Abstract
Objective
To investigate the early and long-term outcomes of the deferred Norwood procedure by bilateral pulmonary artery banding (BPAB) versus the neonatal Norwood procedure.
Methods
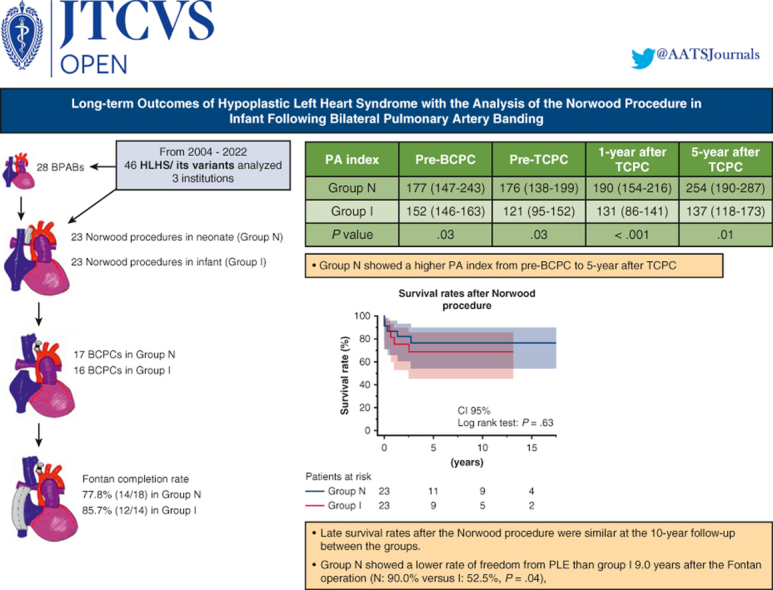
This retrospective study examined 46 patients with hypoplastic left heart syndrome and its variants undergoing the Norwood procedure for single ventricle physiology between 2004 and 2022 at 3 institutions. The patients were divided into 2 groups: neonatal Norwood procedure (group N; n = 23) and staged Norwood procedure in infants following BPAB (group I; n = 23). Preoperative risk factors, surgical results, survival rates, Fontan candidacy, and long-term complications were compared.
Results
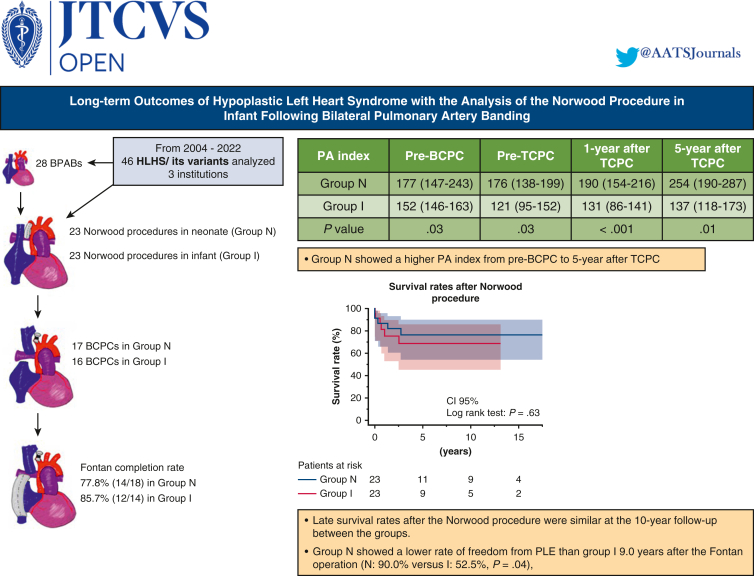
Early survival rates after the Norwood procedure were 91.3% (21 of 23) in both groups. Late survival rates after the Norwood procedure were similar at the 10-year follow-up (group N, 76.3%; group I, 68.7%; P = .63). Fontan completion rates also were comparable in the 2 groups (group N, 77.8%; group I, 85.7%; P = .67). Group N showed a higher median pulmonary artery (PA) index before bidirectional cavopulmonary connection (group N, 177 [interquartile range (IQR), 147-243] mm2/m2; group I, 152 [IQR, 146-163] mm2/m2; P = .03); this trend continued until 5 years after Fontan completion (P = .01). Group N also had a lower rate of freedom from protein-losing enteropathy (PLE) at 9.0 years after the Fontan operation (90.0% vs 52.5% for group I; P = .04), although the incidences of other Fontan-associated events were not significantly different.
Conclusions
Fontan candidacy and survival rates were similar regardless of the timing of the Norwood procedure. Early performance of the Norwood procedure may lead to lower rates of late Fontan-associated events, such as PLE.
Key Words: bilateral pulmonary artery banding, complications associated with Fontan circulation, hypoplastic left heart syndrome, Norwood procedure
Graphical Abstract
Late survival rates after the Norwood procedure were similar in the groups.
Central Message.
Delaying the Norwood procedure after bilateral pulmonary artery banding could lead to a higher risk of late Fontan-associated adverse events, including protein-losing enteropathy.
Perspective.
A prolonged duration of bilateral pulmonary artery band placement before the Norwood procedure for hypoplastic left heart syndrome has a detrimental effect on pulmonary artery growth and the incidence of long-term Fontan-associated adverse events in Norwood survivors.
Bilateral pulmonary artery (PA) banding (BPAB) has the advantage of delaying the Norwood procedure for hypoplastic left heart syndrome (HLHS) and its variants, with some centers selecting a staged approach using BPAB as the first palliation regardless of risk factors.1 The advantages of the staged Norwood procedure following BPAB include avoidance of cardiopulmonary bypass (CPB) in early neonates, which influences multiple immature organs;2 stabilized hemodynamics; and allowance for body growth in patients with risk factors for the Norwood procedure, such as prematurity and low birth weight.3, 4, 5 On the other hand, disadvantages of the staged Norwood procedure include potential bleeding due to surgical adhesion, prolonged CPB, decreased PA growth, and risks for inadequate cerebral and coronary flow maintained with prostaglandin E1 in patients with aortic atresia.2,6 In contrast, the primary Norwood procedure offers certain advantages, including nonsurgical adhesion and potential PA growth with adjusted pulmonary blood flow; however, this procedure has been associated with high rates of low cardiac output syndrome and the need for extracorporeal membrane oxygenation (ECMO).7,8
The advantages and disadvantages of the rapid 2-stage Norwood procedure are the same as those for the primary Norwood procedure, except for the presence of slight adhesions. The comprehensive stage II procedure offers some advantages, including the absence of shunt-related complications and lower rates of the 6 major Society of Thoracic Surgeons Congenital Heart Surgery Database postoperative complications; however, it also has been associated with some disadvantages, including a greater prevalence of ECMO compared with bidirectional cavopulmonary connection.8
As mentioned earlier, despite the reported advantages of BPAB under certain clinical conditions, the long-term effects of long-duration band placement on PA growth and Fontan circulation remain unclear.1,9 In this study, we analyzed and compared surgical outcomes between infants who underwent the deferred Norwood procedure by BPAB and neonates who underwent the Norwood procedure.
Methods
Ethical Statement
This retrospective multi-institutional study was approved by the Institutional Review Board of Kitasato University Hospital (approval B22-061; October 24, 2022). Informed consent for publication of the study data was obtained using a form of the opt-out approach on the websites approved by the Institutional Review Board.
Patient Selection
The study cohort comprised 46 patients who underwent the modified Norwood procedure for HLHS or a variant with single ventricular physiology at 3 institutions—Kitasato University Hospital, Gunma Children's Medical Center, and Jichi Children's Medical Center, Japan—between 2004 and 2022 were included. No patients in this cohort underwent the comprehensive stage II procedure. Patients undergoing the Norwood procedure followed by biventricular repair were excluded.
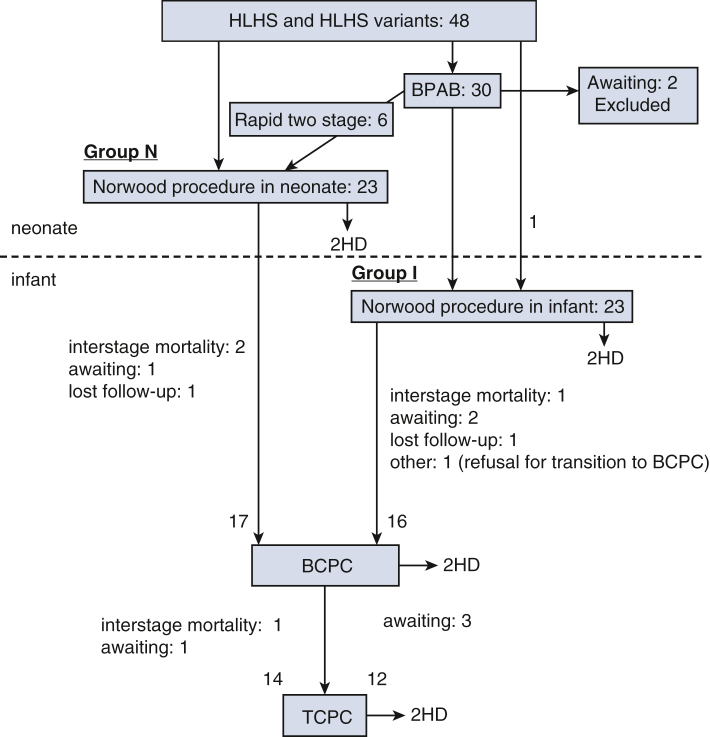
The patients were divided into 2 groups based on the timing of the Norwood procedure: Norwood procedure in neonates (group N; n = 23), including six rapid 2-stage Norwood procedures, and staged Norwood procedure in infants after BPAB (group I; n = 23) (Figure 1). Preoperative risk factors are listed in Table 1. Surgical results for each stage, Fontan candidacy, and long-term complications were compared between the 2 groups.
Figure 1.
Flowchart of surgical pathways and outcomes in this cohort. HLHS, Hypoplastic left heart syndrome; BCPC, bidirectional cavopulmonary connection; HD, hospital death; BPAB, bilateral pulmonary artery banding; TCPC, total cavopulmonary connection.
Table 1.
Patient demographics
| Variable | Group N (N = 23) | Group I (N = 23) | P value |
|---|---|---|---|
| Male sex, n (%) | 10 (43.5) | 14 (60.9) | .38 |
| Birth weight, kg, median (IQR) | 2.95 (2.33-3.25) | 2.81 (2.49-3.26) | .69 |
| Prematurity (<37 wk), n (%) | 0 (0) | 3 (13.0) | .23 |
| Genetic syndrome, n (%) | 3 (13.0) | 3 (13.0) | .99 |
| Heterotaxia, n (%) | 3 (13.0) | 1 (4.3) | .35 |
| HLHS, n (%) | 13 (56.5) | 12 (52.2) | .99 |
| AA, MA, n/N (%) | 4/13 (30.8) | 4/12 (33.3) | |
| AA, MS, n/N (%) | 1/13 (7.7) | 4/12 (33.3) | |
| AS, MS, n/N (%) | 7/13 (53.8) | 4/12 (33.3) | |
| AS, MA, n/N (%) | 1/13 (7.7) | 0/12 (0) | |
| HLHS variant, n (%) | 10 (43.5) | 11 (47.8) | .99 |
| CoA/IAA, n/N (%) | 5/10 (50.0) | 4/11 (36.3) | |
| Unbalanced AVSD, n/N (%) | 1/10 (10.0) | 4/11 (36.4) | |
| TGA, n/N (%) | 2/10 (20.0) | 2/11 (18.2) | |
| Shone's complex, n/N (%) | 2/10 (20.0) | 0/11 (0) | |
| DILV, n/N (%) | 0/10 (0) | 1/11 (9.1) | |
| AVVR ≥ moderate, n (%) | 1 (4.3) | 5 (21.7) | .19 |
| Ascending aorta <2 mm, n (%) | 4 (17.4) | 3 (13.0) | .99 |
| Common atrioventricular valve, n/N (%) | 2/23 (8.7) | 3/23 (13.0) | .99 |
| Restrictive atrial septal defect, n (%) | 3 (13.0) | 5 (21.7) | .70 |
| TAPVC, n (%) | 0 (0) | 0 (0) | - |
| Extracardiac anomaly, n (%) | 2 (8.7) | 2 (8.7) | .99 |
HLHS, Hypoplastic left heart syndrome; AA, aortic atresia; AS, aortic stenosis; MA, mitral atresia; MS, mitral stenosis; CoA/IAA, coarctation/interruption of the aorta; AVSD, atrioventricular septal defect; TGA, transposition of the great arteries; DILV, double-inlet left ventricle; AVVR, atrioventricular valve regurgitation; TAPVC, total anomalous pulmonary venous connection.
Norwood Procedures and Timing
The timing of the Norwood procedure was based on the surgeon’s preference. After BPAB, Norwood procedures were basically planned in patients who had a body weight (BW) >4.0 kg, were 3 months post-BPAB, and were hemodynamically stable. No patients in this cohort received ductal stenting, and all patients with BPAB received a continuous intravenous infusion of prostaglandin (PG) E1 to maintain the patency of the ductus from birth to the Norwood procedure.
Group N consisted of 22 neonatal Norwood cases at Kitasato University Hospital, including 6 rapid 2-stage cases and 1 case at Gunma Children's Medical Center. For the rapid 2-stage cases, BPAB was performed at 1 to 6 days of age due to prematurity in 2 patients, low birth weight in 2 patients, preshock status in 1 patient, and low birth weight with suspected chromosome anomaly in 1 patient. Group I included 20 infant Norwood cases at Gunma Children's Medical Center, 2 cases at Kitasato University Hospital, and 1 case at Jichi Children's Medical Center. During the observation period, 4 attending surgeons at the 3 institutions performed the Norwood, BCPC, and Fontan procedures (extracardiac total cavopulmonary connection [TCPC] with a fenestration). These 4 surgeons had received the same surgical training and operated similarly. Our institutional group policy stipulates that 1 of the 4 attending surgeons should visit other hospitals to assist with surgery whenever Norwood procedures are performed to maintain the operative quality for HLHS and its variants. In group N, surgeon A performed 17 primary Norwood procedures and 5 rapid 2-stage Norwood procedures, whereas surgeon B performed 1 rapid 2-stage Norwood procedure. In group I, surgeons C, B, and D performed 14, 7, and 2 staged infantile Norwood procedures, respectively.
Operative Characteristics and Management
Group I patients underwent BPAB at the neonatal stage. Two 2-mm-wide PA bands were created from a 3.5-mm Gore-Tex tube (W.L. Gore & Associates). For neonates weighing approximately 3 kg, through a median sternotomy, each PA was banded with a circular length of 10 mm adjusted with surgical clips on the band to obtain adequate circulation, which was confirmed by transesophageal echocardiography and arterial oxygen saturation.
In the Norwood procedure, a 3.5-mm Gore-Tex tube was anastomosed to the right innominate artery and connected to an arterial cannula after dissection around the heart and vessels. Once CPB was initiated, the patient was cooled to moderate hypothermia (28-30°C). The main PA was transected at the level of the PA bifurcation. For all patients with BPAB, branched PAs were debanded and dilated with a Hegar dilator inserted from each orifice of both PAs. After cardiac arrest, the ascending aorta was incised longitudinally to the level of the aortic sinus, and the ascending aorta was anastomosed side-to-side to the transected main PA for coronary circulation. The aortic arch was repaired under high-flow regional cerebral perfusion as described previously.10 After the descending aorta was clamped under high-flow regional cerebral perfusion, ductal tissues were removed, and the aortic arch was created with the descending aorta. The main PA was anastomosed directly to the aortic arch without any foreign patches. For the blood source of pulmonary circulation, the right ventricular PA conduit or modified Blalock–Taussig–Thomas (BTT) shunt was created.
BCPC was performed using CPB. Branch PA plasty was required in 3 patients, and a tricuspid valvuloplasty was needed in 1 patient. Extracardiac TCPC was performed with a fenestrated Gore-Tex graft when the BW exceeded 9 to 10 kg. To assess Fontan circulation, a follow-up catheter examination was conducted at 1, 5, and 10 years after TCPC. Data for representative events associated with Fontan circulation were collected retrospectively from patient notes in the institutional chart system.11 Arrhythmias, such as atrial fibrillation atrial flutter, sinus node dysfunction with bradycardia, paroxysmal supraventricular tachycardia, ventricular tachycardia, and ventricular fibrillation, after TCPC were collected if confirmed on electrocardiography or Holter electrocardiography. Protein-losing enteropathy (PLE) was diagnosed by increased levels of alpha-1 antitrypsin in a single stool collection with evidence of edema and hypoalbuminemia or increased alpha-1 antitrypsin clearance in 24-hour stool collection without other comprehensible causes of symptoms.11 Fontan-associated liver disease (FALD) was defined by hepatic abnormalities, ie, heterogeneous lobular parenchyma, segmental atrophy, or hypertrophy, hepatic vein dilatation, and abnormal hepatic vein architectures on ultrasound or magnetic resonance imaging,12 or elevations of ≥2 values of the aspartate aminotransferase, alanine aminotransferase, total bilirubin, or gamma-guanosine triphosphate in 2 consecutive blood tests according to the criteria for FALD described by Oka and colleagues.13
Statistical Analysis
Continuous variables were expressed as mean ± SD for normally distributed values and as median and interquartile range (IQR; 25th and 75th percentiles) for non-normally distributed values. Categorical variables were expressed as numbers and percentages. The Mann–Whitney U test was used to compare the groups for nonparametric data. Statistical analysis was performed using JMP Pro version 16 (SAS institute). Differences with P < .05 were considered statistically significant.
Results
Preoperative and Perioperative Demographics of the Norwood Procedure
There were no significant differences in preoperative risk factors, including prematurity (<37 weeks), genetic syndrome, heterotaxia, presence of ≥moderate atrioventricular valve regurgitation, ascending aorta diameter <2 mm, and a common atrioventricular valve, between groups N and I (Table 1).1,14,15 Thirty patients underwent BPAB at a median age of 6 days (IQR, 4-7 days) and had a median BW of 2.8 kg (IQR, 2.5-3.2 kg). Six of these patients proceeded to the neonatal Norwood procedure, which was defined as a rapid 2-stage procedure. During the interstage between BPAB and the Norwood procedure, 6 of the 28 patients (21.4%) developed peripherally inserted central catheter infection or fever, and 1 patient (3.6%) developed ostial thickening due to prolonged administration of PGE1. Moreover, 4 patients (14.3%) had ductus closing requiring an increase in PGE1 dose to >10 ng/kg/minute, whereas patents whose ductus remained stable received continuous infusion of PGE1 ranging from 2 to 5 ng/kg/minute. Respiratory depression was observed in 3 patients (10.7%) who required tracheal intubation and management in the pediatric intensive care unit. The other 25 patients (89.3%) were managed in the pediatric ward with a continuous PGE1 infusion until the Norwood procedure. Catheter balloon atrioseptectomy before the staged Norwood procedure was required in 5 patients (17.9%) due to restrictive atrial septum. Nutritional management was performed via a nasogastric tube or oral intake with milk administration at 130 to 150 mL/kg, except for 1 patient who experienced necrotizing enterocolitis at 1.5 months before the Norwood procedure and required intravenous hyperalimentation due to fasting management until the staged Norwood procedure. Despite nutritional management, weight gain was generally poor, with a daily BW increase of 11.7 g/day (IQR, 1.3-17.5 g/day) after the BPAB. Patients with desaturation or narrow pulmonary arteries on echocardiography were treated aggressively with catheter balloon dilation if they failed to reach the target weight for the Norwood procedure. Moreover, 6 patients (21.4%) received bilateral balloon dilatation of the PA, 3 patients (10.7%) underwent catheter balloon right PA dilation, and 1 patient (3.6%) underwent catheter left PA dilatation, whereas 1 patient (3.6%) required extensive left PA augmentation during the Norwood procedure.
In this cohort, 46 patients underwent the Norwood procedure (Figure 1). As shown in Table 2, the median age and BW at the time of the Norwood procedure were 8 (IQR: 3-16) days and 2.9 (IQR: 2.6-3.2) kg in group N and 112 (IQR: 78-144) days and 4.4 (IQR: 3.8-5.0) kg in group I (both P < .001), respectively. In group I, 22 patients underwent the Norwood procedure at a median interval from BPAB of 93 (IQR: 47-137) days. The operation time (393 [IQR: 318-438] vs 554 [IQR: 483-619] min) and cardiopulmonary bypass time (229 [IQR: 171-263] vs 332 [IQR: 273-397] min) were longer in group I than in group N (both P < .0001), possibly reflecting additional dissection time of adhesion around the heart post-BPAB (Table E1). On the contrary, the aortic cross-clamp time, lower body circulatory arrest time, use of postoperative ECMO, and use of BTT shunt as a pulmonary blood source showed no significant difference between the two groups. The 30-day survival rates after the Norwood procedure were 91.3% (21/23) in both groups. In all 4 patients, the cause of early death was the failure of separation from the CPB due to low cardiac output and desaturation. Four patients in group N did not undergo BCPC, including 2 with interstage mortality, 1 who was on the BCPC waiting list, and 1 who was lost to follow-up. Of the 5 patients in group I who had not undergone BCPC, 1 had interstage mortality, 2 were on the BCPC waiting list, and 1 was lost to follow-up, and in 1 patient the family refused to proceed with BCPC.
Table 2.
Operative data for the Norwood procedure, BCPC, and TCPC
| Variable | Group N (N = 23) | Group I (N = 23) | P value |
|---|---|---|---|
| Age at Norwood procedure, d, median (IQR) | 8 (3-16) | 112 (78-144) | <.0001 |
| Weight at Norwood procedure, kg, median (IQR) | 2.9 (2.55-3.17) | 4.4 (3.83-5.00) | <.0001 |
| Rapid 2-stage Norwood procedure, n/N (%) | 6/23 (26) | − | − |
| Interval between BPAB and Norwood procedure, d, median (IQR) | 14 (6-24)∗ | 97 (86-141) | − |
| Operative time, min, median (IQR) | 393 (318-438) | 554 (483-619) | <.0001 |
| Cardiopulmonary bypass time, min, median (IQR) | 229 (171-263) | 332 (273-397) | <.0001 |
| Aortic cross-clamp time, min, median (IQR) | 83 (71-108) | 87 (70-105) | .74 |
| Lower body circulatory arrest time, min, median (IQR) | 72 (61-79) | 78 (66-101) | .23 |
| Blalock–Taussig–Thomas shunt, n/N (%) | 5/23 (22) | 7/23 (30) | .74 |
| 3.5 mm | 3 | – | |
| 4.0 mm | 2 | 6 | |
| 5.0 mm | – | 1 | |
| RV-PA shunt, n (%) | 18/23 (78) | 16/23 (70) | .74 |
| 5.0 mm | 9 | 1 | |
| 6.0 mm | 9 | 7 | |
| 7.0 mm | — | 3 | |
| 8.0 mm | — | 5 | |
| Postoperative ECMO support, n/N (%) | 4/23 (17) | 6/23 (26) | .72 |
| Survival rate of the Norwood procedure, n/N (%) | 21/23 (91.3) | 21/23 (91.3) | 1.00 |
| Interstage mortality before BCPC, n/N (%) | 2/20 (10) | 1/17 (5.9) | .99 |
| Age at BCPC, mo, median (IQR) | 4.6 (3.7-7.2) | 9.4 (7.0-15.7) | .01 |
| Body weight at BCPC, kg, median (IQR) | 4.9 (4.1-7.2) | 5.8 (4.7-7.4) | .31 |
| PA augmentation, n/N (%) | 5/17 (29.4) | 6/16 (37.5) | .72 |
| Survival rate of BCPC, n/N (%) | 16/17 (94.1) | 15/16 (93.8) | .99 |
| Interstage mortality before TCPC, n/N (%) | 1/16 (6.3) | 0/12 (0) | .99 |
| Age at TCPC, y, median (IQR) | 1.5 (1.1-2.1) | 3.3 (2.1-4.3) | .004 |
| Body weight at TCPC, kg, median (IQR) | 9.0 (7.7-10.8) | 10.8 (9.7-12.3) | .14 |
| Fontan completion rate, n/N (%) | 14/18 (77.8) | 12/14 (85.7) | .67 |
| PA augmentation, n/N (%) | 3/14 (21.4) | 2/12 (16.7) | .99 |
| Survival rate of TCPC, n/N (%) | 13/14 (92.9) | 11/12 (91.7) | .99 |
IQR, Interquartile range; BPAB, bilateral pulmonary artery banding; RV, right ventricle; PA, pulmonary artery; ECMO, extracorporeal membrane oxygenation; BCPC, bidirectional cavopulmonary connection; TCPC, total cavopulmonary connection.
Six cases.
Demographics of BCPC and the Fontan Procedure
Seventeen patients in group N and 16 patients in group I underwent BCPC. Two interstage deaths occurred between the Norwood procedure and BCPC in group N and 1 death occurred in group I, with no significant difference in mortality between the 2 groups. The patients in group N proceeded to BCPC with cardiac catheterization if they were age >3 months. In contrast, if the BW of patients in group I increased to >5 kg, the surgeons considered proceeding with BCPC. However, although the waiting period between the Norwood procedure and BCPC tended to be longer in group I (median, 175 [IQR, 126-326] days vs 129 [IQR, 112-189] days for group N; P = .29), the BW gain per month was significantly lower in group I (0.21 [IQR, 0.05-0.34] kg/month vs 0.40 [IQR, 0.23-0.46] kg/month for group N; P = .02), even though the 2 groups were receiving the same type of nutritional support using via feeding tube. These results might have influenced the later timing of BCPC in group I, as well as difference in the timing of the Norwood procedure. At BCPC, group I was significantly older than group N (median, 9.36 [IQR, 6.96-15.72] months vs 4.56 [IQR, 3.72-7.20] months; P = .01). This trend also was observed in the timing of the Fontan procedure (median, 1.5 [IQR, 1.1-2.1] years in group N vs 3.3 [IQR, 2.1-4.3] years in group I; P = .004). In contrast, BW at the time of BCPC was not significantly different in the 2 groups (4.9 [IQR, 4.1-7.2] kg in group N vs 5.8 [IQR, 4.7-7.4] kg in group I; P = .31). The mortality rates after BCPC were 5.9% (1 of 17 cases due to infection) in group N and 6.3% (1 of 16 cases due to pulmonary hemorrhage) in group I, showing no significant difference (P = .99).
The Fontan completion rates of Norwood survivors excluding awaiting cases did not differ between the 2 groups (77.8% (14 of 18) in group N vs 85.7% (12 of 14) in group I; P = .67). Two deaths occurred after TCPC, with 1 case in group N due to low output syndrome and 1 case in group I due to postoperative infection.
Catheter Data for Each Stage after the Norwood Procedure
The PA index (Nakata index) was larger in group N than in group I (median, 177 [IQR, 147-243] mm2/m2 vs 152 [IQR, 146-163] mm2/m2), with a P value of .03 pre-BCPC (Table 3). This trend continued from pre-BCPC to even 5 years after TCPC (Figure 2, Figure 3, Figure 4); however, throughout the observational period, PA pressures and resistance showed no significant difference between the 2 groups. The total number of additional catheter interventions to augment the PA after the Norwood procedure was higher in group I than in group N during the observational period (3 [IQR, 0-4] vs 0 [IQR, 0-2]; P = .02).
Table 3.
Catheterization data at each stage after the Norwood procedure
| Variables | Group N | Group I | P value |
|---|---|---|---|
| Pre-BCPC | N = 17 | N = 14 | |
| PA index, mm2/m2, median (IQR) | 177 (147-243) | 152 (146-163) | .03 |
| Rp, Wood units·m2, median (IQR) | 1.51 (0.98-2.25) | 1.90 (1.39-2.71) | .21 |
| SVEF, %, median (IQR) | 62 (55-70) | 59 (48-65) | .46 |
| SVEDP, mm Hg, median (IQR) | 8 (6-10) | 6 (5-10) | .42 |
| AVVR ≥ moderate, n (%) | 1 (5.9) | 2 (14.3) | .58 |
| Reduced VF, n (%) | 1 (5.9) | 2 (14.3) | .58 |
| Pre-TCPC | N = 14 | N = 12 | |
| PA index, mm2/m2, median (IQR) | 176 (138-199) | 121 (95-152) | .03 |
| Rp, Wood units·m2, median (IQR) | 1.30 (.73-1.73) | 1.42 (.95-2.17) | .34 |
| PA pressure, mmHg, median (IQR) | 12 (11-12) | 11 (9-14) | .88 |
| SVEF, %, median (IQR) | 56 (52-65) | 63 (45-65) | .73 |
| SVEDP, mm Hg, median (IQR) | 7 (6-9) | 7 (5-9) | .75 |
| AVVR ≥ moderate, n (%) | 0 (0) | 1 (8.3) | .46 |
| Reduced VF, n (%) | 1 (7.1) | 2 (16.7) | .58 |
| 1 y after TCPC | N = 12 | N = 8 | |
| PA index, mm2/m2, median (IQR) | 190 (154-216) | 131 (86-141) | <.001 |
| Rp, Wood units·m2, median (IQR) | 1.21 (.80-1.67) | 1.64 (.91-2.95) | .18 |
| PA pressure, mmHg, median (IQR) | 10 (9-12) | 10 (9-13) | .73 |
| SVEF, %, median (IQR) | 64 (60-76) | 55 (50-60) | .08 |
| SVEDP, mm Hg, median (IQR) | 7 (4-8) | 7 (4-8) | .97 |
| AVVR ≥ moderate, n (%) | 0 (0) | 0 (0) | 1.00 |
| Reduced VF, n (%) | 0 (0) | 2 (25.0) | .15 |
| 5 y after TCPC | N = 9 | N = 5 | |
| PA index, mm2/m2, median (IQR) | 254 (190-287) | 137 (118-173) | .01 |
| Rp, Wood units·m2, median (IQR) | 1.45 (.63-1.92) | 1.45 (1.13-1.82) | .88 |
| PA pressure, mmHg, median (IQR) | 9 (8-12) | 10 (9-12) | .46 |
| SVEF, %, median (IQR) | 52 (51-64) | 53 (48-58) | .35 |
| SVEDP, mm Hg, median (IQR) | 7 (6-8) | 7 (6-11) | .32 |
| AVVR ≥ moderate, n (%) | 0 (0) | 0 (0) | 1.00 |
| Reduced VF, n (%) | 0 (0) | 2 (40.0) | .11 |
BCPC, Bidirectional cavopulmonary connection; PA, pulmonary artery; Rp, pulmonary artery resistance; SVEF, ejection fraction of single ventricle; SVEDP, systemic ventricular end-diastolic pressure; AVVR, atrioventricular valve regurgitation; VF, ventricular function; TCPC, total cavopulmonary connection.
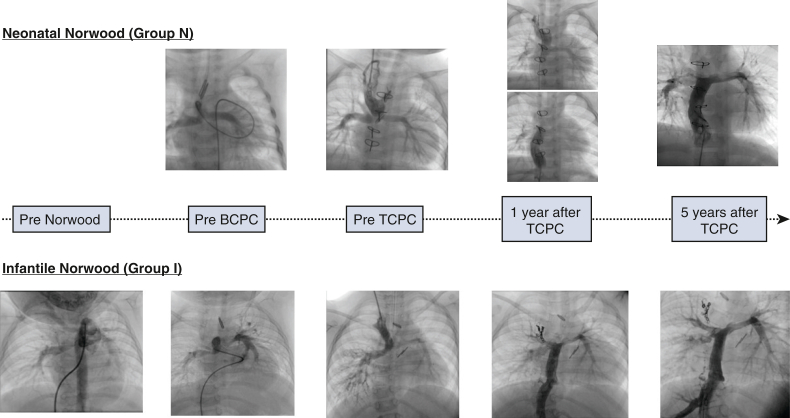
Figure 2.
Representative images of the pulmonary arteries on catheter angiography in the Group N and I. BCPC, Bidirectional cavopulmonary connection; TCPC, total cavopulmonary connection.
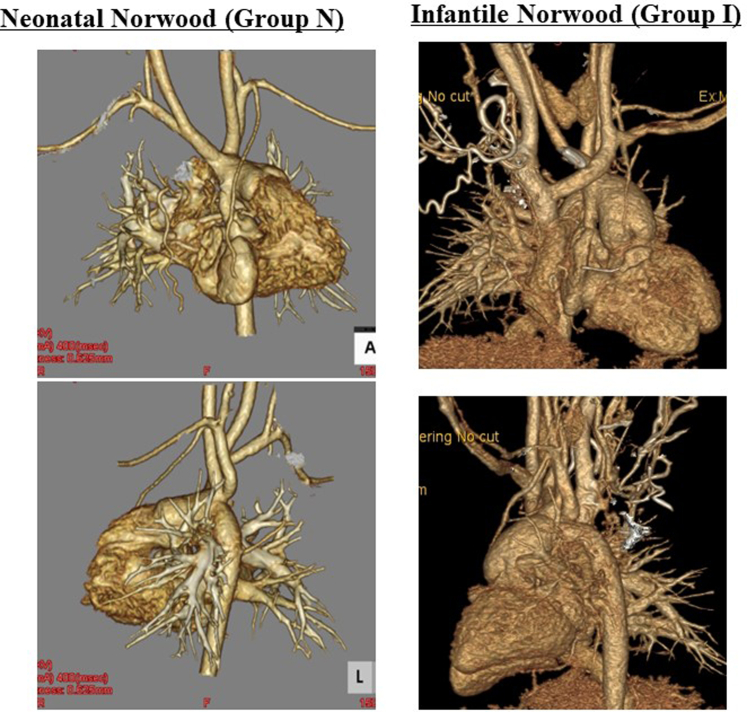
Figure 3.
Representative computed tomography images of the aortic arch anatomy 1 year after the Fontan procedure in the groups N and I.
Figure 4.
Summary of major findings. BPAB, Bilateral pulmonary artery banding; HLHS, hypoplastic left heart syndrome; BCPC, bidirectional cavopulmonary connection; TCPC, total cavopulmonary connection; PLE, protein-losing enteropathy.
Mortality and Morbidity Rates and Risk Factors for Postoperative Events Associated With the Fontan Procedure
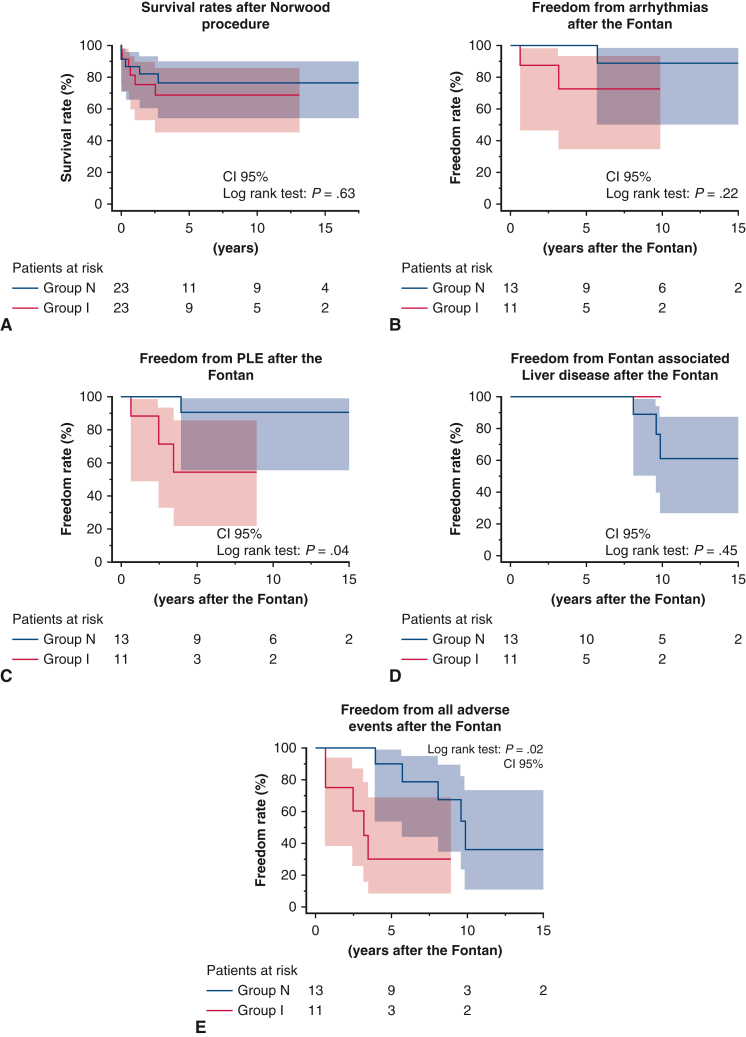
The median duration of follow-up after the Norwood procedure was 3.7 years (IQR, 2.1-12.7 years) in group N and 2.3 years (IQR, 0.6-6.6.2 years) in group I (P = .20). Figure 5, A shows Kaplan–Meier survival plots of outcomes after the Norwood procedure in the 2 groups. The survival rates were 76.3% at 3 to 10 years after the Norwood procedure in group N and 75.6% at 3 years and 68.7% at 10 years after the Norwood procedure in group I, with no significant difference between the 2 groups (P = .63) (Figure 4). We analyzed the rates of freedom from arrhythmias, PLE, and FALD to investigate the effect of the timing of the Norwood procedure on outcomes after Fontan completion. As shown in Figure 5, B, rates of freedom from arrhythmias and FALD after the Fontan procedure were comparable in the 2 groups, whereas the rate of freedom from PLE after Fontan were significantly lower in group I (52.5%) than in group N (90.0%) 5 years after Fontan completion (P = .04). Altogether, the number of all Fontan-associated events was higher in group I than in group N (P = .02) (Figure 5, E).
Figure 5.
Kaplan–Meier curves for survival and events after the Norwood and Fontan procedures. (A) Survival rates after the Norwood procedure. (B) Freedom from arrhythmias after the Fontan procedure. (C) Freedom from PLE after the Fontan procedure. (D) Freedom from Fontan-associated liver disease after the Fontan procedure. (E) Freedom from all Fontan-associated events after the Fontan procedure. CI, Confidence interval; PLE, protein-losing enteropathy.
Outcomes of Patients Who Underwent BPAB and the Primary Norwood Procedure
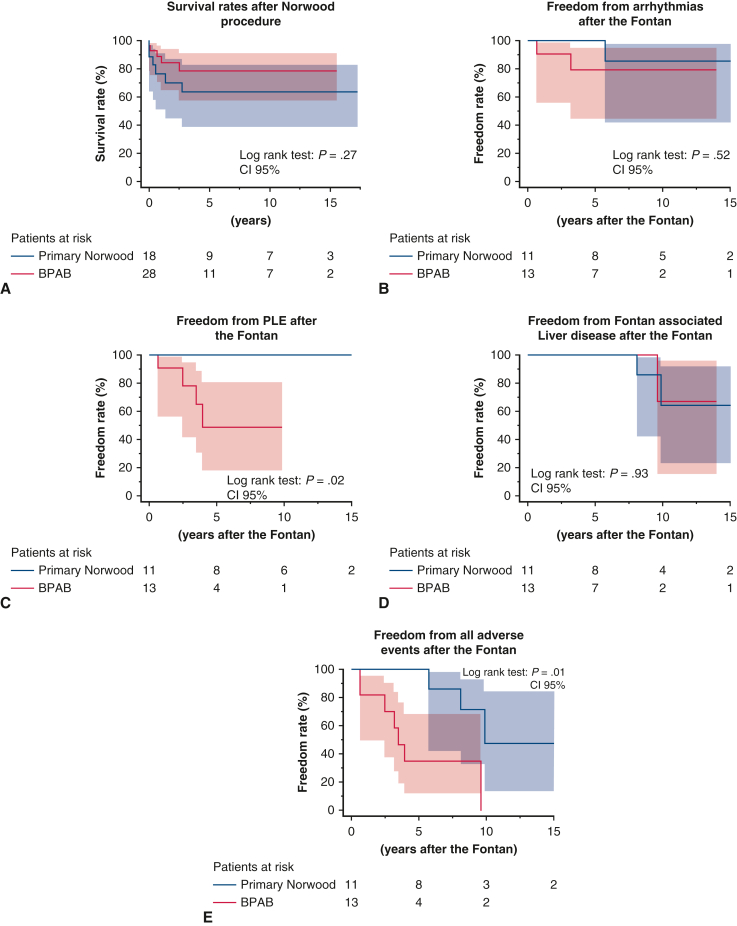
We conducted a subanalysis to compare early and late outcomes between patients who underwent BPAB, including the rapid 2-stage Norwood procedure, and those who underwent the primary Norwood procedure. Data are provided in Figure E1. The results of this subanalysis are close to those of our analysis of groups N and I.
Discussion
In this study, we analyzed surgical outcomes for HLHS and its variants by comparing patients who underwent a staged Norwood procedure in infancy via bilateral PA banding as the first surgical palliation regardless of risk factors and patients who underwent the neonatal Norwood procedure. In previously published data comparing staged and primary Norwood procedures, BPAB tended to be selected in high-risk cases, which could induce significant selection bias; therefore, long-term outcomes were not comparable between staged and primary Norwood, because such risk factors as prematurity and lower BW could influence long-term outcomes, such as Fontan-associated events.1,6,9,16 Furthermore, the rotation of attending surgeons to each institution as a first surgical assistant when performing the Norwood procedure resulted in a relatively homogeneous surgical quality and consequent equivalent outcomes in this multicenter retrospective cohort, which was the different point from the Japanese research using the national database system.1
Although no differences in early surgical outcomes, Fontan completion rates, and long-term survival rates were noted despite the different timing of the Norwood procedure, patients undergoing the Norwood procedure in infancy showed significantly reduced PA growth even 5 years after Fontan procedures. In addition, although no differences in the incidence of arrhythmia or FALD (representative postoperative complications after Fontan) were noted post-Fontan, the timing of the Norwood procedure might have influenced the incidence of PLE in this study.
As shown in data from the Japan Congenital Cardiovascular Surgery Database, many Japanese centers selected BPAB as the first palliative surgery regardless of preoperative risk factors to wait until infancy when the Norwood procedures with a BTT shunt or RV-PA shunt.1 Moreover, 80% of Japanese centers chose the staged Norwood strategy, with BPAB performed in >50% of patients with HLHS and its variants, and 62% of the centers performed BPAB in all cases irrespective of non-preoperative risk factors.1,17 One of the advantages of BPAB for HLHS and its variants is that centers with even a smaller number of HLHS cases than overseas might be able to provide steady and less complicated perioperative and postoperative management than neonatal Norwood.18 BPAB offers not only steady hemodynamics despite ductus dependency during the neonatal stage, but also the avoidance of CPB in neonates.15,19 However, one disadvantage of BPAB is that it requires a continuous infusion of PGE1 and long hospitalization until the Norwood procedure, as the patent ductus arteriosus stent is not mainstream in Japan.
Although the interstage death between BPAB and the Norwood procedure was not investigated in this study, a high rate of interstage death after BPAB (13.2%) cannot be ignored.1 In addition, infants who underwent the Norwood procedure had a lower PA index throughout the observation period, even 5 years after TCPC (Table 3). The long duration of banding may hinder proper PA growth, as reflected in the greater number of catheter interventions to enlarge PAs throughout the observation period even at 5 years post-Fontan.15,16,20 Furthermore, the duration of banding in our study, with a median of 97 days, was longer than those in other studies with BPAB (median, 97 days vs 56-70 days), suggesting that this might have affected longer-term complications after Fontan completion.6,15,16,21
PLE is one of the serious long-term complications of Fontan completion, which manifests as excessive protein loss from the gut causing fat malabsorption, severe diarrhea, immunodeficiency, and gastrointestinal bleeding secondary to coagulant disorder.11,22 PLE appears to be triggered by infection, accompanied by high central venous pressure (CVP) and low cardiac output leading to portal hypertension, lymphatic insufficiency, and eventually intestinal congestion.11,23 Ohuchi and colleagues reported that one of the causes for PLE might be high CVP (≥12 mm Hg) post-Fontan, and PLE could be prevented by intensive surgical/catheter interventions to alter hemodynamic such as impaired ventricular function and high CVP.23 We found later timing of the Norwood procedure in the PLE-onset group than in the non-PLE-onset group (median: 139 [33-194] vs 24 [6-105] days, P = .046) in this study. Although our data did not show a statistically significant difference, possibly owing to the small number of patients, the PLE-onset group tended to have a smaller PA index 5 years after Fontan (142 [IQR, 16-190] mm2/m2 vs 222 [IQR, 141-278] mm2/m2; P = .2) and higher PA pressure before Fontan (13 [11-16] vs 11 [10-12] mm Hg; P = .2). Kido and colleagues24 recently reported that a small left PA index was associated with adverse events after Fontan, including chylothorax, PLE, and thrombosis in their cohort of 247 non-fenestrated Fontan patients and 102 HLHS patients (41%). Thus, they emphasized the need for surgical interventions or catheterization for small-sized PAs to improve late outcomes after the Fontan procedure. Although we could not show evidence of impaired systemic ventricular function, such as higher ventricular end-diastolic pressure and lower systemic ventricular ejection fraction in the PLE-onset group because of the small number of patients with PLE, we assumed that a longer duration of banding affects systemic ventricular function because of the narrow ascending aorta, resulting in reduced retrograde coronary blood flow, and inhibits PA growth, resulting in high CVP and poor Fontan outcomes, such as PLE.25,26 Therefore, careful consideration should be given for surgical plans to avoid delaying the timing of the Norwood procedure too much after BPAB.
The current study might not have compared 2 different approaches, but rather 2 different institutions or surgeons, given that the operative and bypass times for the Norwood procedures were longer in group I than in group N. The longer cardiopulmonary and operative times in group I might have been related to the the need for more prolonged PA band placement. To support this, we analyzed the time spent on each surgical step (Table E1). Of note, the differences in time from the start of surgery to the start of CPB could be attributed to the prolonged dissection of adhesions around the bilateral pulmonary arteries related to the longer time taken for band placement. This is supported by the lack of differences between the 2 groups in aortic cross-clamp time and lower body circulatory arrest time, which reflect the time for aortic reconstruction. The time between aortic declamping and separation from CPB was longer in group I, given that the hemostatic maneuvers took longer because of the bleeding tendency resulting from the longer CPB time.
Study Limitations
First, differences in surgical techniques such as aortic arch reconstruction might have affected the surgical results owing to the long observation period and multiple attending surgeons. However, we believe that the rotation of attending surgeons to assist with the Norwood procedure at other institutions helps maintain surgical quality as homogenous as possible. Second, this study could not rule out selection biases. One attending surgeon at 1 center selected the neonatal Norwood procedure, whereas the other 3 attending surgeons from the other 2 centers selected the infantile Norwood procedure. The 3 surgeons believed that the surgical outcomes of the Norwood procedure would be better when patients weighed >4 kg, despite the lack of clear scientific evidence supporting this belief. Consequently, the 3 surgeons selected the infant Norwood procedure. The methods of Norwood aortic reconstruction, BCPC, and TCPC were the same at all 3 centers; however, the choice of shunt size differed because of the different weights at the time of the Norwood procedure. This also can be a source of bias, given that it concerns the growth of the PA. Another source of selection bias might be exclusion of the 3 interstage deaths between BPAB and the Norwood procedure from group I. Two of the 3 patients had a preoperative risk of low BW. Finally, they received palliative care, owing to heart failure in 2 patients and multiple organ failure in 1 patient.
Third, to investigate the risk factors and freedom rates from Fontan-associated events, we defined postoperative arrhythmias, FALD, and PLE as Fontan-associated events; however, other Fontan-associated events, such as kidney dysfunction and thromboembolism,11 also might affect long-term Fontan outcomes. Finally, this study was conducted retrospectively and included a relatively small number of patients compared with other studies, which might have introduced type II errors and biases.
Conclusions
Fontan candidacy and survival rates were comparable regardless of the Norwood procedure timing. Early Norwood procedure timing may lead to lower rates of late Fontan-associated events such as PLE.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix 1
Table E1.
Time management of steps of the Norwood procedure
| Variable | Group N (N = 23) | Group I (N = 23) | P value |
|---|---|---|---|
| Op start – CPB on, min, median (IQR) | 88 (76-94) | 112 (79-140) | .03 |
| CPB on – ACC, min, median (IQR) | 87 (59-109) | 112 (87-134) | .07 |
| Declamp – CPB off, min, median (IQR) | 59 (49-88) | 115 (73-188) | .005 |
| CPB off – chest closed, min, median (IQR) | 93 (53-153) | 100 (50-163) | .88 |
Op, Operation; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp.
Figure E1.
Kaplan–Meier curves of survival and adverse events after the Norwood and Fontan procedures. (A) Survival rates after the Norwood procedure. (B) Freedom from arrhythmias after the Fontan procedure. (C) Freedom from PLE after the Fontan procedure. (D) Freedom from Fontan-associated liver disease after the Fontan procedure. (E) Freedom from all Fontan-associated events after the Fontan procedure. BPAB, bilateral pulmonary artery banding; CI, confidence interval; PLE, protein-losing enteropathy.
References
- 1.Hirata Y., Miyata H., Hirahara N., Murakami A., Kado H., Sakamoto K., et al. Long-term results of bilateral pulmonary artery banding versus primary Norwood procedure. Pediatr Cardiol. 2018;39:111–119. doi: 10.1007/s00246-017-1735-1. [DOI] [PubMed] [Google Scholar]
- 2.Wernovsky G., Ozturk M., Diddle J.W., Muñoz R., d'Udekem Y., Yerebakan C. Rapid bilateral pulmonary artery banding: a developmentally based proposal for the management of neonates with hypoplastic left heart. JTCVS Open. 2023;14:398–406. doi: 10.1016/j.xjon.2023.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argo M.B., Barron D.J., Eghtesady P., Alsoufi B., Honjo O., Yerebakan C., et al. Norwood operation versus comprehensive stage II after bilateral pulmonary artery banding palliation for infants with critical left heart obstruction. J Thorac Cardiovasc Surg. 2023 doi: 10.1016/j.jtcvs.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai T., Sakurai H., Yamana K., Nonaka T., Noda R., Otsuka R., et al. Expectations and limitations after bilateral pulmonary artery banding. Eur J Cardio Thorac Surg. 2016;50:626–631. doi: 10.1093/ejcts/ezw056. [DOI] [PubMed] [Google Scholar]
- 5.Ceneri N.M., Desai M.H., Tongut A., Ozturk M., Ramakrishnan K., Staffa S.J., et al. Hybrid strategy in neonates with ductal-dependent systemic circulation and multiple risk factors. J Thorac Cardiovasc Surg. 2022;164:1291–1303.e6. doi: 10.1016/j.jtcvs.2021.11.103. [DOI] [PubMed] [Google Scholar]
- 6.Davies R.R., Radtke W.A., Klenk D., Pizarro C. Bilateral pulmonary arterial banding results in an increased need for subsequent pulmonary artery interventions. J Thorac Cardiovasc Surg. 2014;147:706–712. doi: 10.1016/j.jtcvs.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Baba K., Kotani Y., Chetan D., Chaturvedi R.R., Lee K.J., Benson L.N., et al. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation. 2012;126:S123–S131. doi: 10.1161/CIRCULATIONAHA.111.084616. [DOI] [PubMed] [Google Scholar]
- 8.Cua C.L., McConnell P.I., Meza J.M., Hill K.D., Zhang S., Hersey D., et al. Hybrid palliation: outcomes after the comprehensive stage 2 procedure. Ann Thorac Surg. 2018;105:1455–1460. doi: 10.1016/j.athoracsur.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Alphonso N., Angelini A., Barron D.J., Bellsham-Revell H., Blom N.A., Brown K., et al. Guidelines for the management of neonates and infants with hypoplastic left heart syndrome: the European Association for Cardio-Thoracic Surgery (EACTS) and the Association for European Paediatric and Congenital Cardiology (AEPC) hypoplastic left heart syndrome guidelines task force. Eur J Cardio Thorac Surg. 2020;58:416–499. doi: 10.1093/ejcts/ezaa188. [DOI] [PubMed] [Google Scholar]
- 10.Miyaji K., Miyamoto T., Kohira S., Yoshii T., Itatani K.I., Sato H., et al. The effectiveness of high-flow regional cerebral perfusion in Norwood stage I palliation. Eur J Cardio Thorac Surg. 2011;40:1215–1220. doi: 10.1016/j.ejcts.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Martino D., Rizzardi C., Vigezzi S., Guariento C., Sturniolo G., Tesser F., et al. Long-term management of Fontan patients: the importance of a multidisciplinary approach. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.886208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schleiger A., Kramer P., Salzmann M., Danne F., Schubert S., Bassir C., et al. Evaluation of Fontan failure by classifying the severity of Fontan-associated liver disease: a single-centre cross-sectional study. Eur J Cardio Thorac Surg. 2021;59:341–348. doi: 10.1093/ejcts/ezaa310. [DOI] [PubMed] [Google Scholar]
- 13.Oka N., Miyamoto T., Tomoyasu T., Hayashi H., Miyaji K. Risk factors for mid-term liver disease after the Fontan procedure. Int Heart J. 2020;61:979–983. doi: 10.1536/ihj.20-059. [DOI] [PubMed] [Google Scholar]
- 14.Ono M., Kido T., Wallner M., Burri M., Lemmer J., Ewert P., et al. Preoperative risk factors influencing inter-stage mortality after the Norwood procedure. Interact Cardiovasc Thorac Surg. 2021;33:218–226. doi: 10.1093/icvts/ivab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoashi T., Imai K., Okuda N., Komori M., Kurosaki K., Ichikawa H. Intermediate-term outcomes of deferred Norwood strategy. Eur J Cardio Thorac Surg. 2022;62 doi: 10.1093/ejcts/ezac099. [DOI] [PubMed] [Google Scholar]
- 16.Davies R.R., Radtke W., Bhat M.A., Baffa J.M., Woodford E., Pizarro C. Hybrid palliation for critical systemic outflow obstruction: neither rapid stage 1 Norwood nor comprehensive stage 2 mitigate consequences of early risk factors. J Thorac Cardiovasc Surg. 2015;149:182–191. doi: 10.1016/j.jtcvs.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura N., Hirata Y., Inuzuka R., Tachimori H., Hirano A., Sakurai T., et al. Effect of procedural volume on the outcomes of congenital heart surgery in Japan. J Thorac Cardiovasc Surg. 2023;165:1541–1550.e3. doi: 10.1016/j.jtcvs.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Mascio C.E., Irons M.L., Ittenbach R.F., Gaynor J.W., Fuller S.M., Kaplinski M., et al. Thirty years and 1663 consecutive Norwood procedures: has survival plateaued? J Thorac Cardiovasc Surg. 2019;158:220–229. doi: 10.1016/j.jtcvs.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 19.Ota N., Murata M., Tosaka Y., Ide Y., Tachi M., Ito H., et al. Is routine rapid-staged bilateral pulmonary artery banding before stage 1 Norwood a viable strategy? J Thorac Cardiovasc Surg. 2014;148:1519–1525. doi: 10.1016/j.jtcvs.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 20.Knirsch W., De Silvestro A., von Rhein M. Neurodevelopmental and functional outcome in hypoplastic left heart syndrome after Hybrid procedure as stage I. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.1099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki T., Takahashi Y., Ando M., Wada N., Kawase Y., Seki H. Bilateral pulmonary artery banding for hypoplastic left heart syndrome and related anomalies. Gen Thorac Cardiovasc Surg. 2008;56:158–162. doi: 10.1007/s11748-007-0207-6. [DOI] [PubMed] [Google Scholar]
- 22.Ohuchi H. Seeking a better quality of life for patients after the Fontan operation: lessons learned from serial assessment of Fontan Pathophysiology. Pediatr Card Card Surg. 2016;32:141–153. [Google Scholar]
- 23.Ohuchi H., Yasuda K., Miyazaki A., Kitano M., Sakaguchi H., Yazaki S., et al. Haemodynamic characteristics before and after the onset of protein losing enteropathy in patients after the Fontan operation. Eur J Cardio Thorac Surg. 2013;43:e49–e57. doi: 10.1093/ejcts/ezs714. [DOI] [PubMed] [Google Scholar]
- 24.Kido T., Stern C., Heinisch P.P., Burri M., Vodiskar J., Strbad M., et al. The impact of pulmonary artery size on midterm outcomes after nonfenestrated Fontan operation. J Thorac Cardiovasc Surg. 2023;165:1651–1660.e2. doi: 10.1016/j.jtcvs.2022.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Latus H., Nassar M.S., Wong J., Hachmann P., Bellsham-Revell H., Hussain T., et al. Ventricular function and vascular dimensions after Norwood and hybrid palliation of hypoplastic left heart syndrome. Heart. 2018;104:244–252. doi: 10.1136/heartjnl-2017-311532. [DOI] [PubMed] [Google Scholar]
- 26.Dave H., Rosser B., Knirsch W., Hubler M., Pretre R., Kretschmar O. Hybrid approach for hypoplastic left heart syndrome and its variants: the fate of the pulmonary arteries. Eur J Cardio Thorac Surg. 2014;46:14–19. doi: 10.1093/ejcts/ezt604. [DOI] [PubMed] [Google Scholar]