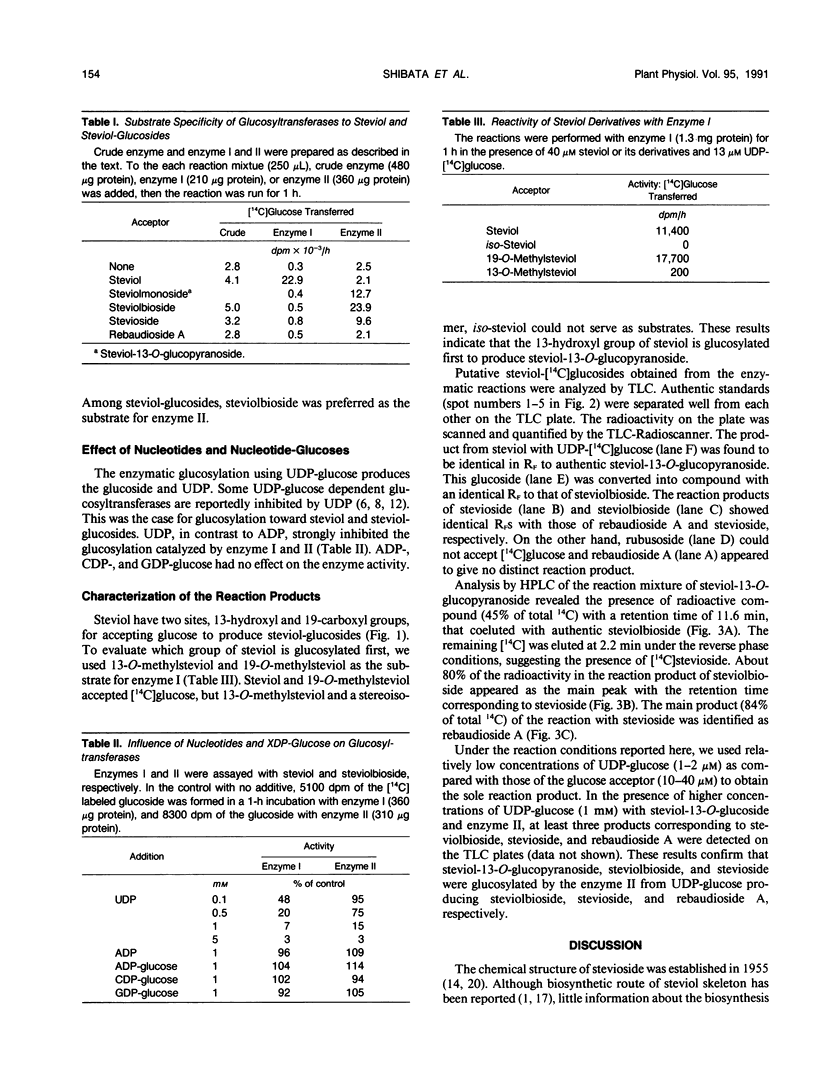
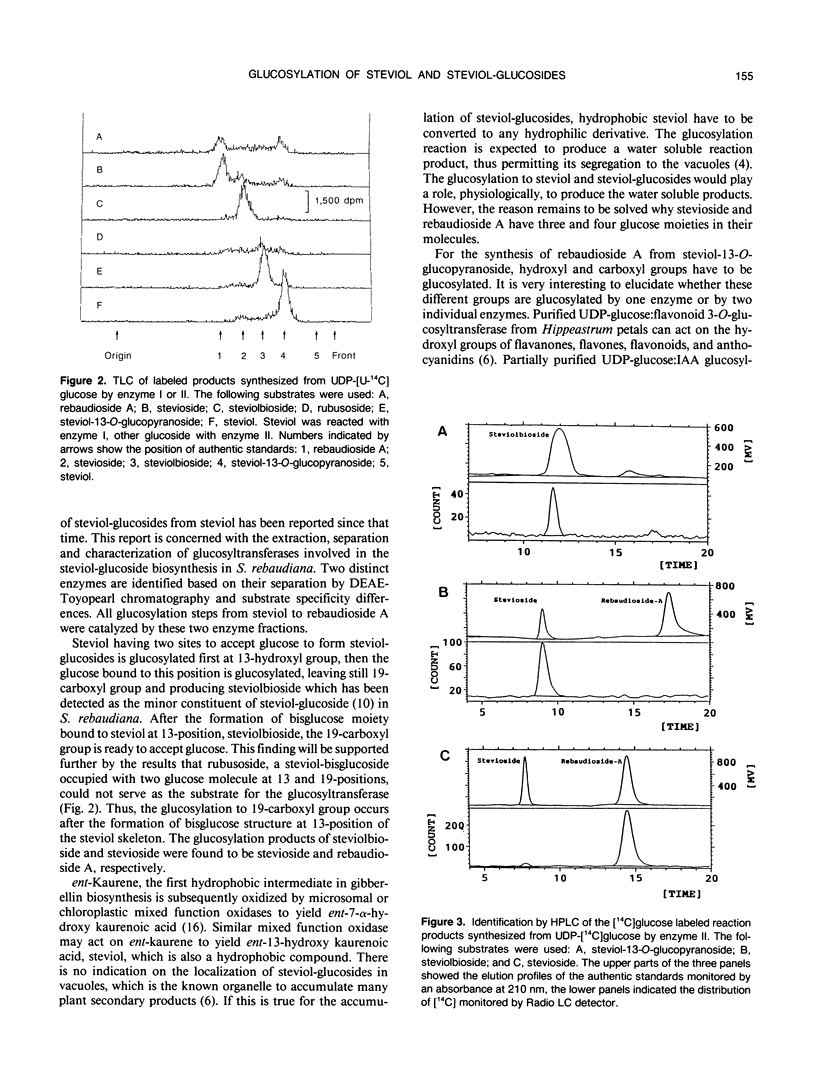
To evaluate and characterize stevioside biosynthetic pathway in Stevia rebaudiana Bertoni cv Houten, two enzyme fractions that catalyze glucosylation of steviol (ent-13-hydroxy kaur-16-en-19-oic acid) and steviol-glucosides (steviol-13-O-glucopyranoside, steviolbioside and stevioside), utilizing UDP-glucose as the glucose donor, were prepared from the soluble extracts of S. rebaudiana leaves. Enzyme fraction I, passed through DEAE-Toyopearl equilibrated with 50 millimolar K-phosphate pH 7.5, catalyzed the glucosylation to steviol and 19-O-methylsteviol, but not to iso-steviol and 13-O-methylsteviol, indicating that 13-hydroxyl group of the steviol skeleton is glucosylated first from UDP-glucose to produce steviol-13-O-glucopyranoside. Enzyme fraction II, eluted from the DEAE-Toyopearl column with 0.15 molar KCI, catalyzed the glucose transfer from UDP-glucose to steviol-13-O-glucopyranoside, steviolbioside and stevioside, but not to rubusoside (13, 19-di-O-glucopyranoside) and rebaudioside A. The reaction products glucosylated from steviol-13-O-glucopyranoside, steviolbioside and stevioside were identified to be steviolbioside, stevioside and rebaudioside A, respectively. These results indicate that in the steviol-glucoside biosynthetic pathway, steviol-13-O-glucopyranoside produced from the steviol glucosylation is successively glucosylated to steviolbioside, then to stevioside producing rebaudioside A.
Full text
PDF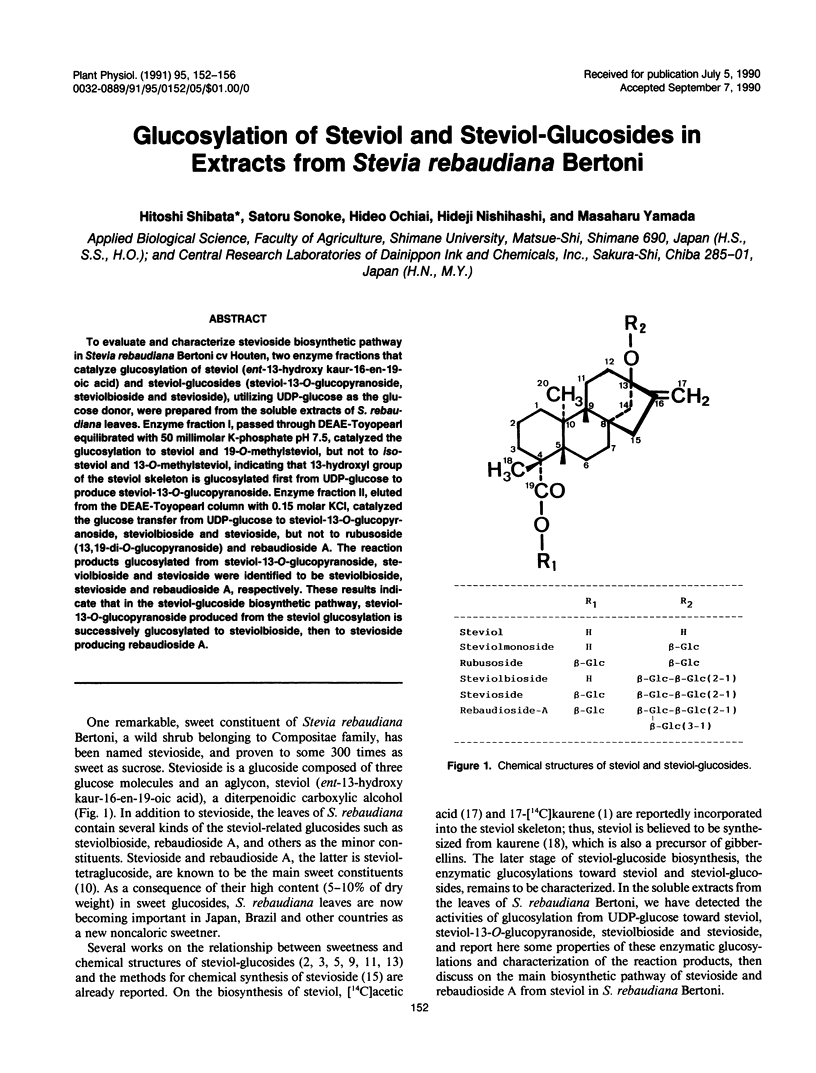


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DuBois G. E., Stephenson R. A. Diterpenoid sweeteners. Synthesis and sensory evaluation of stevioside analogues with improved organoleptic properties. J Med Chem. 1985 Jan;28(1):93–98. doi: 10.1021/jm00379a017. [DOI] [PubMed] [Google Scholar]
- Imanari T., Tamura Z. Gas chromatography of glucuronides. Chem Pharm Bull (Tokyo) 1967 Nov;15(11):1677–1681. doi: 10.1248/cpb.15.1677. [DOI] [PubMed] [Google Scholar]
- Leznicki A. J., Bandurski R. S. Enzymic synthesis of indole-3-acetyl-1-O-beta-d-glucose. II. Metabolic characteristics of the enzyme. Plant Physiol. 1988;88:1481–1485. doi: 10.1104/pp.88.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddat M., Heftmann E., Lang A. Biosynthesis of steviol. Arch Biochem Biophys. 1965 Jun;110(3):496–499. doi: 10.1016/0003-9861(65)90441-8. [DOI] [PubMed] [Google Scholar]
- Tamura Z., Imanari T. Gas chromatography of O-glucuronides. Chem Pharm Bull (Tokyo) 1964 Nov;12(11):1386–1388. doi: 10.1248/cpb.12.1386. [DOI] [PubMed] [Google Scholar]


