Abstract
Objectives
The use of bioprosthetic aortic valve replacement (AVR) is inherently associated with a risk of structural valve degeneration (SVD) and the need for aortic valve (AV) reintervention. We sought to evaluate whether AV reintervention, in the form of repeat surgical AVR (SAVR) or valve-in-valve transcatheter aortic valve replacement (ViV-TAVR), negatively affects patients’ subsequent long-term survival after index SAVR.
Methods
We identified patients who had undergone bioprosthetic SAVR from 2002 to 2017 at our institution. Median longitudinal follow-up after index SAVR was 7.3 years (10.9 years for those with and 7.2 years for those without AV reintervention), and median follow-up after AV reintervention was 1.9 years. Cox regression analyses using AV reintervention (re-SAVR and ViV-TAVR) as a time-varying covariate were used to determine the impact of reintervention on subsequent survival.
Results
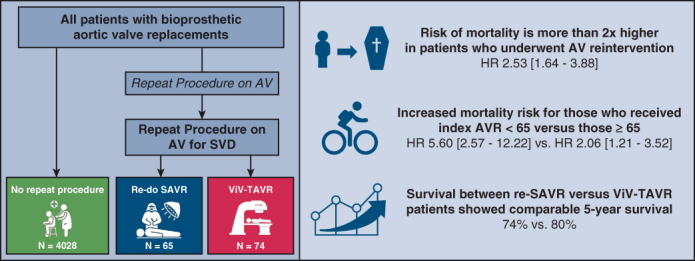
Of 4167 patients who underwent index SAVR, 139 (3.3%) required AV reintervention for SVD, with re-SAVR being performed in 65 and ViV-TAVR in 74. Median age at the index SAVR was 73 years (interquartile range, 64-79 years), and 2541 (61%) were male. Overall, there were total of 1171 mortalities observed, of which 13 occurred after re-SAVR and 9 after ViV-TAVR. AV reintervention was associated with a greater risk of subsequent mortality compared with those patients who did not require AV reintervention (hazard ratio, 2.53; 95% confidence interval, 1.64-3.88, P < .001). This increased risk of subsequent mortality was more pronounced for those who received their index AVR when <65 years of age (hazard ratio, 5.60; 95% confidence interval, 2.57-12.22, P < .001) versus those ≥65 years (2.06, 1.21-3.52, P = .008). Direct comparison of survival between those who underwent re-SAVR versus ViV-TAVR showed 5-year survival to be comparable (re-SAVR: 74% vs ViV-TAVR: 80%, P = .67).
Conclusions
Among patients receiving bioprosthetic AVR, an AV reintervention for SVD is associated with an increased risk of subsequent mortality, regardless of re-SAVR or ViV-TAVR, and this risk is greater among younger patients. These findings should be balanced with individual preferences at index AVR in the context of patients’ lifetime management of aortic stenosis.
Key Words: aortic valve replacement, transcatheter aortic valve replacement, structural valve degeneration
After AVR, AV reintervention is associated with an increased risk of mortality.
Central Message.
An AV reintervention for SVD is associated with an increased risk of subsequent mortality, regardless of re-SAVR or ViV-TAVR, and this risk is greater among younger patients.
Perspective.
Little is known about the impact of AV reinterventions for SVD on long-term survival after AVR. We found that after index AVR, the risk of mortality for patients who underwent AV reintervention is more than 2-fold greater compared with patients who did not undergo one. AV reintervention is not benign, and its risks must be balanced with individual preferences in the context of lifetime management of aortic stenosis.
See Discussion on page 103.
The use of bioprosthetic valves for aortic valve replacement (AVR) has gained substantial popularity, especially among younger patients.1,2 The obvious benefit of avoiding lifelong anticoagulation is offset by bioprostheses' limited durability and the risk of structural valve degeneration (SVD), which has traditionally required reoperative surgery during patients’ lifetimes. Repeat surgical aortic valve replacement (re-SAVR) is inherently associated with a greater risk of mortality and morbidity than primary AVR.3 Meanwhile, the recent increased use of bioprosthetic valve is linked with the enthusiasm that the deteriorated valve can be treated with valve-in-valve transcatheter aortic valve replacement (ViV-TAVR).4, 5, 6 However, ViV-TAVR comes with several caveats: not all patients are eligible, there is a greater risk of coronary obstruction and elevated residual gradients, whereas long-term outcomes following ViV-TAVR remain unknown at this time.6,7
Despite bioprosthetic AVR becoming an increasingly popular choice for young patients, less is known about the impact of aortic valve (AV) reinterventions for SVD on long-term survival. Moreover, there is growing interest in the concept of lifetime management of aortic stenosis, whereby prosthesis choice, mode of therapy, and strategies for reinterventions are tailored to individuals, looking decades into the future for younger patients. Alternative surgical choices such as mechanical prosthesis, Ross procedure, and even the Ozaki technique have been regaining interest in this context, especially in younger patients. Therefore, understanding the impact of AV reintervention procedures will help the discussion and decision-making process for patients and clinicians. The aim of this study was to review long-term data in patients who underwent bioprosthetic SAVR and determine whether AV reintervention procedures for SVD—re-SAVR or ViV-TAVR—influenced subsequent mortality.
Methods
This study was approved by expedited review with waived consent requirement by the Mass General Brigham Institutional Review Board [2010P000292] initially on November 9, 2021.
Study Design and Patient Population
We identified all patients ≥18 years of age at Brigham and Women’s Hospital who underwent index bioprosthetic AVR between January 1, 2002, and June 30, 2017, either as an isolated procedure or in combination with coronary artery bypass grafting (CABG), ascending aorta replacement, or aortic root replacement. These patients undergoing concomitant procedures were included since previous CABG or aortic surgery do not negate either modality of AV reintervention—be it re-SAVR or ViV-TAVR—should they present with SVD. Patients undergoing concomitant mitral, tricuspid, and pulmonary valve surgery; mechanical assist devices; or cardiac transplantation at the index surgery were excluded.
Of these patients, we identified those who underwent repeat procedures on the aortic valve for SVD. Data points were collected manually. The diagnosis of SVD was made based on the Valve Academic Research Consortium 3 definition.7 The decision to offer either mode of therapy was based on a multidisciplinary heart team evaluation that considered patients’ comorbidities, life expectancy, surgical risk, TAVR access feasibility, aortic root anatomy, and the need to address coexistent cardiac disease. Patients undergoing reintervention for prosthetic valve endocarditis, paravalvular leak, aortic aneurysm, and aortic dissection were excluded. Patients were also excluded from analysis if they met an indication for coexisting valvular disease to be addressed in addition to aortic bioprosthesis SVD.
Patient demographics, laboratory values, operative details, and in-hospital outcomes were recorded at the time of presentation and obtained from hospital electronic medical records. Data on postdischarge outcomes and 30-day mortality were collected obtained from routine follow-up, our internal research data repository, and by query of our institution’s Research Patient Data Repository, which includes National Death Index data. Time-to-event was calculated in days from the date of the index AVR to the date of reintervention, death, or December 31, 2020, if no events occurred. For patients with no National Death Index data, observation time was censored at the date of last known clinical contact. All variables collected were coded according to the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database version 2.81 or the STS/American College of Cardiology Transcatheter Valve Therapy Registry, version 2.1, specifications, unless otherwise noted.
Statistical Analysis
Descriptive statistics were presented as number and percent for categorical data and median (interquartile range [IQR]) for continuous data. Categorical variables were compared using χ2 and Fisher exact test when appropriate, whereas continuous data were compared using the Wilcoxon nonparametric rank-sum test. All analyses were performed using IBM SPSS Statistics for Windows, Version 28 (IBM Corp) and R statistical package, version 3.3.1.
To determine the impact of AV reintervention on survival, a Cox proportional hazard model treating AV reintervention as a time-dependent covariate was used. The time intervals between index AVR and AV reintervention and between AV reintervention to death or end of follow-up were calculated for subjects who underwent AV reinterventions, whereas a placeholder time of 99 years (exceeding the longest observation interval) was used for those who did not undergo AV reintervention. Because of the limited number of events, a select set of covariables (age at index AVR, sex, left ventricular ejection fraction, and preprocedure creatinine) were chosen based on their established impact on prognosis to avoid overfitting.8, 9, 10 This analysis was repeated among patients <65 and ≥65 years of age at index AVR.
The Kaplan–Meier method was used to estimate survival probabilities after AV reintervention, and group comparisons were performed using the log-rank test. Multivariable Cox regression analysis was performed to examine the association between the type of AV reintervention (re-SAVR vs ViV-TAVR) and mortality after AV reintervention. Variables included in this analysis were age at repeat procedure, sex, left ventricular ejection fraction, and preprocedure creatinine.
Results
Patient Sample and Early Outcomes
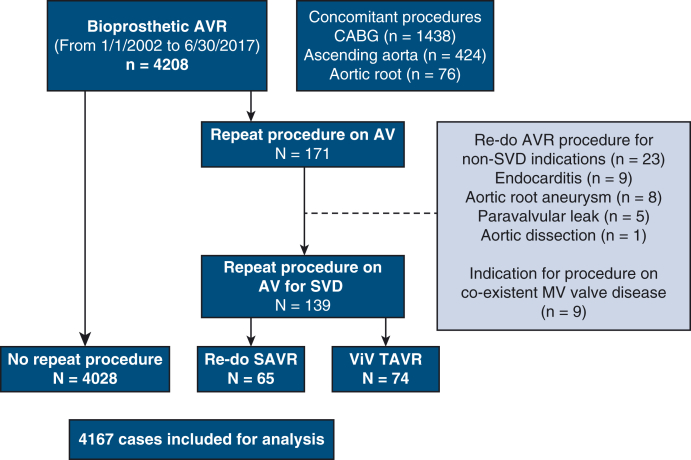
We identified 4208 patients who underwent bioprosthetic SAVR at our center during the study period. Figure 1 is a diagrammatic representation of the study population. The clinical characteristics of these patients are presented in Table 1. Median age at the index SAVR was 73 years (IQR, 64-79), and 2541 (61%) were male. Aortic stenosis was present in 3565 (85.6%) patients. Concomitant CABG was performed in 1438 (34.5%), ascending aorta replacement in 424 (10.1%), and aortic root replacement in 76 (1.8%) cases. Thirty-day postoperative outcomes of the entire study group are presented in Table E1. At 30 days, 11 patients had died (0.3%), whereas stroke occurred in 109 (2.6%) of patients.
Figure 1.
Flow diagram illustrating the study population. AVR, Aortic valve replacement; CABG, coronary artery bypass grafting; AV, aortic valve; SVD, structural valve degeneration; MV, mitral valve; VIV-TAVR, valve-in-valve transcatheter aortic valve replacement.
Table 1.
Clinical characteristics of study patients who underwent bioprosthetic AVR
| Clinical characteristic | Total (n = 4167) |
No repeat Procedure (n = 4028) |
Repeat procedure (n = 139) | P value |
|---|---|---|---|---|
| Age, y, median (IQR) | 73 (64-79) | 73 (65-80) | 62 (54-69) | <.001 |
| Male, n (%) | 2541 (61.0) | 2460 (61.1) | 81 (58.2) | .54 |
| Smoker, n (%) | 1961 (47.1) | 1896 (47.1 | 65 (46.8) | >.99 |
| Diabetes, n (%) | 980 (23.5) | 950 (23.6) | 30 (21.6) | .68 |
| Hypercholesterolemia, n (%) | 3052 (73.2) | 1964 (48.8) | 88 (63.3) | .008 |
| Hypertension, n (%) | 3072 (73.7) | 2987 (74.1) | 85 (61.1) | .001 |
| Creatinine, mg/dL, median (IQR) | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | 1.0 (0.8-1.1) | .029 |
| Dialysis, n (%) | 46 (1.1) | 46 (1.1) | 0 (0.0) | .41 |
| Previous stroke, n (%) | 197 (4.7) | 192 (4.8) | 5 (3.6) | .68 |
| Endocarditis, n (%) | 105 (2.5) | 96 (2.4) | 9 (6.5) | .008 |
| Chronic lung disease: moderate/severe, n (%) | 87 (2.1) | 86 (2.1) | 1 (0.7) | .25 |
| Previous MI, n (%) | 415 (10.0) | 405 (10.0) | 10 (7.2) | .17 |
| Previous PCI, n (%) | 435 (10.4) | 427 (10.6) | 8 (6) | .029 |
| CHF, n (%) | 125 (3.0 | 123 (3.1) | 2 (1.4) | .44 |
| Aortic stenosis, n (%) | 3565 (85.6) | 3461 (85.9) | 104 (74.8) | <.002 |
| Aortic regurgitation: moderate/severe, n (%) | 1013 (24.3) | 967 (24.0) | 46 (33.1) | .014 |
| Aortic valve prosthesis size, mm, n (%) | ||||
| 19 | 322 (87.7 | 306 (7.6) | 16 (11.5) | – |
| 21 | 975 (23.4) | 939 (23.3) | 36 (25.9) | – |
| 23 | 1316 (31.6) | 1274 (31.6) | 42 (30.2) | – |
| 25 | 1050 (25.2) | 1023 (25.4 | 27 (19.4 | – |
| 29 | 81 (1.9) | 81 (2.0) | 0 (0.0) | – |
| Unknown | 423 (10.1) | 405 (10.0) | 18 (12.9) | .11 |
| Concomitant CABG, n (%) | 1438 (34.5) | 1388 (34.4) | 50 (36.0) | .71 |
| Ascending aorta replacement, n (%) | 424 (10.1) | 421 (10.4) | 3 (2.2) | <.001 |
| Aortic root replacement, n (%) | 76 (1.8) | 76 (1.8) | 0 (0.0) | .18 |
IQR, Interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; CHF, congestive heart failure; CABG, coronary artery bypass grafting; AVR, aortic valve replacement.
Longitudinal Follow-Up
Median overall longitudinal follow-up for the entire study population was 7.3 years (IQR, 4.8-10.7 years) and was 100% complete for survival. Follow-up was 10.9 years (IQR, 8.1-14.4) for those who underwent AV reintervention and 7.3 years (IQR, 4.8-10.7) for those without reintervention. During follow-up, 171 patients underwent AV reintervention; 139 for SVD and 23 for reasons other than SVD. Nine patients required coexisting native mitral valve disease (7 had severe mitral regurgitation, whereas 2 had severe mitral stenosis) to be addressed surgically, in addition to an indication for a procedure for aortic valve bioprosthesis SVD (Figure 1). Of those 139 who underwent AV reinterventions for SVD, re-SAVR was performed in 65 (47%) and ViV-TAVR in 74 (53%). During follow-up, there were 1171 patients who died without undergoing AV reintervention.
Characteristics and Outcomes After Reintervention
The clinical characteristics of the patients who underwent AV reintervention are presented in Table 2. All patients had initially received stented prostheses at index AVR. Patients undergoing ViV-TAVR were generally older, with greater median STS scores (re-SAVR: 2.3% [IQR, 1.5%-4.3%] vs ViV-TAVR: 4.5% [IQR, 2.7%-6.7%], P < .001). Of the 65 patients undergoing re-SAVR, 3 underwent concomitant CABG. Two patients underwent concomitant ascending aorta replacement. This was performed in 1 patient due to ascending aorta injury and an inability to repair it primarily, whereas in the other patient, aortic replacement was performed due to extensive aorta calcification. The 30-day outcomes of those who underwent an AV reintervention are presented in Table E2. Thirty-day mortality (re-SAVR 6.2% vs ViV-TAVR 1.4%, P = .19), stroke (re-SAVR 4.6% vs ViV-TAVR 1.4%, P = .34), and permanent pacemaker rates (re-SAVR 1.5% vs ViV-TAVR 4.1%, P = .62) were not statistically significantly different. A greater proportion of patients who underwent re-SAVR required transfusion of red blood cells compared with those who underwent ViV-TAVR (re-SAVR 34% vs ViV-TAVR 4.1%, P < .001).
Table 2.
Clinical characteristics of patients undergoing re-SAVR versus ViV-TAVR
| Clinical characteristic | re-SAVR (n = 65) | VIV-TAVR (n = 74) | P value |
|---|---|---|---|
| Time to repeat procedure, y median (IQR) | 7.6 (5.7-9.8) | 8.5 (5.5-11.3) | .24 |
| Age, y median (IQR) | 68 (59-72) | 74 (67-79) | <.001 |
| Female, n (%) | 31 (47.) | 25 (33.8) | .12 |
| BMI, kg/m2, median (IQR) | 29 (26-34) | 26 (24-30) | .01 |
| STS score, %, median (IQR) | 2.3 (1.5-4.3) | 4.5 (6.7-2.7) | <.001 |
| Diabetes, n (%) | 20 (30.8) | 27 (36.5) | .59 |
| Hypertension, n (%) | 51 (78.5) | 68 (91,9) | .03 |
| Creatinine, n (%) | 0.99 (0.84-1.14) | 1.15 (0.90-1.55) | .002 |
| Peripheral vascular disease, n (%) | 3 (4.6) | 15 (20.2) | .0097 |
| Previous stroke, n (%) | 2 (3.1) | 9 (12.2) | .061 |
| Previous MI, n (%) | 4 (6.2) | 15 (20.2) | .024 |
| Previous PCI, n (%) | 6 (9.2) | 13 (17.6) | .15 |
| Previous CABG, n (%) | 19 (29.2) | 32 (43.2) | .053 |
| Bioprosthetic stenosis, n (%) | 45 (69.2) | 53 (71.6) | .85 |
| Bioprosthetic AI grade, n (%) | |||
| None/trivial | 20 (30.8) | 35 (47.3) | – |
| Mild | 15 (23.0) | 13 (17.6) | – |
| Moderate | 21 (32.3) | 11 (14.9) | – |
| Severe | 9 (13.8) | 15 (20.2) | .040 |
| Initial implant size, mm, n (%) | |||
| 19 | 9 (13.8) | 7 (9.5) | – |
| 21 | 18 (27.7) | 18 (24.3) | – |
| 23 | 15 (23.3) | 27 (36.4) | – |
| 25 | 15 (23.3) | 12 (16.2) | – |
| 27 | 6 (9.2) | 4 (5.4) | – |
| Unknown | 2 (3.1) | 6 (8.1) | .32 |
SAVR, Surgical aortic valve replacement; VIV-TAVR, valve-in-valve transcatheter aortic valve replacement; IQR, interquartile range; BMI, body mass index; STS, Society of Thoracic Surgeons; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; AI, aortic insufficiency.
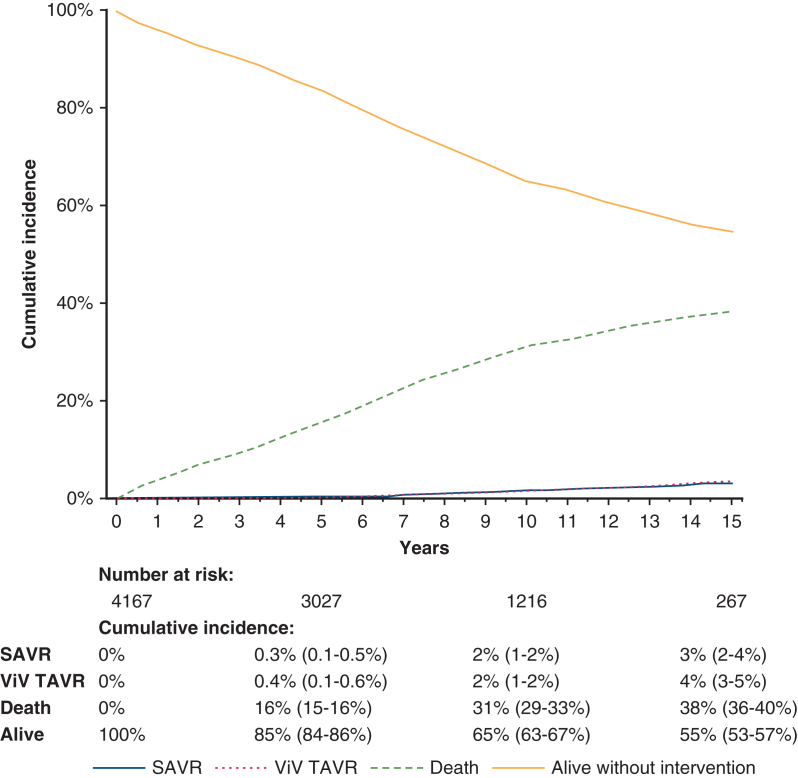
Figure 2 presents the cumulative incidence of the competing events of re-SAVR, ViV-TAVR, death without repeat procedure, and remaining alive without a repeat procedure. At 15 years, the cumulative rates were 3% (95% confidence interval [CI], 2%-4%) for re-SAVR, 4% (3%-5%) for ViV-TAVR, 38% (36%-41%) for death without repeat procedure, and 55% (53%-57%) for remaining alive without a repeat procedure.
Figure 2.
Cumulative incidence curves demonstrating the incidence of the competing events of undergoing reoperative surgical aortic valve replacement (SAVR), valve-in-valve transcatheter aortic valve replacement (ViV-TAVR), death, or remaining alive without undergoing a repeat procedure after index AVR. 95% confidence intervals are shown in parentheses. AVR, Aortic valve replacement.
Survival After Repeat AV Procedure
Median follow-up of the 139 patients after their AV reintervention was 1.9 years. Mortality occurred in a total of 21 patients; 13 occurred after re-SAVR and 9 after ViV-TAVR. An AV reintervention was associated with a greater risk of late mortality (hazard ratio [HR], 2.53; 95% CI, 1.64-3.88, P < .001) compared with the group with no reintervention. This increased risk of subsequent late mortality was more pronounced for those who received their index AVR when <65 years of age (HR, 5.60; 95% CI, 2.57-12.22, P < .001) versus those ≥65 (2.06; 95% CI, 1.21-3.52, P = .008) as shown in Table 3. However, the individual impact of re-SAVR (2.48; 95% CI, 1.43-4.32, P < .001) and ViV-TAVR (2.46; 95% CI, 1.27-4.75, P = .008) on subsequent late mortality was comparable.
Table 3.
Results of Cox regression examining association between repeat AV procedure and subsequent mortality (repeat AV intervention modeled as a time-varying covariate) 95% CIs are in parentheses.
| Repeat AV procedure | HR (95% CI) | P value |
|---|---|---|
| All repeat AV intervention | 2.53 (1.64-3.88) | <.001 |
| SAVR | 2.48 (1.43-4.32) | <.001 |
| ViV-TAVR | 2.46 (1.27-4.75) | .008 |
| Patients <65 y at initial AVR | 5.60 (2.57-12.22) | <.001 |
| SAVR | 3.72 (1.14-12.18) | .030 |
| ViV-TAVR | 6.77 (2.60-17.67) | <.001 |
| Patients ≥65 y at initial AVR | 2.06 (1.21-3.52) | .008 |
| SAVR | 2.43 (1.29-4.56) | .006 |
| ViV-TAVR | 1.44 (0.54-3.85) | .47 |
HR, Hazard ratio; CI, confidence interval; AV, aortic valve; SAVR, surgical aortic valve replacement; VIV-TAVR, valve-in-valve transcatheter aortic valve replacement; AVR, aortic valve replacement.
Comparison Between Re-SAVR and ViV-TAVR
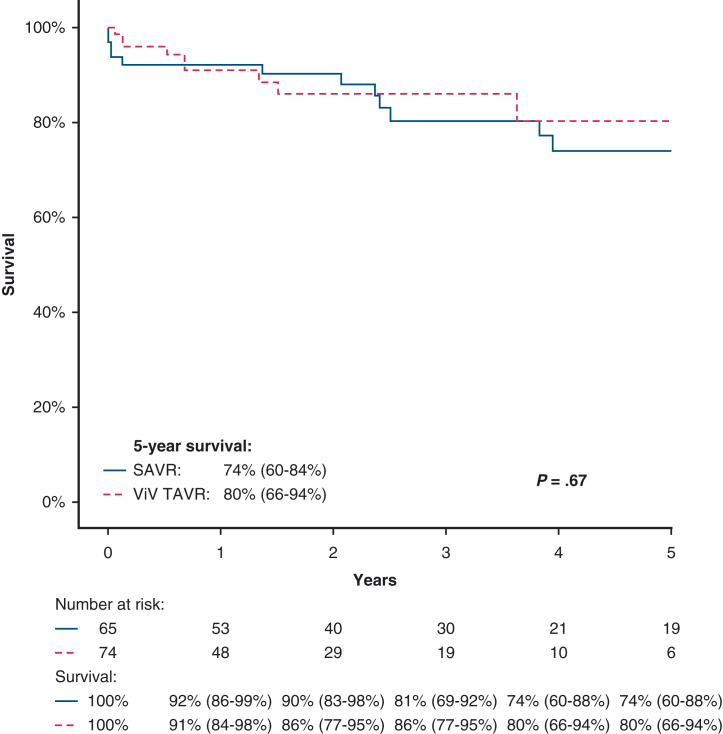
Using the Kaplan–Meier method, a direct comparison of survival between those who underwent re-SAVR versus ViV-TAVR showed 5-year survival to be comparable (re-SAVR 74% vs ViV-TAVR 80%, P = .67). Kaplan–Meier survival curves comparing survival after re-SAVR and ViV-TAVR is presented in Figure 3. Cox regression showed the type of AV reintervention not to be independently associated with late mortality (HR for ViV-TAVR: 0.52, 95% CI, 0.19-1.45, P = .21).
Figure 3.
Comparison of survival after reoperative surgical aortic valve replacement (re-SAVR) versus valve-in-valve transcatheter aortic valve replacement (ViV-TAVR). 95% confidence intervals are shown in parentheses.
Discussion
This study represents a large, single-center experience with bioprosthetic SAVR with longitudinal outcomes with a median follow-up period of over 10 years. To our knowledge, this is the first study to specifically examine the impact of repeat AV procedures on longitudinal survival over the course of patients’ lifetime after index AVR. Our study has several important findings. First, the rate of reoperation for SVD remains relatively rare, with a substantial proportion of patients remaining alive or dying without undergoing AV reintervention. Second, the risk of mortality for patients who underwent AV reintervention is more than 2-fold greater compared with patients who did not undergo one. This increased risk was more pronounced for those <65 years of age, with the risk of late mortality being more than 5 times greater compared with patients who did not undergo a repeat procedure. Third, the survival was comparable between either re-SAVR versus ViV-TAVR. The impact of reintervention for SVD in patients with bioprosthetic AVR is not benign, and this must be balanced with individual preferences in the context of lifetime management of aortic stenosis.
In this study, the need to undergo AV reintervention due to SVD was relatively rare. Of the 4167 patients included, only 192 underwent AV reintervention, with 139 receiving it for SVD. At 15 years, the cumulative rate of requiring a re-SAVR or ViV-TAVR was 3.0% and 3.7%, respectively. As illustrated in Figure 1, a significant proportion of patients died without requiring a repeat procedure. The rate of reoperation for SVD seen in our study is consistent with those observed in several previous studies investigating bioprosthetic durability, ranging from 3% to 10% at 10 years.11, 12, 13, 14 The incidence of repeat AVR in our study is also consistent with that of a large multicenter nationally representative STS database analysis,3 whereby between 2011 and 2013, 54,183 patients were reported to have undergone primary AVR, while in the same time period, 3383 patients underwent repeat AVR after having previously received an AVR, although this was not a longitudinal follow-up.
Our study’s major finding—that the risk of mortality for patients who underwent AV reintervention is greater compared with patients who did not undergo one—has important implications for the lifetime management of AV disease. The use of aortic bioprostheses continues to grow in popularity and be applied to younger patients.1,2 This is likely driven by the appeal of avoiding lifelong anticoagulation associated with mechanical prostheses, which seems to be a larger decision factor compared with the risk and burdensome nature of AV reinterventions in the future.15 However, our analysis provides a critical reminder that undergoing an AV reintervention is not benign and is associated with heightened mortality risk, particularly in younger patients. Whether this increased mortality risk after AV reintervention is secondary to the inherent risks of a repeat invasive procedure16, 17, 18 or the deleterious physiological impact of the long-standing valvular dysfunction due to SVD preceding the repeat procedure remains difficult to differentiate.19 Goldstone and colleagues,1 using a multicenter administrative database, previously demonstrated that for patients >55 years of age, long-term survival was similar between recipients of a mechanical versus bioprosthetic valve. Likewise, our institution has previously shown that for patients <65 years of age, overall long-term survival was similar between bioprosthetic and mechanical AVR.20 Our result cautions against this perception since the strategy of using a bioprosthetic SAVR may negatively affect life expectancy if repeat AV intervention for SVD is needed, especially in younger patients. This negative prognostic impact of AV reinterventions on late mortality was less pronounced for patients ≥65 years of age. This suggests that for a significant proportion of patients, especially those who are older and have comorbidities that make life expectancy relatively shorter, the issue of SVD may lie beyond their expected life span.
The outcomes following either re-SAVR or ViV-TAVR in our study were comparable with the published results in respect to major early mortality and major complications as well as mid-term survival.3,5,17,21,22 These outcomes were achieved despite patients who underwent ViV-TAVR exhibiting a greater proportion of comorbidities and having greater median STS scores, again verifying the generally good safety profile of ViV-TAVR.4,5 Promising data concerning the outcomes of ViV-TAVR have served to fuel the increasing usage of bioprosthetic AVR, which is often justified based on the assumption that ViV-TAVR will eventually serve as the first-line method to address SVD23 and thus avoid the perceived riskier and more morbid option of re-SAVR over the patient’s lifetime.5,16,23 Indeed, recent meta-analyses have shown ViV-TAVR to be associated with a reduced risk of early mortality and morbidity compared with re-SAVR in the short term.17,18 However, these potential early benefits have not been found to translate into any measurable differences in long-term mortality, albeit with relative short follow-up periods.4,5,17,18 This could be in part due to the negative prognostic impact of long-standing valvular dysfunction due to SVD before actually undergoing ViV-TAVR19 or the risk of prosthesis–patient mismatch.17,24 However, the most likely explanation is the sicker profile of patients who undergo ViV-TAVR, and thus calls for studies that investigate the long-term outcomes in comparable groups between re-SAVR and ViV-TAVR in the future.
Our study highlights the value of a careful and thoughtful discussion with patients on several key points during the contemporary evaluation of the treatment options for AS. This discussion with patients and the selection of valve prosthesis should extend beyond merely the risks and inconvenience of lifelong anticoagulation associated with mechanical prostheses but to the overall longitudinal strategy in patients' lifetime management of aortic valve disease, which encompasses decades of follow-up and the potential for multiple repeat procedures for SVD. Patients should be informed that AV reintervention for SVD is associated with inherent mortality risk and that this exists even for ViV-TAVR, despite it being perceived as a relatively noninvasive procedure. In younger patients, alternative surgical options such as mechanical valve or Ross procedure should also be discussed and offered to patients where appropriate, especially with recent reports showing that the Ross procedure can achieve a 15-year survival of over 90% and superior long-term survival—that which restores normal life expectancy—compared with both stented bioprosthesis and mechanical AVR at experienced centers, with relatively low re-intervention rates on both the autograft and the pulmonary homograft.25, 26, 27 While the multidisciplinary structural heart team is positioned well to guide patient management, ultimately, a shared decision-making model—whereby patients and physicians collaborate to align treatment safety and efficacy with patients’ preferences and values—has the potential to further enhance the quality of care.28
Limitations
This study is limited by several factors. First, as a single-institution study at an experienced, comprehensive valve center, our results may not be generalizable to all institutions or operators. Second, our follow-up method may not have captured AV reinterventions performed at external institutions and thus underestimated reoperation rates. However, even allowing for a liberal assumption that our capture was 85% to 90% rather than 100% of events, those misclassified patients would amount to approximately 0.5% or less of the comparison group, a variance that should be well within the 95% CI ranges of our effect estimate overall. In addition, the fact that the rate of repeat AVR seen in our study is relatively consistent with previously published reports is reassuring, suggesting that the number of repeat cases occurring at external centers is relatively small.3 Third, the number of deaths after AV reintervention precluded developing fully robust Cox proportional hazards models. Thus, we cannot rule out confounding from unmeasured variables. Fourth, important clinical end points in addition to mortality, such as functional status, hospitalizations, and health care use parameters, were not available and thus could not be analyzed. In addition, due to the lack of comprehensive echocardiographic follow-up for all patients, we could not assess SVD that did not require reintervention. It is likely that the proportion of those with SVD in the study population exceeds that of those who actually received a procedure. Therefore, the impact of SVD itself on prognosis could not be directly analyzed here.
Conclusions
In this large single-center experience with bioprosthetic SAVR, AV reintervention for SVD is associated with an increased risk of subsequent late mortality, regardless of whether the reintervention is re-SAVR or ViV-TAVR. This risk is greater among younger patients. Therefore, selecting a bioprosthetic AVR initially in these subgroups increases mortality risk for those who eventually require AV reintervention. These findings should be balanced with individual preferences at index AVR in the context of patients’ lifetime management of aortic stenosis.
Conflict of Interest Statement
Dr Kaneko is a consultant for Edwards Life Sciences, Medtronic, 4C Medical, Abbott, and Baylis. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix 1
Table E1.
Intraoperative and early postoperative outcomes of the entire study cohort
| Cases (n = 4167) | |
|---|---|
| Cardiopulmonary bypass time, min | 115 (87-153) |
| Aortic crossclamp time, min | 85 (65-116) |
| 30-d mortality, n (%) | 11 (0.3) |
| Reoperation for bleeding, n (%) | 85 (2.0) |
| Stroke, n (%) | 109 (2.6) |
| Renal failure, n (%) | 81 (1.9) |
| Dialysis, n (%) | 10 (0.2) |
| Prolonged ventilation, n (%) | 252 (6.0) |
| Heart block requiring permanent pacemaker, n (%) | 35 (0.8) |
| Readmission within 30 d, n (%) | 442 (10.6) |
Table E2.
Early postoperative outcomes of patients who underwent re-SAVR versus ViV-TAVR
| SAVR (n = 65) | ViV (n = 74) | P value | |
|---|---|---|---|
| 30-d mortality, n (%) | 4 (6.2) | 1 (1.4) | .19 |
| Stroke, n (%) | 3 (4.6) | 1 (1.4) | .34 |
| Pacemaker, n (%) | 1 (1.5) | 3 (4.1) | .62 |
| Dialysis, n (%) | 1 (1.5) | 1 (1.4) | >.99 |
| Major bleeding,∗n (%) | 1 (1.5) | 1 (1.4) | >.99 |
| Red blood cell transfusion, n (%) | 22 (33.8) | 3 (4.1) | <.001 |
SAVR, Surgical aortic valve replacement; VIV-TAVR, valve-in-valve transcatheter aortic valve replacement.
Reoperation for bleeding in a patient undergoing SAVR or major access-site bleed in a patient undergoing TAVR.
References
- 1.Goldstone A., Chiu P., Baiocchi M., Lingala B., Patrick W., Fischbein M., et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377:1847–1857. doi: 10.1056/NEJMoa1613792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isaacs A., Shuhaiber J., Salemi A., Isom O., Sedrakyan A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015;149:1262–1269.e3. doi: 10.1016/j.jtcvs.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko T., Vassileva C., Englum B., Kim S., Yammine M., Brennan M., et al. Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve-in-valve procedures. Ann Thorac Surg. 2015;100:1298–1304. doi: 10.1016/j.athoracsur.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 4.Saleem S., Ullah W., Syed M., Megaly M., Thalambedu N., Younas S., et al. Meta-analysis comparing valve-in-valve TAVR and redo-SAVR in patients with degenerated bioprosthetic aortic valve. Catheter Cardiovasc Interv. 2021;98:940–947. doi: 10.1002/ccd.29789. [DOI] [PubMed] [Google Scholar]
- 5.Sedeek A., Greason K., Sandhu G., Dearani J., Holmes D.J., Schaff H. Transcatheter valve-in-valve vs surgical replacement of failing stented aortic biological valves. Ann Thorac Surg. 2019;108:424–430. doi: 10.1016/j.athoracsur.2019.03.084. [DOI] [PubMed] [Google Scholar]
- 6.Wernly B., Zappe A., Unbehaun A., Sinning J., Jung C., Kim W., et al. Transcatheter valve-in-valve implantation (VinV-TAVR) for failed surgical aortic bioprosthetic valves. Clin Res Cardiol. 2019;108:83–92. doi: 10.1007/s00392-018-1326-z. [DOI] [PubMed] [Google Scholar]
- 7.VARC-3 Writing Committee, Généreux P., Piazza N., Alu M., Nazif T., Hahn R., et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77:2717–2746. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 8.Bienjonetti-Boudreau D., Fleury M., Voisine M., Paquin A., Chouinard I., Tailleur M., et al. Impact of sex on the management and outcome of aortic stenosis patients. Eur Heart J. 2021;42:2683–2691. doi: 10.1093/eurheartj/ehab242. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg J., DeSimone J., Kramer R., Discipio A., Russo L., Dacey L., et al. Impact of preoperative left ventricular ejection fraction on long-term survival after aortic valve replacement for aortic stenosis. Circ Cardiovasc Qual Outcomes. 2013;6:35–41. doi: 10.1161/CIRCOUTCOMES.112.965772. [DOI] [PubMed] [Google Scholar]
- 10.Pineda A., Kevin Harrison J., Kleiman N., Reardon M., Conte J., O'Hair D., et al. Clinical impact of baseline chronic kidney disease in patients undergoing transcatheter or surgical aortic valve replacement. Catheter Cardiovasc Interv. 2019;93:740–748. doi: 10.1002/ccd.27928. [DOI] [PubMed] [Google Scholar]
- 11.Yankah C., Pasic M., Musci M., Stein J., Detschades C., Siniawski H., et al. Aortic valve replacement with the mitroflow pericardial bioprosthesis: durability results up to 21 years. J Thorac Cardiovasc Surg. 2008;136:688–696. doi: 10.1016/j.jtcvs.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson W., Burr L., Miyagishima R., Germann E., Macnab J., Stanford E., et al. Carpentier-Edwards supra-annular aortic porcine bioprosthesis: clinical performance over 20 years. J Thorac Cardiovasc Surg. 2005;130:994–1000. doi: 10.1016/j.jtcvs.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Forcillo J., Pellerin M., Perrault L., Cartier R., Bouchard D., Demers P., et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg. 2013;96:486–493. doi: 10.1016/j.athoracsur.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Mykén P., Bech-Hansen O. A 20-year experience of 1712 patients with the biocor porcine bioprosthesis. J Thorac Cardiovasc Surg. 2009;137:76–81. doi: 10.1016/j.jtcvs.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 15.Anaya J., Moonsamy P., Sepucha K., Axtell A., Ivan S., Milford C., et al. Pilot study of a patient decision aid for valve choices in surgical aortic valve replacement. Ann Thorac Surg. 2019;108:730–736. doi: 10.1016/j.athoracsur.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Park C., Suri R., Burkhart H., Greason K., Dearani J., Schaff H., et al. Identifying patients at particular risk of injury during repeat sternotomy: analysis of 2555 cardiac reoperations. J Thorac Cardiovasc Surg. 2010;140:1028–1035. doi: 10.1016/j.jtcvs.2010.07.086. [DOI] [PubMed] [Google Scholar]
- 17.Sá M., Van den Eynde J., Simonato M., Cavalcanti L., Doulamis I., Weixler V., et al. Valve-in-valve transcatheter aortic valve replacement versus redo surgical aortic valve replacement: an updated meta-analysis. JACC Cardiovasc Interv. 2021;14:211–220. doi: 10.1016/j.jcin.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Thandra A., Abusnina W., Jhand A., Shaikh K., Bansal R., Pajjuru V., et al. Valve-in-valve transcatheter aortic valve replacement versus redo surgical valve replacement for degenerated bioprosthetic aortic valve: an updated meta-analysis comparing midterm outcomes. Catheter Cardiovasc Interv. 2021;97:1481–1488. doi: 10.1002/ccd.29541. [DOI] [PubMed] [Google Scholar]
- 19.Dvir D., Bourguignon T., Otto C., Hahn R., Rosenhek R., Webb J., et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388–399. doi: 10.1161/CIRCULATIONAHA.117.030729. [DOI] [PubMed] [Google Scholar]
- 20.McClure R., McGurk S., Cevasco M., Maloney A., Gosev I., Wiegerinck E., et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis. J Thorac Cardiovasc Surg. 2014;148:1931–1939. doi: 10.1016/j.jtcvs.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Al-Abcha A., Saleh Y., Boumegouas M., Prasad R., Herzallah K., Baloch Z., et al. Meta-analysis of valve-in-valve transcatheter aortic valve implantation versus redo-surgical aortic valve replacement in failed bioprosthetic aortic valve. Am J Cardiol. 2021;146:74–81. doi: 10.1016/j.amjcard.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko T., Makkar R., Krishnaswami A., Hermiller J., Greenbaum A., Babaliaros V., et al. Valve-in-surgical-valve with SAPIEN 3 for transcatheter aortic valve replacement based on Society of Thoracic Surgeons predicted risk of mortality. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.010288. [DOI] [PubMed] [Google Scholar]
- 23.Vincent F., Ternacle J., Denimal T., Shen M., Redfors B., Delhaye C., et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 2021:1043–1061. doi: 10.1161/CIRCULATIONAHA.120.048048. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann H., Daneshvar S., Fonarow G., Stebbins A., Vemulapalli S., Desai N., et al. Prosthesis–patient mismatch in patients undergoing transcatheter aortic valve replacement: from the STS/ACC TVT Registry. J Am Coll Cardiol. 2018;72:2701–2711. doi: 10.1016/j.jacc.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 25.El-Hamamsy I., Toyoda N., Itagaki S., Stelzer P., Varghese R., Williams E., et al. Propensity-matched comparison of the Ross procedure and prosthetic aortic valve replacement in adults. J Am Coll Cardiol. 2022;79:805–815. doi: 10.1016/j.jacc.2021.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Buratto E., Shi W., Wynne R., Poh C., Larobina M., O'Keefe M., et al. Improved survival after the ross procedure compared with mechanical aortic valve replacement. J Am Coll Cardiol. 2018;71:1337–1344. doi: 10.1016/j.jacc.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Skillington P., Mokhles M., Takkenberg J., Larobina M., O'Keefe M., Wynne R., et al. The Ross procedure using autologous support of the pulmonary autograft: techniques and late results. J Thorac Cardiovasc Surg. 2015;149:S46–S52. doi: 10.1016/j.jtcvs.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 28.Coylewright M., O'Neill E., Sherman A., Gerling M., Adam K., Xu K., et al. The learning curve for shared decision-making in symptomatic aortic stenosis. JAMA Cardiol. 2020;5:442–448. doi: 10.1001/jamacardio.2019.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]