Abstract
Objective
Patients with esophageal cancer may be reluctant to proceed with surgery due to high complication rates. This study aims to compare outcomes between eligible surgical candidates who proceeded with surgery versus those who refused surgery.
Methods
Characteristics and survival of patients with locally advanced (cT3N0M0, cT1-3N+M0) mid-/distal esophageal adenocarcinoma in the National Cancer Database (2006-2019) who either proceeded with or refused surgery after chemoradiotherapy were evaluated with logistic regression, Kaplan–Meier curves, and Cox proportional hazards methods.
Results
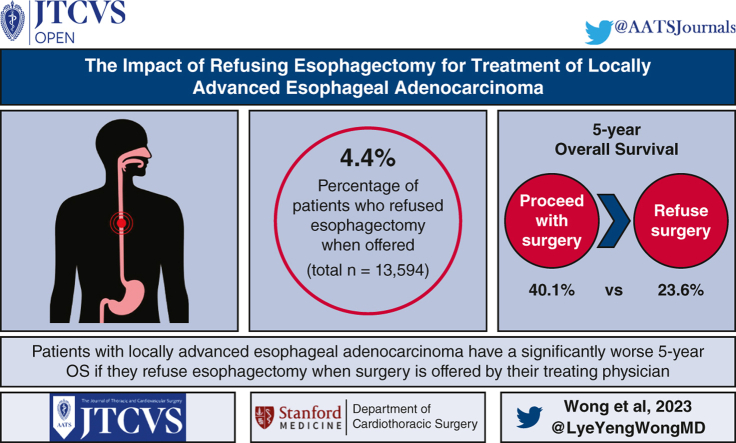
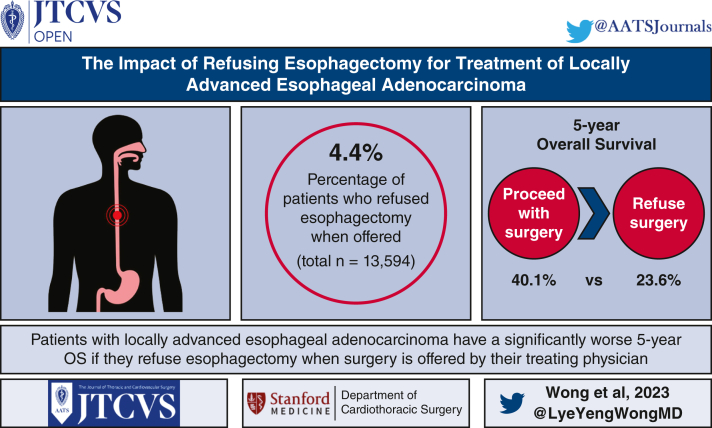
Of the 13,594 patients included in the analysis, 595 (4.4%) patients refused esophagectomy. Patients who refused surgery were older, had less distance to travel to their treatment facility, were more likely to have cN0 disease, and were more likely to be treated at a community rather than academic or integrated network program, but did not have significantly different comorbid disease distributions. On multivariable analysis, refusing surgery was independently associated with older age, uninsured, lower income, less distance to a hospital, and treatment in a community program versus an academic/research or integrated network program. Esophagectomy was associated with better survival (5-year survival 40.1% [39.2-41] vs 23.6% [19.9-27.9], P < .001) and was also independently associated with better survival in the Cox model (hazard rate, 0.78 [95% confidence interval, 0.7-0.87], P < .001).
Conclusions
The results of this study can inform selected patients with resectable esophageal adenocarcinoma that their survival will be significantly diminished if surgery is not pursued. Many factors associated with refusing surgery are non-clinical and suggest that access to or support for care could influence patient decisions.
Key Words: esophagectomy, refusal, esophageal cancer
Graphical abstract
Survival of patients who proceed versus who refuse esophagectomy when offered.
Central Message.
Patients with locally advanced esophageal adenocarcinoma have a significantly worse 5-year survival if they refuse esophagectomy when surgery is offered by their treating physician.
Perspective.
Patients and referring providers may be deterred from esophagectomy as treatment for esophageal cancer due to daunting postoperative complications. Because of this selection bias, it is difficult to provide patients with an accurate estimate of surgical benefit. Our study shows that patients eligible for surgery who refuse esophagectomy when offered have a survival that is half of their counterparts.
Esophageal adenocarcinoma is one of the fastest-growing cancers in the Western world, and the overall prognosis is somewhat grim.1,2 One reason for an overall 5-year relative survival rate of only 21.7% even in recent years of 2013 to 2019 is that less than 20% of patients are diagnosed at an early localized stage.3 Structurally, the esophagus does not have a serosal layer, leading to early spread of malignancy through the extensive submucosal plexus. Patients often also don't develop symptoms that lead to diagnosis until cancer invades into or through esophageal muscle. Many patients therefore are diagnosed with locally advanced esophageal cancer, which necessitates multimodal therapy to optimize the chance for cure.4 Although many studies have shown the benefits of chemotherapy and radiation in the treatment of esophageal cancer, surgery also plays a critical role in improving survival and achieving long-term cure.5, 6, 7
Patients unfortunately are often reluctant to proceed with potentially curative surgery. Esophagectomy is a complex procedure that has an average postoperative complication rate cited up to 50%.8 In addition, undergoing esophagectomy is associated with significant quality-of-life changes, such as reflux and changes in meal times and frequency.2 These factors may deter referring providers from encouraging patients to undergo surgical resection. Providing patients with an accurate estimate of surgical benefit is also challenging because studies that have shown the impact of surgery on survival is often fraught with selection bias secondary to unmeasured patient and tumor preoperative characteristics. As an example, patients deemed healthier and having resectable tumors may be offered surgery at a greater rate, such that observed survival benefits are inflated due to confounding factors. This study aims to provide more accurate estimates of the benefit of surgery for locally advanced esophageal adenocarcinoma by using a multi-institutional national dataset to compare outcomes between patients who proceeded with surgery versus patients deemed acceptable for but who refused surgery after completing chemoradiation.
Methods
Data Source
The National Cancer Database (NCDB) is a reputable and highly recognized resource in the United States that captures the majority of new cancer diagnoses from more than 1500 facilities yearly. The NCDB is governed by the American Cancer Society and the Commission on Cancer of the American College of Surgeons. Data from 2006 to 2019 were included in this study. Patients are deidentified in this database, so this study was exempt from the Stanford Institutional Review Board.
Patient Selection
All patients >18 years of age diagnosed with locally advanced (cT3N0M0, cT1-3N+M0) mid- to distal esophageal adenocarcinoma using Seventh edition American Joint Committee on Cancer guidelines who either proceeded with or refused surgery after chemoradiotherapy were evaluated. The NCDB provides a specific code for the following definition: “Surgery of the primary site was not performed; it was recommended by the patient's physician, but this treatment was refused by the patient, the patient's family member, or the patient's guardian. The refusal was noted in patient record,” which was used to create the cohort for this study. Patients with clinical T2N0 disease were excluded, as some of these patients proceed to surgery directly without undergoing neoadjuvant treatment and because up to 50% of these patients are inaccurately staged.9 Patients with proximal tumors in the cervical location, previous malignancies, incomplete data, and who did not pursue definitive treatment were excluded.
Perioperative Outcomes and Survival Analyses
One aim of the study was to investigate factors that influence patient decisions to proceed with surgery, which could allow more focus on specific areas in which the patient treatment decision process could be improved. We estimated variables that were independently associated with refusing surgery using multivariable logistic regression and forest plots. Variables included in this model were those that have been shown or are clinically felt to be likely to influence treatment selection and were age, sex, race, insurance status, comorbidity score, education level, income, tumor location, clinical stage, nodal status, distance to hospital, and hospital type.
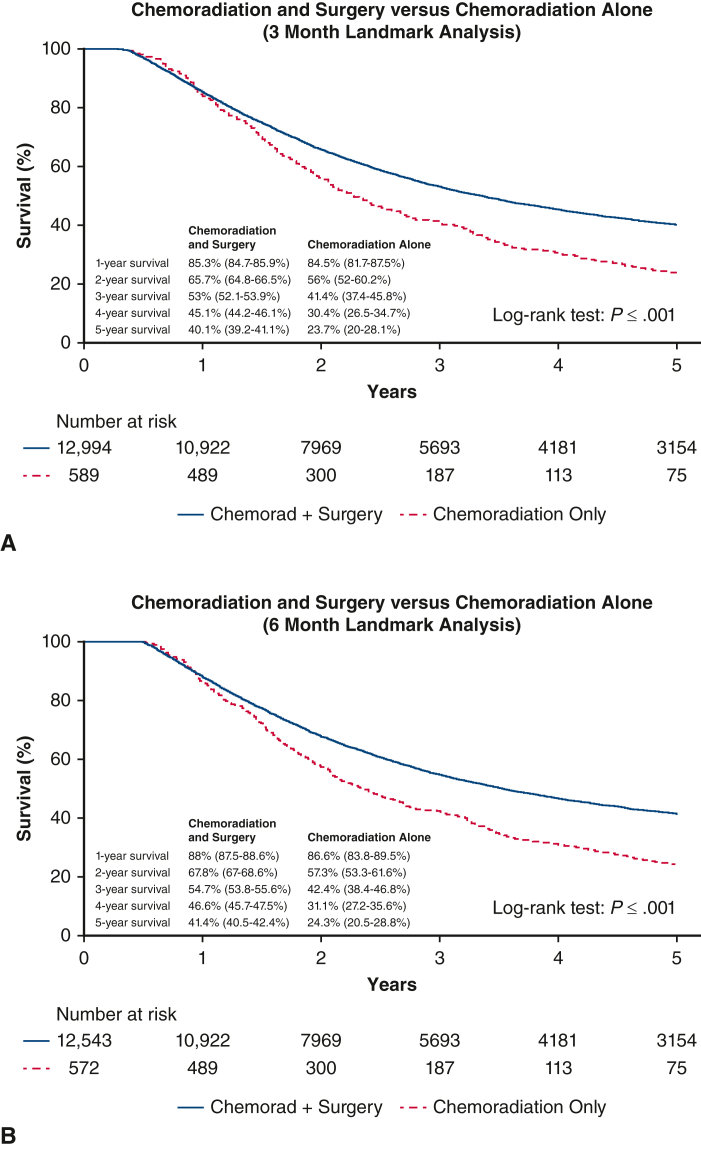
The primary hypothesis tested in the study was that patients deemed acceptable for but who refuse surgery and have chemoradiation alone have poorer survival than patients who proceeded with surgery after chemoradiation. Survival was compared between these groups using Kaplan–Meier curves and the log-rank test, with the primary end point being 5-year overall survival (OS) calculated from the date of diagnosis. In addition, the potential independent benefit of surgery was evaluated with Cox proportional hazards methods. Variables chosen a priori for inclusion in the Cox model were patient (age, sex, and comorbidities) and tumor characteristics (location, T stage, and nodal involvement) previously shown to be associated with survival, along with the study variable of interest: completion of esophagectomy. Two landmark studies in the survival analyses were also performed, where patients who survived less than 3 or 6 months were left truncated, to account for potential bias where differences in treatment-related mortality between chemoradiation alone and chemoradiation and surgery patients could bias the results such worse short-term outcomes related to either therapy regimen could falsely make the alternative therapy appear more beneficial.
Propensity Matching Analysis
Patients were matched based on propensity scores using a 1:1 nearest neighbor algorithm (R software: MatchIt-Nonparametric Preprocessing for Parametric Casual Inference) such that the chemoradiation alone who refused surgery group consisting of 595 patients and the matched chemoradiation with surgery group consisting of 595 patients. The following covariates were used for matching: age, sex, race, education, income, comorbidity profile, insurance, tumor location, T stage, nodal status, and facility type. Following propensity matching, balance was assessed between groups based on standardized differences (R software: nonrandom-stratification and matching by the propensity score). Survival between groups was assessed with the Kaplan–Meier method and log-rank test.
Statistical Analysis
The data were analyzed using R, version 3.6.1 (R Foundation for Statistical Computing). Baseline demographic and preoperative clinical characteristics between the 2 groups were compared with Wilcoxon rank-sum test for continuous variables and the Pearson χ2 test for discrete variables. The Fisher exact test was used for discrete variables with fewer than 5 outcomes.
Results
Patient Cohort and Characteristics Stratified by Surgery Versus Refused Surgery
In total, 13,594 patients met inclusion criteria, with 595 (4.4%) in the refused surgery group and 12,999 (95.6%) in the surgery group (Table 1). Patients who refused surgery were more likely to be older, with a median age 71 years (interquartile range, 64, 77) versus age 63 years (56, 69) in the surgery group (P < .001). The refused surgery group was more likely to live closer to their treatment facility (11 vs 18.2, P < .001), have no nodal disease on clinical staging (N0 rate 31.8% vs 27.3%, P = .02), and receive treatment at a community hospital (51.2% vs 32.5%, P < .001). There were no differences in univariate analysis of the groups with regard to sex, race, education level, income, insurance status, tumor location, tumor size, clinical T stage, or comorbidity status.
Table 1.
Characteristics of entire cohort, stratified by patients who refused surgery versus completed surgery for esophageal adenocarcinoma
| Variable | Refused esophagectomy (n = 595) | Esophagectomy (n = 12,999) | P value |
|---|---|---|---|
| Age, y | 71 (64, 77) | 63 (56, 69) | <.001 |
| Female sex | 75 (12.6%) | 1453 (11.2%) | .312 |
| Race | .637 | ||
| White | 570 (96.4%) | 12,531 (97.1%) | |
| Black | 9 (1.5%) | 179 (1.4%) | |
| Other | 12 (2%) | 201 (1.6%) | |
| Education above median | 312 (61.8%) | 7195 (64%) | .336 |
| Income above median | 300 (59.4%) | 7083 (63%) | .111 |
| Distance to treatment facility | 11 (4.9, 24.1) | 18.2 (7.5, 46) | <.001 |
| Location | .245 | ||
| Distal esophagus | 563 (94.6%) | 12,430 (95.6%) | |
| Mid-esophagus | 32 (5.4%) | 569 (4.4%) | |
| Tumor size, cm | .64 | ||
| 0-4.9 | 135 (58.7%) | 3409 (57.1%) | |
| 5+ | 95 (41.3%) | 2557 (42.9%) | |
| Clinical T stage | .074 | ||
| 1 | 22 (3.8%) | 295 (2.3%) | |
| 2 | 76 (13.1%) | 1602 (12.7%) | |
| 3 | 481 (83.1%) | 10,711 (85%) | |
| Clinical N stage | .02 | ||
| 0 | 189 (31.8%) | 3562 (27.4%) | |
| 1 | 406 (68.2%) | 9437 (72.6%) | |
| Insured | 577 (97.6%) | 12,614 (98.2%) | .435 |
| Facility type | <.001 | ||
| Community program | 58 (9.8%) | 547 (4.3%) | |
| Comprehensive community program | 246 (41.4%) | 3614 (28.2%) | |
| Integrated network program | 108 (18.2%) | 2328 (18.2%) | |
| Research/academic program | 182 (30.6%) | 6309 (49.3%) | |
| Charlson comorbidity score | .065 | ||
| 0 | 410 (68.9%) | 9298 (71.5%) | |
| 1 | 124 (20.8%) | 2705 (20.8%) | |
| 2+ | 61 (10.3%) | 996 (7.7%) |
Impact of Surgery on Perioperative Esophagectomy Outcomes
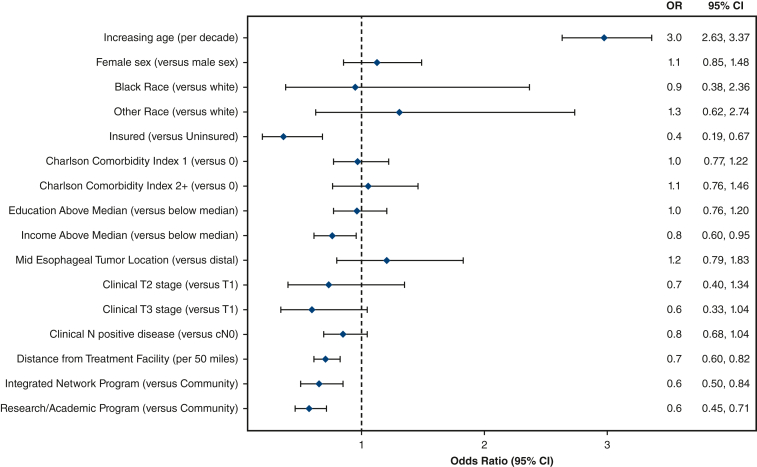
On multivariable analysis, the strongest association with refusing surgery was increasing age (odds ratio [OR], 2.98; 95% confidence interval [CI], 2.63-3.37; P < .001). Figure 1 shows the forest plot from this multivariable logistic regression. In contrast, patients who were more likely to proceed with surgery were those with insurance (OR, 2.83; 95% CI, 1.49-5.37; P = .001), income above the median (OR, 1.32; 95% CI, 1.05-1.66; P = .017), who lived farther from the hospital (OR, 1.42; 95% CI, 1.22-1.65; P < .001), and those treated in an integrated network program (OR, 1.54; 95% CI, 1.19-2.00; P = .001) or research/academic hospital (OR, 1.77; 95% CI, 1.42-2.20; P < .001) (Table 2). Variables that were not associated with the pursuit of surgery included sex, race, comorbidity status, education level, tumor location, and clinical T or N stage.
Figure 1.
Forest plot from multivariable logistic regression showing variables that are independently associated with patients refusing surgery. CI, Confidence interval.
Table 2.
Variables independently associated with patients proceeding with surgery after chemoradiation
| Variable | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Increasing age (per decade) | 0.34 (0.30-0.38) | <.001 |
| Female sex (vs male sex) | 0.89 (0.67-1.18) | .4 |
| Race (vs White) | ||
| Black | 1.06 (0.42-2.66) | .9 |
| Other | 0.77 (0.36-1.61) | .5 |
| Insured (vs uninsured) | 2.83 (1.49-5.37) | .001 |
| Charlson Comorbidity Index (vs 0) | ||
| 1 | 1.04 (0.82-1.30) | .8 |
| 2+ | 0.95 (0.68-1.32) | .8 |
| Education above median (vs below median) | 1.04 (0.83-1.31) | .72 |
| Income above median (vs below median) | 1.32 (1.05-1.66) | .017 |
| Mid-esophageal tumor location (vs distal) | 0.83 (0.55-1.26) | .4 |
| Clinical T stage (vs T1) | ||
| T2 | 1.37 (0.74-2.53) | .3 |
| T3 | 1.70 (0.96-3.01) | .07 |
| Clinical N-positive disease (vs cN0) | 1.18 (0.96-1.46) | .112 |
| Distance from treatment facility (per 50 miles) | 1.42 (1.22-1.65) | <.001 |
| Treatment facility type (vs community) | ||
| Integrated network program | 1.54 (1.19-2.00) | .001 |
| Research/academic program | 1.77 (1.42-2.2) | <.001 |
Survival Stratified by Completion of Esophagectomy
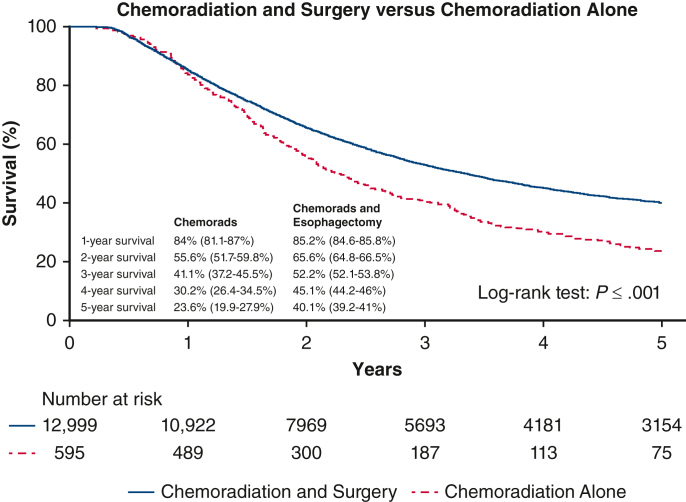
Figure 2 shows the Kaplan–Meier survival curves stratified by the group of patients who proceeded with surgery after chemoradiation versus those who refused surgery. The surgery group had a significantly better median survival of 71.2 months versus 49 months for the refused surgery group (P < .001) and 5-year OS rate of 40.1% versus 23.6%, respectively. On Cox proportional hazards model, esophagectomy was found to be independently associated with better survival with a hazards ratio (HR) 0.78 (95% CI, 0.7-0.87; P < .001) (Table 3). Being female also independently predicted improved survival (HR, 0.8; 95% CI, 0.8-0.9; P < .001), whereas increased age (HR, 1.2; 95% CI, 1.1-1.2; P < .001), clinical T3 stage (HR, 1.4; 95% CI, 1.2-1.6; P < .001), nodal positivity (HR, 1.2; 95%; CI, 1.2-1.3; P < .001), comorbidity score 1 and 2 (HR, 1.2; 95% CI, 1.1-1.2 and HR, 1.3; 95% CI, 1.2-1.4, respectively, P < .001), and having a mid-esophageal tumor (HR, 1.2; 95%, CI 1.0-1.3; P = .004) independently predicted worse survival. Landmark analyses that excluded patients who survived less than 3 or 6 months also continued to show significant better survival for the patients who had surgery (Figure E1).
Figure 2.
Kaplan–Meier survival curves showing 5-year OS with 95% confidence intervals stratified by the group of patients who proceeded with surgery after chemoradiation versus those who refused surgery.
Table 3.
Cox proportional hazards for overall survival in patients treated with induction chemoradiation with or without surgery for locally advanced esophageal cancer
| Variable | Odds ratio | 95% CI lower | 95% CI higher | P value |
|---|---|---|---|---|
| Age (per decade) | 1.2 | 1.1 | 1.2 | <.001 |
| Female (vs male) | 0.8 | 0.8 | 0.9 | <.001 |
| Clinical T stage (vs T1) | ||||
| T2 | 1.1 | 0.9 | 1.3 | .35 |
| T3 | 1.4 | 1.2 | 1.6 | <.001 |
| Clinical N positive | 1.3 | 1.2 | 1.3 | <.001 |
| Charlson comorbidity index (vs 0) | ||||
| 1 | 1.2 | 1.1 | 1.2 | <.001 |
| 2+ | 1.3 | 1.2 | 1.4 | <.001 |
| Mid-esophagus location (vs distal location) | 1.2 | 1.0 | 1.3 | .004 |
| Esophagectomy | 0.8 | 0.7 | 0.9 | <.001 |
| Adjuvant chemotherapy | 1.1 | 1.0 | 1.2 | .12 |
CI, Confidence interval.
Figure E1.
Landmark analyses showing Kaplan–Meier survival curves with 95% confidence intervals with left truncating of patients who survived less than (A) 3 months and (B) 6 months.
Propensity Score Analysis
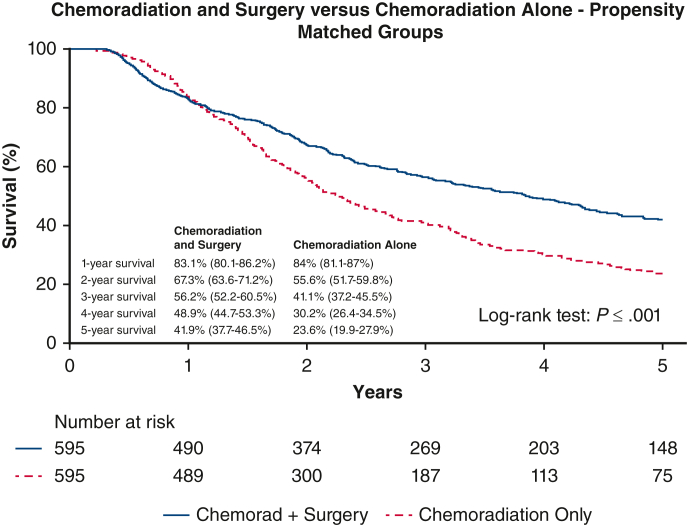
Propensity score matching led to groups that were well matched as a comprehensive cohort (Table 4). Esophagectomy after chemoradiation continued to have a statistically significant association with improved survival in the matched groups (median survival of 69.5 months vs 48.7 months [P < .001] and 5-year OS, 41.9% [95% CI, 37.7-46.5] vs 23.6% [19.9-27.9]) (Figure 3). See Figure 4 for a graphical abstract of the study.
Table 4.
Baseline characteristics of propensity-matched patients treated with chemoradiation and surgery versus chemoradiation alone
| Variable | Chemoradiation and surgery (n = 595) | Chemoradiotherapy only (n = 595) | P value | Standardized mean differences |
|---|---|---|---|---|
| Age, y, median (IQR) | 71 (65, 77) | 71 (64, 77) | .601 | 0.027 |
| Female | 59 (9.9%) | 75 (12.6%) | .169 | 0.085 |
| Race | .529 | 0.069 | ||
| White | 566 (95.6%) | 570 (96.4%) | ||
| Black | 8 (1.4%) | 9 (1.5%) | ||
| Other | 18 (3%) | 12 (2%) | ||
| Insured | 582 (98.3%) | 577 (97.6%) | .533 | 0.053 |
| Charlson comorbidity index | .78 | 0.041 | ||
| 0 | 420 (70.6%) | 410 (68.9%) | ||
| 1 | 120 (20.2%) | 124 (20.8%) | ||
| ≥2 | 55 (9.2%) | 61 (10.3%) | ||
| Income above median | 308 (59.9%) | 300 (59.4%) | .917 | 0.044 |
| Education above median | 323 (62.8%) | 312 (61.8%) | .776 | 0.048 |
| Facility type | .435 | 0.102 | ||
| Community program | 63 (10.6%) | 58 (9.8%) | ||
| Comprehensive community | 243 (41%) | 246 (41.4%) | ||
| Integrated network program | 89 (15%) | 108 (18.2%) | ||
| Research/academic | 198 (33.4%) | 182 (30.6%) | ||
| Tumor location | .214 | 0.072 | ||
| Distal esophagus | 572 (96.1%) | 563 (94.6%) | ||
| Mid-esophagus | 23 (3.9%) | 32 (5.4%) | ||
| Clinical T stage | .968 | 0.018 | ||
| T1 | 21 (3.6%) | 22 (3.8%) | ||
| T2 | 74 (12.8%) | 76 (13.1%) | ||
| T3 | 485 (83.6%) | 481 (83.1%) | ||
| Clinical N stage | .531 | 0.025 | ||
| N negative | 179 (30.1%) | 189 (31.8%) | ||
| N positive | 416 (69.9%) | 406 (68.2%) |
IQR, Interquartile range.
Figure 3.
Kaplan–Meier survival curves showing 5-year OS with 95% confidence intervals between propensity-matched patients, stratified by whether patients had esophagectomy after chemoradiation versus just chemoradiotherapy alone.
Figure 4.
Patients who refuse esophagectomy when recommended by their surgeons have worse survival. OS, Overall survival.
Discussion
Esophageal adenocarcinoma is an increasingly common malignancy that often requires multimodal treatment including chemoradiation and surgery to optimize the chance of cure.6 However, the survival benefit of performing esophagectomy in addition to chemoradiation has not been clearly delineated in previous literature due to confounding patient and tumor factors.10,11 Our study aimed to overcome this selection bias by assessing 2 groups of patients of comparable surgical candidacy to evaluate the impact of completing esophagectomy on long-term outcomes and survival by only comparing surgical patients with patients who were offered but refused surgery. Our results found that approximately 5% of patients with potentially resectable locally advanced esophageal adenocarcinoma refused recommended esophagectomy. Independent associations of refusing surgery for treatment were older age, no insurance status, income below the median, living closer to the treating hospital, and being treated in a community program versus an academic/research or integrated network program. Esophagectomy was associated almost double 5-year survival in univariate analysis and was also independently associated with better survival in the Cox model.
Health disparities leading to worse survival outcomes have been studied in oncologic literature, but the focus has historically been on demographic factors such as sex and race.12,13 In our study, we found a strong association between older age and refusal of surgery. This finding suggests that although surgeons may not discriminate based on age, patients or their referring providers may have unaddressed concerns which contribute to their reluctance to proceed with surgery. Concerns for older patients regarding their ability to tolerate surgery or whether the potential benefit of surgery is worth it to them are certainly valid, but our results can be used to at least demonstrate to them that surgery has an independent survival benefit even when age is considered.14, 15, 16
Our study also found other socioeconomic-related factors influenced treatment. Having insurance and income above the median were 2 significant findings in our study associated with the pursuit of surgery, indicating that socioeconomic disparities continue to be a contributing factor in patient decision-making. Under-resourced patients may not have the financial means to travel to comprehensive cancer centers and seek second opinions, which correlates closely to the finding in our study that those who refused surgery are more likely to live closer to their treatment facility. Thus, the onus falls on providers to provide individualized resources so that patients can make informed decisions prioritizing their health without the sway of unmeasurable social constraints. Our results show that providers should particularly examine social factors when patients refuse surgery and investigate if support can be provided that would allow patients to proceed with the therapy that was recommended.
Another significant finding that affects patients' rate of refusal for esophagectomy is the type of facility in which they seek treatment. Other studies have shown that low-volume centers are associated with greater rates of refusal, and similarly in our results, we found that community hospitals were associated with higher rates of refusal compared to academic and research centers.17 Community hospitals are generally lower resourced and may not have the multidisciplinary approach to surgical oncology that promotes multimodal treatment. Thus, this persistent finding in the literature sheds light on the gaps between different hospitals due to the relative decentralization of health care in the United States. In more rural areas where academic centers are geographically sparse, these results highlight the need for coordinated efforts to provide all patients the gold standard treatment for the treatment of esophageal cancer.
There are several limitations to this study, including the inherent limitation of conducting retrospective analyses from large databases that lack granular data. We might not have captured all patients who refused surgery, due to not having direct access to clinical data and perhaps incomplete coding by registrars. However, because we know that providers recommended esophagectomy to all patients included in the cohort, we can assume that disease progression did not occur. We also lacked data on the exact chemotherapy and radiation regimens that patients underwent, including specific agents as well as timing of chemotherapy and radiation. In addition, we do not have variables that indicate frailty of patients as a preoperative risk factor so we cannot extrapolate how that may play into the role of proceeding with or refusing surgery. The NCDB does not detail exact comorbidities, and the authors recognize that the Charlson comorbidity scoring system is suboptimal in assessing patient baseline robustness and ability to tolerate a complex surgery. Lastly, the NCDB does not report recurrence data, so our survival analyses cannot determine cause-specific survival or disease-free survival. Ultimately, we acknowledge that the results may not be a perfect representation of the current state of esophageal cancer care, as the interdependence of social factors creates confounding for which we cannot wholly account. Nevertheless, we believe that this study quantifies important gaps in esophageal adenocarcinoma care that are affecting survival outcomes. We show that a variety of patient demographic and socioeconomic factors play a role in long-term oncologic outcomes, and even with the impressive ongoing advancements in esophageal cancer care, these issues will require attention in order to continue improving the lives of patients diagnosed with this morbid disease.
Esophagectomy has been shown in the literature to be critical to the potential cure of esophageal adenocarcinoma, and in this cohort of patients with comparable surgical candidacy, we demonstrate that long-term outcomes are much worse when patients refuse surgery.7,10 The current treatment landscape for esophageal adenocarcinoma is becoming increasingly complex, in that chemotherapy is sometimes used before chemoradiation or after surgery, and immunotherapy is also increasingly used for specific patients. Therefore, the study can not necessarily be viewed as indicating that esophagectomy is needed to obtain cure in all patients, but instead to focus on the concept of patient preoperative decision-making when chemotherapy and radiation therapy has been given. By selecting patients who were all recommended to undergo esophagectomy, we attempted to remove provider bias in this process and focus instead on the association of patient factors, both clinical and nonclinical, on willingness to undergo a complex surgery. More trials are needed to study the rationale behind patient decision-making, but physicians also hold great responsibility in ensuring heath equity and access to all patients with cancer. The results of this study can be used to inform selected patients with resectable, locally advanced mid- to distal esophageal adenocarcinoma that their 5-year survival will be significantly lower if they do not pursue esophagectomy after chemoradiation. Other than patient age, the factors associated with refusing surgery are nonclinical and related to socioeconomic factors and characteristics of their treatment facilities, suggesting that access to or support for care could influence patient decisions. These data can potentially be used by both patients and clinicians when choosing treatment for locally advanced esophageal adenocarcinoma to optimize oncologic outcomes, by providing specific and accurate data regarding the consequence of refusing surgery on survival.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
References
- 1.Ajani J.A., D'Amico T.A., Bentrem D.J., Chao J., Corvera C., Das P., et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 2.Lagergren J., Smyth E., Cunningham D., Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396. doi: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 3.SEER∗Explorer An interactive website for SEER Cancer Statistics [internet]. Surveillance Research Program, National Cancer Institute; April 19, 2023. Data source(s): SEER incidence data, November 2022 submission (1975-2020), SEER 22 Registries. https://Seer.Cancer.Gov/Statistics-Network/Explorer/
- 4.Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 5.Luketich J.D., Pennathur A., Awais O., Levy R.M., Keeley S., Shende M., et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi H., Takeuchi H. Future perspectives of surgery for esophageal cancer. Ann Thorac Cardiovasc Surg. 2018;24:219–222. doi: 10.5761/atcs.ed.18-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markar S.R., Karthikesalingam A., Low D.E. Enhanced recovery pathways lead to an improvement in postoperative outcomes following esophagectomy: systematic review and pooled analysis. Dis Esophagus. 2015;28:468–475. doi: 10.1111/dote.12214. [DOI] [PubMed] [Google Scholar]
- 9.Wolfson P., Ho K.M.A., Bassett P., Haidry R., Olivo A., Lovat L., et al. Accuracy of clinical staging for T2N0 oesophageal cancer: systematic review and meta-analysis. Dis Esophagus. 2021;34:doab002. doi: 10.1093/dote/doab002. [DOI] [PubMed] [Google Scholar]
- 10.Borggreve A.S., Kingma B.F., Domrachev S.A., Koshkin M.A., Ruurda J.P., van Hillegersberg R., et al. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci. 2018;1434:192–209. doi: 10.1111/nyas.13677. [DOI] [PubMed] [Google Scholar]
- 11.Eyck B.M., van Lanschot J.J.B., Hulshof M.C.C.M., van der Wilk B.J., Shapiro J., van Hagen P., et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39:1995–2004. doi: 10.1200/JCO.20.03614. [DOI] [PubMed] [Google Scholar]
- 12.Taioli E., Wolf A.S., Camacho-Rivera M., Kaufman A., Lee D.S., Bhora F., et al. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol. 2016;113:659–664. doi: 10.1002/jso.24203. [DOI] [PubMed] [Google Scholar]
- 13.Savitch S.L., Grenda T.R., Scott W., Cowan S.W., Posey J., III, Mitchell E.P., et al. Racial disparities in rates of surgery for esophageal cancer: a study from the national cancer database. J Gastrointest Surg. 2021;25:581–592. doi: 10.1007/s11605-020-04653-z. [DOI] [PubMed] [Google Scholar]
- 14.Tang Z., Lu M., Qu C., Zhang Y., Li L., Li S., et al. Enhanced recovery after surgery improves short-term outcomes in patients undergoing esophagectomy. Ann Thorac Surg. 2022;114:1197–1204. doi: 10.1016/j.athoracsur.2021.08.073. [DOI] [PubMed] [Google Scholar]
- 15.Dezube A.R., Cooper L., Mazzola E., Dolan D.P., Lee D.N., Kucukak S., et al. Long-term outcomes following esophagectomy in older and younger adults with esophageal cancer. J Gastrointest Surg. 2022;26:1119–1131. doi: 10.1007/s11605-022-05295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwabara S., Kobayashi K., Sudo N. Outcomes of elderly patients following thoracoscopic esophagectomy for esophageal cancer. Langenbeck's Arch Surg. 2023;408:56. doi: 10.1007/s00423-023-02797-5. [DOI] [PubMed] [Google Scholar]
- 17.Salti I., Petesch T., Naffouje S.A., Kamarajah S.K., Dahdaleh F. Effect of health disparities on refusal of trimodality therapy in localized esophageal adenocarcinoma: a propensity score matched analysis of the National Cancer Database. Am Surg. 2020 doi: 10.1177/00031348221117040. [DOI] [PubMed] [Google Scholar]