Abstract
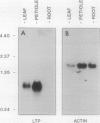
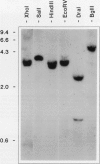
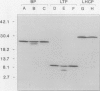
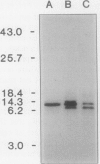
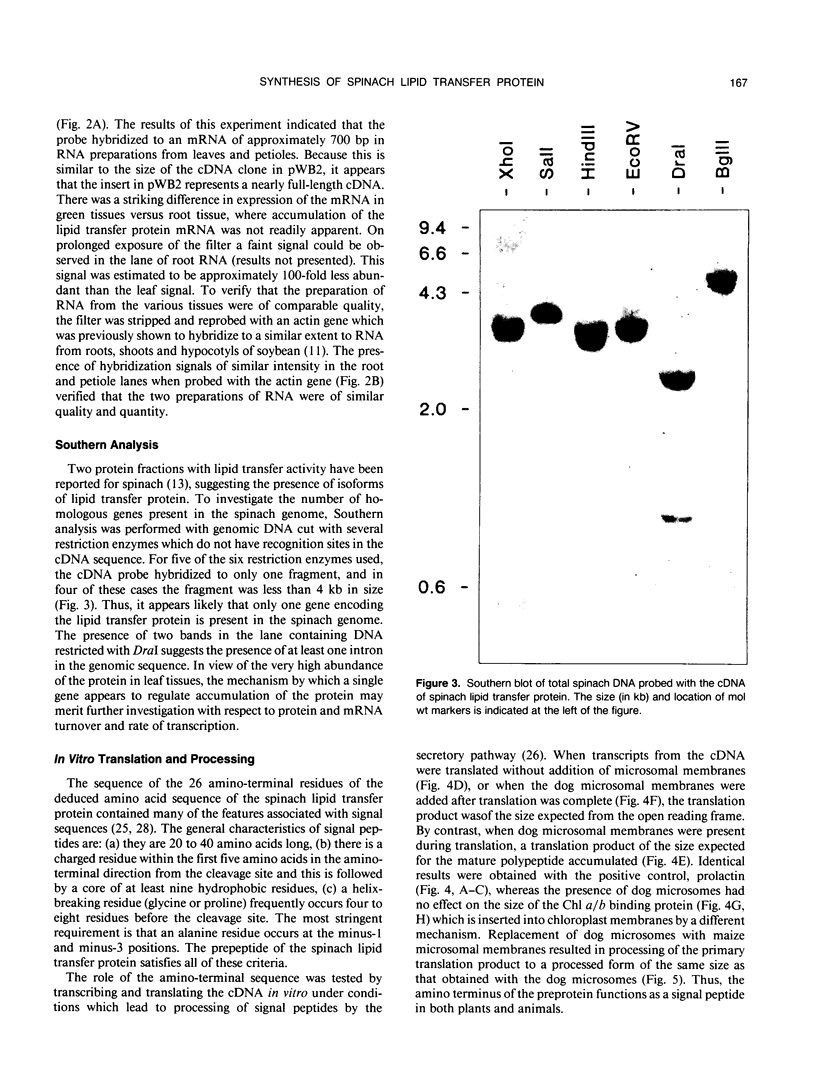
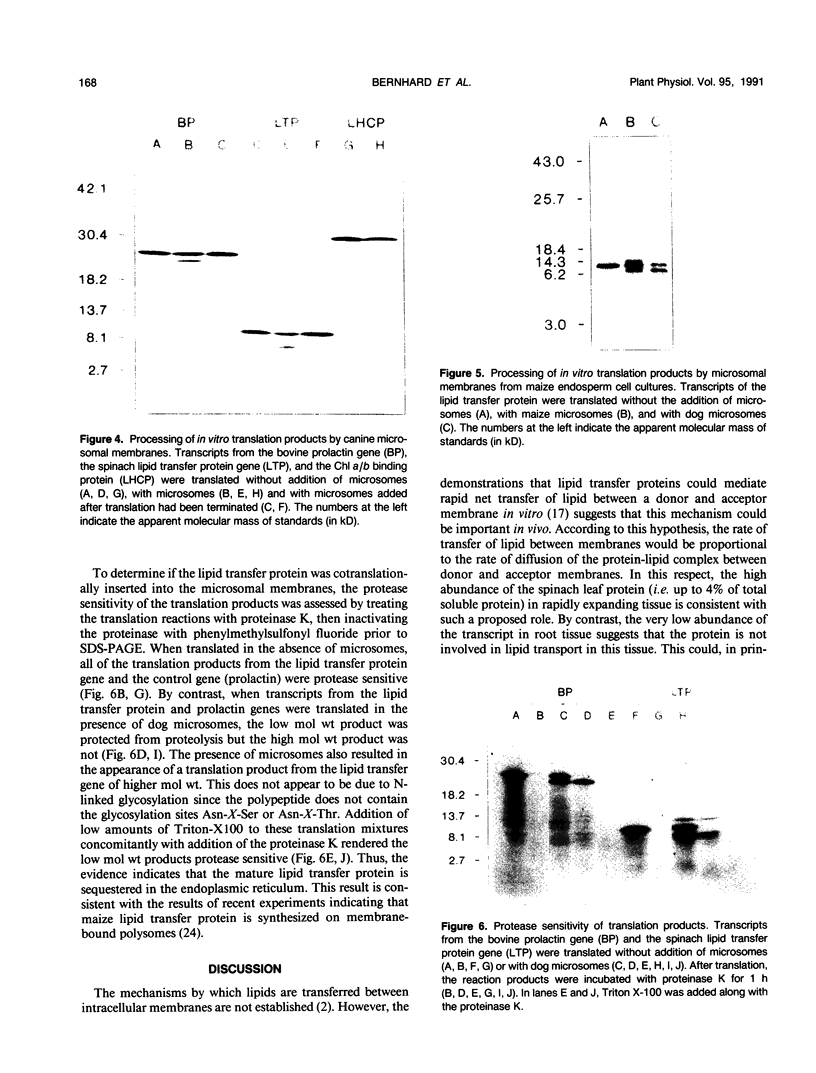
A cDNA clone encoding a nonspecific lipid transfer protein from spinach (Spinacia oleracea) was isolated by probing a library with synthetic oligonucleotides based on the amino acid sequence of the protein. Determination of the DNA sequence indicated a 354-nucleotide open reading frame which encodes a 118-amino acid residue polypeptide. The first 26 amino acids of the open reading frame, which are not present in the mature protein, have all the characteristics of a signal sequence which is normally associated with the synthesis of membrane proteins or secreted proteins. In vitro transcription of the cDNA and translation in the presence of canine pancreatic microsomes or microsomes from cultured maize endosperm cells indicated that proteolytic processing of the preprotein to the mature form was associated with cotranslational insertion into the microsomal membranes. Because there is no known mechanism by which the polypeptide could be transferred from the microsomal membranes to the cytoplasm, the proposed role of this protein in catalyzing lipid transfer between intracellular membranes is in doubt. Although the lipid transfer protein is one of the most abundant proteins in leaf cells, the results of genomic Southern analysis were consistent with the presence of only one gene. Analysis of the level of mRNA by Northern blotting indicated that the transcript was several-fold more abundant than an actin transcript in leaf and petiole tissue, but was present in roots at less than 1% of the level in petioles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard W. R., Somerville C. R. Coidentity of putative amylase inhibitors from barley and finger millet with phospholipid transfer proteins inferred from amino acid sequence homology. Arch Biochem Biophys. 1989 Mar;269(2):695–697. doi: 10.1016/0003-9861(89)90154-9. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Bouillon P., Drischel C., Vergnolle C., Duranton H., Kader J. C. The primary structure of spinach-leaf phospholipid-transfer protein. Eur J Biochem. 1987 Jul 15;166(2):387–391. doi: 10.1111/j.1432-1033.1987.tb13527.x. [DOI] [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C. R., Slack C. R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the '16:3' plant Arabidopsis thaliana. Biochem J. 1986 Apr 1;235(1):25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr F. A., Burr B. In vitro uptake and processing of prezein and other maize preproteins by maize membranes. J Cell Biol. 1981 Aug;90(2):427–434. doi: 10.1083/jcb.90.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Genton C., Kruithof E. K., Schleuning W. D. Phorbol ester induces the biosynthesis of glycosylated and nonglycosylated plasminogen activator inhibitor 2 in high excess over urokinase-type plasminogen activator in human U-937 lymphoma cells. J Cell Biol. 1987 Mar;104(3):705–712. doi: 10.1083/jcb.104.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr Phospholipid transfer proteins: mechanism of action. J Bioenerg Biomembr. 1986 Apr;18(2):71–91. doi: 10.1007/BF00743477. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader J. C., Julienne M., Vergnolle C. Purification and characterization of a spinach-leaf protein capable of transferring phospholipids from liposomes to mitochondria or chloroplasts. Eur J Biochem. 1984 Mar 1;139(2):411–416. doi: 10.1111/j.1432-1033.1984.tb08020.x. [DOI] [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Complete nucleotide sequence of a soybean actin gene. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1022–1026. doi: 10.1073/pnas.79.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchang F., This P., Stiefel V., Arondel V., Morch M. D., Pages M., Puigdomenech P., Grellet F., Delseny M., Bouillon P. Phospholipid transfer protein: full-length cDNA and amino acid sequence in maize. Amino acid sequence homologies between plant phospholipid transfer proteins. J Biol Chem. 1988 Nov 15;263(32):16849–16855. [PubMed] [Google Scholar]
- Vergnolle C., Arondel V., Tchang F., Grosbois M., Guerbette F., Jolliot A., Kader J. C. Synthesis of phospholipid transfer proteins from maize seedlings. Biochem Biophys Res Commun. 1988 Nov 30;157(1):37–41. doi: 10.1016/s0006-291x(88)80007-x. [DOI] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W., Gadella T. W., Jr Properties and modes of action of specific and non-specific phospholipid transfer proteins. Experientia. 1990 Jun 15;46(6):592–599. doi: 10.1007/BF01939698. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]