Abstract
Objective
Innovative technology such as normothermic regional perfusion and the Organ Care System has expanded donation after circulatory death heart transplantation. We wanted to investigate the impact of donation after circulatory death heart procurement in concurrent lung donation and implantation at a national level.
Methods
We reviewed the United Network for Organ Sharing database for heart donation between December 2019 and March 2022. Donation after circulatory death donors were separated from donation after brain death donors and further categorized based on concomitant organ procurement of lung and heart, or heart only.
Results
A total of 8802 heart procurements consisted of 332 donation after circulatory death donors and 8470 donation after brain death donors. Concomitant lung procurement was lower among donation after circulatory death donors (19.3%) than in donation after brain death donors (38.0%, P < .001). The transplant rate of lungs in the setting of concomitant procurement is 13.6% in donation after circulatory death, whereas it is 38% in donation after brain death (P < .001). Of the 121 lungs from 64 donation after circulatory death donors, 22 lungs were retrieved but discarded (32.2%). Normothermic regional perfusion was performed in 37.3% of donation after circulatory death donors, and there was no difference in lung use between normothermic regional perfusion versus direct procurement and perfusion (20.2% and 18.8%). There was also no difference in 1-year survival between normothermic regional perfusion and direct procurement and perfusion.
Conclusions
Although national use of donation after circulatory death hearts has increased, donation after circulatory death lungs has remained at a steady state. The implantation of lungs after concurrent procurement with the heart remains low, whereas transplantation of donation after circulatory death hearts is greater than 90%. The use of normothermic regional perfusion lungs has been controversial, and we report comparable 1-year outcomes to standard donation after circulatory death lungs. Further studies are warranted to investigate the underlying mechanisms of normothermic regional perfusion on lung function.
Key Words: donation after circulatory death, lung transplantation, normothermic regional perfusion, organ donation
National trends demonstrate an increase in DCD hearts, whereas DCD lung use has plateaued.
Central Message.
DCD lungs are an underused source of donors. The use of lungs after concurrent heart procurement remains low (65%), and transplantation of hearts is greater than 90%.
Perspective.
Innovative preservation strategies have expanded the use of DCD hearts in the United States. Analysis of the UNOS database demonstrates that concurrent procurement of lungs after DCD heart procurement is low at 19%. The number of lungs transplanted after DPP versus NRP procurement strategies is not significantly different, and 1-year survival outcomes are similar.
Heart and lung transplant remains the gold standard treatment for patients with end-stage heart or lung failure.1,2 However, the number of patients on transplant waitlists continues to increase, necessitating innovative donor procurement strategies and advancements in organ preservation and evaluation technologies to expand the potential donor pool for both heart and lung transplantation.3 Although donation after circulatory death (DCD) has been implemented in the United States since 2013 for lung transplantation, it remains an underused resource of donor lungs. Furthermore, the recent introduction of DCD heart transplant in the United States4,5 raises the question of its potential impact on the use of lung allografts.
Two recovery strategies have been adopted to increase the use of DCD heart allografts in the United States: direct perfusion and procurement (DPP) using the Organ Care System and normothermic regional perfusion (NRP).6,7 Regardless of the recovery technique used, both have significantly increased the donor pool, demonstrating comparable patient outcomes to donation after brain death (DBD) heart transplants.4 In the NRP technique, after cardiac arrest, the donor undergoes sternotomy and in situ perfusion via central cannulation and extracorporeal circulation (cardiopulmonary bypass). This approach allows for recovery of not only thoracic organs but also abdominal organs that otherwise may be unsalvageable.8
For lung transplantation, the combination of the DCD donation strategy and advanced technology such as ex vivo lung perfusion (EVLP) has opened new avenues for the recovery of extended criteria donors.9,10 Studies have shown outcomes after DCD lung transplantations are comparable to those of DBD donors in the long term.11,12 However, despite its potential as an alternative to increase the number of lungs available for transplantation, DCD lung use remains underused in the United States13,14 and might worsen with the recent increase in heart DCD use.
The reasons underlying this discrepancy and the differences in concurrent lung donation are not well understood. Although the benefit of NRP and Organ Care System for heart allografts after DCD procurement has been demonstrated, little is known about the effects of NRP on lung allografts. During the NRP process, the lungs are subjected to a normothermic low perfusion state until they are weaned off cardiopulmonary bypass, which could exacerbate ischemic damage during the agonal phase. Current publications on lung transplantation after NRP are anecdotal,15 and the exact impact of these different procurement strategies on lung graft status and outcomes remains unknown.
Therefore, the aim of this study was to understand the recent national trends in lung allograft use after the initiation of DCD heart procurement in the United States and to compare the impact of different heart DCD recovery techniques (DPP vs NRP) on concurrent lung recovery. By addressing these knowledge gaps, we aimed to provide valuable insights into optimizing lung allograft use and improving outcomes in the context of DCD heart transplantation.
Patients and Methods
Clinical Data and Definitions
We conducted a retrospective review of 254,932 deceased donors between December 2019 and March 2022 using the Organ Procurement and Transplantation Network/United Network for Organ Sharing (UNOS) database, updated through March 31, 2020. This study was exempt from Institutional Review Board approval because there was no direct involvement with human subjects or identifiable personal information provided. Donor characteristics were extracted from the UNOS deceased donor database and matched with the UNOS thoracic organ database using encrypted donor identifier numbers.
NRP was defined as a heart donation with a time from asystole to crossclamp greater than or equal to 15 minutes among DCD donors.4 Graft failure was defined as failure of the transplanted organ in the UNOS, retransplantation, or patient death. The percentages of recovered, discarded, and implanted lungs were determined by calculating the number of lungs.
Statistical Methods
Summary statistics were used for recipient, donor, and transplant characteristics. Chi-square and nonparametric Wilcoxon rank-sum tests were used for comparisons between group characteristics for frequency and continuous distributions, respectively. Fisher exact tests were used when more than 20% of categorical levels had expected counts less than 5. Survival estimates were constructed using Kaplan–Meier methods, including log-log confidence intervals and log-rank tests to compare survival distributions between groups. The analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc).
Results
Study Population
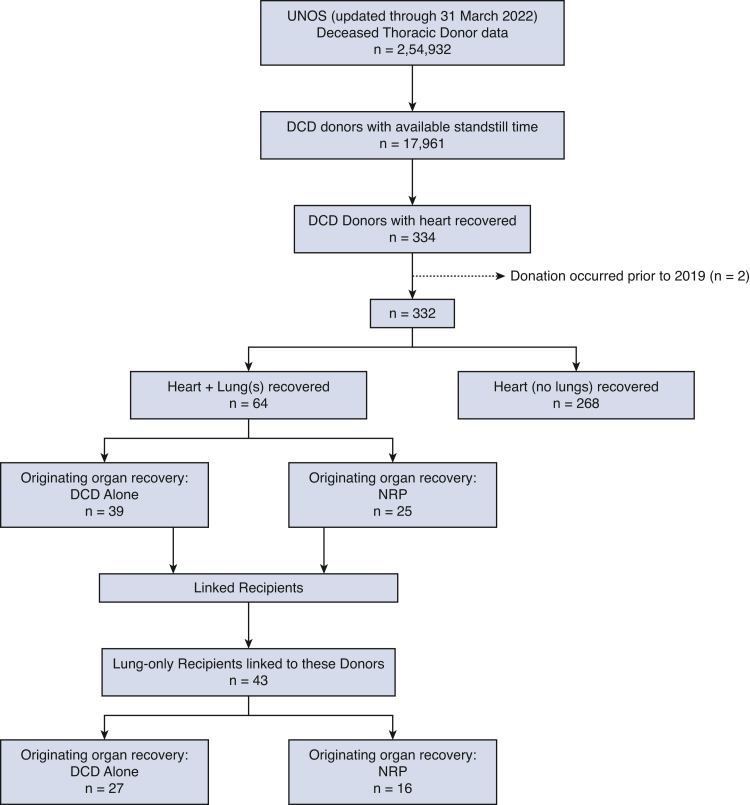
The process of selecting the study population is detailed in Figure 1. Between December 2019 and March 2022, 332 DCD heart donors and 8470 DBD heart donors were identified. Among the DCD donors, 64 had concomitant heart and lung procurements and 268 had heart procurement only. Of the 64 concomitant donations, 25 of these procurements were identified as NRP organ recoveries (37.3%).
Figure 1.
Flowchart diagram of study cohort. UNOS, United Network for Organ Sharing; DCD, donation after circulatory death; NRP, normothermic regional perfusion.
Temporal Trends in Donation After Circulatory Death Lungs
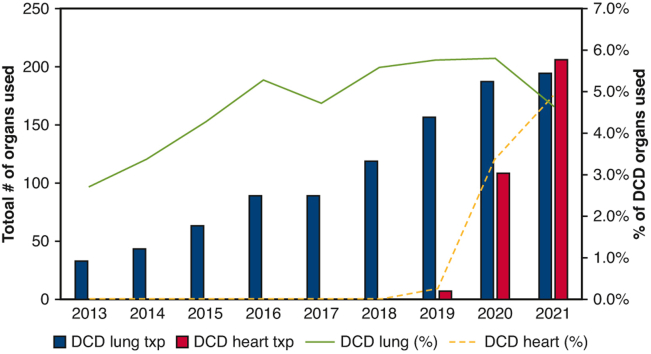
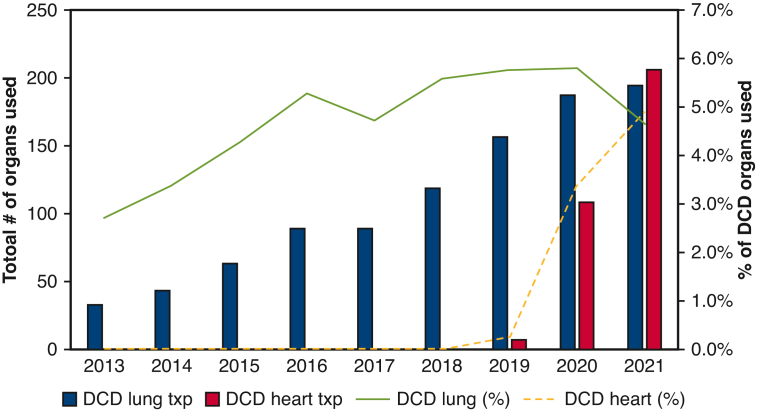
The number of DCD lung transplants increased over time, from 33 in 2013 to 195 in 2021. The national rate of DCD donor lungs used has remained relatively stable, ranging between 4.0% and 5.5%. In contrast, DCD heart procurement, implemented in the United States in December 2018, results in a total of 322 DCD heart transplants performed through 2021. The rate of heart donor per DCD donor rapid increased to 4.9% in 2021 (Figure 2).
Figure 2.
Trends of annual thoracic organ use in the United States. Percentage of transplanted heart and lung over total DCD donors are marked as lines, and the absolute numbers of transplanted organs are shown as bars.
Heart Only Versus Concomitant Heart-Lung Procurement
Concomitant heart-lung procurement was significantly lower among DCD heart procurements (64 patients, 19.3%, P < .001) than DBD heart procurements (3217 patients, 38.0%). Table 1 provides a detailed overview of the demographics based on the presence of dual thoracic organ recovery in DCD heart donors.
Table 1.
Demographic characteristics by presence of dual thoracic organ recovery in donation after circulatory death heart donors
| Characteristics | Heart only N (%) or median (IQR) (N = 268) | Heart + lung N (%) or median (IQR) (N = 64) | P value |
|---|---|---|---|
| Donor age (y) | 29.0 (23.0-35.0) | 27.0 (21.0-35.0) | .142∗ |
| Calculated donor BMI | 27.0 (24.0-31.0) | 25.0 (22.0-28.0) | <.001∗ |
| Male donor | 240 (89.6%) | 56 (87.5%) | .635† |
| Procurement type | .753† | ||
| DCD alone | 169 (63.1%) | 39 (60.9%) | |
| DCD with NRP | 99 (36.9%) | 25 (39.1%) | |
| Year organ was recovered | .291† | ||
| 2019 | 4 (1.5%) | 3 (4.7%) | |
| 2020 | 89 (33.2%) | 17 (26.6%) | |
| 2021 | 144 (53.7%) | 38 (59.4%) | |
| 2022 | 31 (11.6%) | 6 (9.4%) | |
| History of smoking | 25 (9.3%) | 2 (3.1%) | .104† |
| History of diabetes | 7 (2.7%) | 2 (3.2%) | .654‡ |
| History of hypertension | 38 (14.2%) | 7 (10.9%) | .490† |
| Standstill to clamp (min) | 11.0 (9.0-58.0) | 10.5 (8.0-68.0) | .863∗ |
| PaO2 on 1.0 FiO2 | 133.5 (91.4-245.0) | 412.0 (316.5-459.0) | <.001∗ |
| Heart transplanted | 246 (91.8%) | 58 (90.6%) | .763† |
| Lungs recovered | - | 121 | - |
| Single | - | 7 (10.9%) | |
| Bilateral | - | 57 (89.1%) | |
| Lungs discarded | - | 39 (32.2%) | - |
| Single | - | 5 | |
| Bilateral | - | 17 | |
| Lungs transplanted | - | 82 (67.8%) | - |
| Single | - | 8 | |
| Bilateral | - | 37 |
IQR, Interquartile range; BMI, body mass index; DCD, donation after circulatory death; NRP, normothermic regional perfusion.
Wilcoxon.
Chi-square.
Fisher exact.
Of the 121 lungs from 64 DCD donors, 39 lungs from 22 donors were retrieved but ultimately discarded (32.2%), consisting of 5 single lungs and 17 double lungs. Lungs were transplanted from 45 donors of 332 heart DCD donors (8 single and 37 double), resulting in a lung allograft use rate of 13.6%. This is significantly lower than the lung allograft use of 38% observed in DBD donors (P < .001). Among heart-only donors, NRP was used in 36.9% of donors (n = 99), whereas in donors who donated both heart and lungs, NRP was used in 39.1% of cases (n = 25) (P = .753).
Outcomes of Donation After Circulatory Death Lung Transplants by Procurement Method
The study found that 74 lungs were recovered after DPP and 47 lungs were recovered after DCD + NRP. There was no significant difference in the number of discarded lungs between the DPP and DCD + NRP groups (31.1% and 34%, respectively). Among the recovered lungs, 68.9% (3 single lungs and 24 bilateral lungs) were transplanted in the DPP group, and 66.0% (5 single lungs and 13 bilateral lungs) were transplanted in the DCD + NRP group. In the DCD + NRP group, only 52% of the lungs that were recovered bilaterally were actually implanted bilaterally, compared with 61% in the DPP group. However, this difference did not reach statistical significance (P = .384). No EVLP was performed after DPP, whereas it was performed in 15% of the DCD + NRP group (P = .278). The ischemic time was 7.1 hours in the DPP group and 5.6 hours in the DCD + NRP group (P = .005).
Regarding postoperative outcomes, there was no significant difference observed in postoperative length of stay, prolonged intubation (>72 hours postoperatively), reintubation rate, ECMO use at 72 hours postoperatively, and graft failure between the 2 groups. More detailed information on demographics and postoperative outcomes is shown in Tables 2 and 3.
Table 2.
Demographic characteristics by procurement type in donation after circulatory death dual thoracic organ donors
| Characteristics | DPP N (%) or median (IQR) (N = 39) | DCD with NRP N (%) or median (IQR) (N = 25) | Total DCD lung N (%) or median (IQR) (N = 64) | P value |
|---|---|---|---|---|
| Donor age (y) | 30.0 (23.0-35.0) | 21.0 (18.0-34.0) | 27.0 (21.0-35.0) | .051∗ |
| Calculated donor BMI | 25.9 (22.9-29.2) | 24.1 (21.3-26.6) | 25.0 (22.0-27.7) | .103∗ |
| Male donor | 31 (79.5%) | 25 (100.0%) | 56 (87.5%) | .018† |
| Year organ was recovered | .011† | |||
| 2019 | 3 (7.7%) | 0 (0.0%) | 3 (4.7%) | |
| 2020 | 14 (35.9%) | 3 (12.0%) | 17 (26.6%) | |
| 2021 | 21 (53.8%) | 17 (68.0%) | 38 (59.4%) | |
| 2022 | 1 (2.6%) | 5 (20.0%) | 6 (9.4%) | |
| Cause of death | .441† | |||
| Anoxia | 10 (25.6%) | 10 (40.0%) | 20 (31.3%) | |
| Cerebrovascular | 3 (7.7%) | 3 (12.0%) | 6 (9.4%) | |
| Head trauma | 25 (64.1%) | 12 (48.0%) | 37 (57.8%) | |
| History of smoking | 1 (2.6%) | 1 (4.0%) | 2 (3.2%) | .999† |
| History of diabetes | 2 (5.1%) | 0 (0.0%) | 2 (3.1%) | .516† |
| History of hypertension | 4 (10.3%) | 3 (12.0%) | 7 (10.9%) | .999† |
| Standstill to clamp (min) | 8.0 (8.0-10.0) | 72.0 (67.0-118.0) | 10.5 (8.0-68.0) | - |
| PaO2 on 100% FiO2 | 417.0 (318.0-473.0) | 400.0 (218.0-455.0) | 412 (316.5-459.0) | .554∗ |
| Heart transplanted | 34 (87.2%) | 24 (96.0%) | 58 (90.6%) | .391† |
| Lungs recovered | 74 | 47 | 121 | .999† |
| Single | 4 (10.3%) | 3 (12.0%) | 7 (10.9%) | |
| Bilateral | 35 (89.7%) | 22 (88.0%) | 57 (89.1%) | |
| Lungs discarded | 23 (31.1%) | 16 (34.0%) | 39 (32.2%) | .999† |
| Single | 3 | 2 | 5 | |
| Bilateral | 10 | 7 | 17 | |
| Lungs transplanted | 51 (68.9%) | 31 (66.0%) | 82 (67.8%) | .384† |
| Single | 3 | 5 | 8 | |
| Bilateral | 24 | 13 | 37 |
DPP, Direct procurement followed by perfusion; IQR, interquartile range; DCD, donation after circulatory death; NRP, normothermic regional perfusion; BMI, body mass index.
Wilcoxon.
Fisher exact.
Table 3.
Preoperative characteristics and outcomes by procurement type
| Characteristics | DPP N (%) or median (IQR) (N = 27) | DCD with NRP N (%) or median (IQR) (N = 16) | Total DCD lungs N (%) or median (IQR) (N = 43) | P value |
|---|---|---|---|---|
| Recipient age (y) | 61.0 (49.0-66.0) | 60.0 (50.5-64.0) | 61 (50.0-66.0) | .782∗ |
| Male gender | 19 (70.4%) | 15 (93.8%) | 34 (79.1%) | .121† |
| Lung allocation score | 39.5 (33.6-56.2) | 37.5 (34.0-42.6) | 37.9 (33.8-44.0) | .563∗ |
| Primary diagnosis | - | |||
| IPF | 6 (22.2%) | 6 (37.5%) | 12 (27.9%) | |
| COPD | 9 (33.3%) | 4 (25.0%) | 13 (30.2%) | |
| Cystic fibrosis | 1 (3.7%) | 0 (0.0%) | 1 (2.3%) | |
| COVID-19 related | 2 (7.4%) | 3 (18.8%) | 5 (11.7%) | |
| Others | 9 (33.3%) | 3 (18.8%) | 12 (27.9%) | |
| Serum creatinine (mg/dL) | 0.7 (0.6-0.9) | 0.8 (0.7-1.1) | 0.8 (0.6-0.9) | .093∗ |
| Total bilirubin (mg/dL) | 0.5 (0.3-0.6) | 0.4 (0.2-0.6) | 0.4 (0.3-0.6) | .244∗ |
| Life support at transplant | 5 (19.2%) | 2 (13.3%) | 7 (17.1%) | .999† |
| EVLP | .2781† | |||
| Yes | 4 (15.4%) | 0 (0.0%) | 4 (6.6%) | |
| No | 22 (84.6%) | 14 (100.0%) | 36 (59.0%) | |
| Missing | 1 | 2 | 3 | |
| Ischemic time (h) | 7.1 (5.9-9.1) | 5.6 (4.5-6.0) | 6.1 (5.4-8.6) | .005∗ |
| Postoperative length of stay (d) | 25.0 (16.0-47.0) | 17.5 (10.0-29.0) | 35.2 (15.0-42.5) | .112∗ |
| Intubated at 72 h postoperatively | 12 (44.4%) | 3 (18.8%) | 15 (34.9%) | .123‡ |
| ECMO at 72 h | .399† | |||
| Yes | 5 (19.2%) | 1 (7.1%) | 6 (15.0%) | |
| No | 21 (80.8%) | 13 (92.9%) | 34 (85.0%) | |
| missing | 1 | 2 | 3 | |
| Reintubation | 6 (22.2%) | 3 (18.8%) | 9 (20.9%) | .999† |
| Graft failure | 2 (7.4%) | 0 (0.0%) | 2 (4.6%) | .533† |
| Repeat hospitalization | 11 (40.7%) | 3 (18.8%) | 14 (32.5%) | .521† |
DPP, Direct procurement followed by perfusion; IQR, interquartile range; DCD, donation after circulatory death; NRP, normothermic regional perfusion; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; EVLP, ex vivo lung perfusion; ECMO, extracorporeal membrane oxygenation.
Wilcoxon.
Fisher exact.
Chi-square.
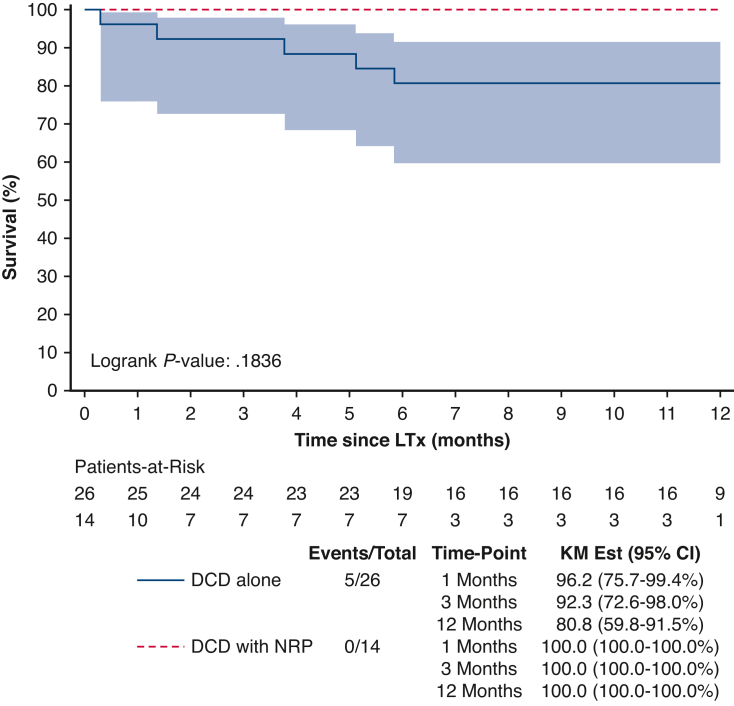
The survivals at 1 month, 3 months, and 1 year were 96.2%, 92.3%, and 80.8% in the DPP group. All recipients who received lungs from DCD donor using NRP were alive at 1 year. However, there was no significant difference in overall 1-year survival between the DPP and DCD + NRP groups (P = .184). Further details on survival and outcomes are described in Figure 3.
Figure 3.
Survival of donation after DCD transplant recipients by procurement method (1-year censor). KM, Kaplan–Meier; CI, confidence interval; DCD, donation after circulatory death; NRP, normothermic regional perfusion; LTx, lung transplantation.
Discussion
The recent Food and Drug Administration approval of ex vivo perfusion and organ care systems has sparked renewed interest in cardiac DCD organ procurement.6,16 Likewise, NRP has emerged as a promising technique to expand the donor pool while avoiding the costs associated with machine preservation.7,17 However, our analysis reveals that the concomitant heart and lung procurement and implantation rate of recovered lung allografts in DCD remains unexpectedly low compared with DBD. Our study showed that only 19% of DCD heart procurements involved concomitant lung retrieval, compared with 38% in DBD cases. Furthermore, more than 30% of the retrieved lungs from DCD donors were discarded, resulting in a low use rate of 13.6% for transplantation from concomitant thoracic organ donors, compared with more than 35% in the context of DBD.
These findings highlight a concerning disparity in the use of DCD lungs and underscore the need to better understand the underlying reasons. Although our analysis does not provide a definitive explanation, there are likely multifactorial factors contributing to this trend. Concerns regarding organ damage during the functional warm ischemia period or logistic issues related to the donation process may play a role in the underuse of DCD lungs during concomitant thoracic organ procurement.13
In a survey from the International Society for Heart and Lung Transplantation DCD working group, logistic issues were considered the most important obstacles to the development of the DCD program.18 The lack of a universally accepted protocol for antemortem interventions or comfort measures during the DCD process significantly complicates the logistics of donation, leading to reduced proactive consideration and optimization of DCD donors by organ procurement organizations.19 Additionally, the prolonged critical care stay, which can exceed 30 hours compared with DBD,20 and the uncertainty surrounding the progression of a donor to circulatory death further complicate the process. The uncertainty might have important management and financial implications, because “dry” or negative runs consume valuable time and resources,21 with DCD procurement reported to result in dry runs of approximately 40%20,22 compared with 6% to 20% in DBD.19,23 Another important challenge in using DCD lungs is the lack of comprehensive organ assessment due to the difficulty of obtaining bronchoscopies or computed tomography in patients who are not legally declared dead, as well as the inability to perform arterial blood gases during the procurement.13
The lower implantation rate of retrieved DCD organs for lung transplantation reported in this study may be due to concerns regarding potential lung damage during the procurement process. Our results are in line with other studies reporting use of DCD lungs ranging between 5%24 and 20%.19 Several factors could contribute to the underuse of DCD lungs, including the variable duration of functional warm ischemia, concerns about significant ischemia–reperfusion injury, and the perceived risk of primary graft dysfunction in this type of donation.13 There is also a theoretical concern about the potential detrimental effect of NRP on lungs. Although NRP can assist heart reperfusion, the systemic inflammatory reaction associated with extracorporeal circulation and the relatively low flow provided to the lungs during NRP might exacerbate ischemia–reperfusion damage or lead to lung edema. In our study, we observed that 28% of the bilateral lungs retrieved after NRP were discarded, and only 52% of the total were actually implanted bilaterally. In contrast, 61% of DPP lungs were implanted bilaterally. Although these differences did not reach statistical significance, the absolute number of discarded or not actually transplanted lungs after NRP may indicate a perceived degree of damage to the organ. However, it is important to note that no definitive evidence is currently available to support this hypothesis, and further preclinical studies would be needed to further elucidate these points.
Another important finding of this study is the greater than 30% rate of organs discarded after DCD. This could further corroborate the hypothesis of lung damage in the procurement process, but because no details on the reasons for nonuse of the lungs are available in the database, no further conclusion could be drawn at this stage. On the other hand, this is among the first reports in the modern era showing similar outcomes between NRP-based procurement and direct rapid procurement for lung transplantation.
A recent analysis of the Scientific Registry of Transplant Recipients database25 on the trends in DCD lung transplantation has provided insights into the trends and outcomes of DCD lung transplantation. The analysis revealed a gradual increase in the use of DCD lungs over time, particularly in conjunction with the use of EVLP. However, despite this increase, there is still a significant underuse of DCD lungs, which accounts for only 4.2% of the total lung transplant volume. Of note, there is geographical variation, with the majority of DCD organs being transplanted in patients in the northern portion of the United States. The study also showed that recipients of DCD organs tend to be older and in sicker conditions compared with those who receive organs from DBD donors. Despite the longer ischemic time, increased postoperative length of stay and higher need of dialysis, both unadjusted and adjusted survival, remain similar to DBD lung transplantation,25 consistent with 2 other previous meta-analyses.26,27
Study Limitations
Among the limitations of this work, the authors acknowledge the retrospective observational nature, which inherently exposes the study to known biases, missing data constraints, and unmeasurable confounders normally affecting retrospective database studies. Also, the large centrally managed dataset used in the analysis is subject to information and selection bias. Moreover, the lack of granularity and detailed reasons for organ unsuitability, underuse, or turn-down prevented extrapolation of possible explanations of the current findings. Additionally, the variable of NRP is not currently collected in the UNOS database, and we calculated the NRP based on the time between asystole to crossclamp. The small sample size and inability to control for other variables may have contributed to the absence of a statistically significant difference in overall survival between recipients of lungs from DCD donors using NRP compared with DCD alone. For these reasons, the conclusions reached remain cautiously speculative and hypothesis generating, but could assist new studies in this field, furthering a wider adoption of DCD in lung transplantation.
Conclusions
This study brings attention to the current low use of DCD lungs in concomitant thoracic organ procurement, despite the comparable outcomes and safety of DCD transplantation. The use of lungs after NRP remains controversial, but our findings suggest that although there are a low number of cases, the early outcomes are not significantly different from standard DCD lungs. Further studies are warranted to investigate the underlying reasons for the low use of DCD lungs and to explore strategies for expanding the donor pool. Additionally, it would be interesting to compare other health systems, such as in Europe and Australia, to see if these observations are worldwide or just in the United States. These findings provide a valuable foundation for developing protocols and making management adjustments that can increase the use of DCD donors in lung transplantation.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This work was supported by the CAO/Lung Transplant Award, Mayo Clinic internal funding to S.A.S.
References
- 1.Hsich E., Singh T.P., Cherikh W.S., Harhay M.O., Hayes D., Jr., Perch M., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-ninth adult heart transplantation report-2022; focus on transplant for restrictive heart disease. J Heart Lung Transplant. 2022;41:1366–1375. doi: 10.1016/j.healun.2022.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsich E., Singh T.P., Cherikh W.S., Harhay M.O., Hayes D., Perch M., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-ninth adult lung transplantation report-2022; focus on lung transplant recipients with chronic obstructive pulmonary disease. J Heart Lung Transplant. 2022;41:1335–1347. doi: 10.1016/j.healun.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valapour M., Lehr C.J., Skeans M.A., Smith J.M., Uccellini K., Lehman R., et al. OPTN/SRTR 2017 annual data report: lung. Am J Transplant. 2019;19:404–484. doi: 10.1111/ajt.15279. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q., Emerson D., Megna D., Osho A., Roach A., Chan J., et al. Heart transplantation using donation after circulatory death in the United States. J Thorac Cardiovasc Surg. 2023;165:1849–1860.e6. doi: 10.1016/j.jtcvs.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrsam J.P., Benden C., Immer F.F., Inci I. Current status and further potential of lung donation after circulatory death. Clin Transplant. 2021;35:e14335. doi: 10.1111/ctr.14335. [DOI] [PubMed] [Google Scholar]
- 6.Langmuur S.J.J., Amesz J.H., Veen K.M., Bogers A.J.J.C., Manintveld O.C., Taverne Y.J.H.J. Normothermic ex situ heart perfusion with the organ care system for cardiac transplantation: a meta-analysis. Transplantation. 2022;106:1745–1753. doi: 10.1097/TP.0000000000004167. [DOI] [PubMed] [Google Scholar]
- 7.Vandendriessche K., Tchana-Sato V., Ledoux D., Degezelle K., Rex S., Neyrinck A., et al. Transplantation of donor hearts after circulatory death using normothermic regional perfusion and cold storage preservation. Eur J Cardiothorac Surg. 2021;60:813–819. doi: 10.1093/ejcts/ezab139. [DOI] [PubMed] [Google Scholar]
- 8.Schurink I.J., de Goeij F.H., Habets L.J., van de Leemkolk F.E., van Dun C.A., Oniscu G.C., et al. Salvage of declined extended-criteria DCD livers using in situ normothermic regional perfusion. Ann Surg. 2022;276:e223–e230. doi: 10.1097/SLA.0000000000005611. [DOI] [PubMed] [Google Scholar]
- 9.Divithotawela C., Cypel M., Martinu T., Singer L.G., Binnie M., Chow C.W., et al. Long-term outcomes of lung transplant with ex vivo lung perfusion. JAMA Surg. 2019;154:1143–1150. doi: 10.1001/jamasurg.2019.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad N.K., Pasrija C., Talaie T., Krupnick A.S., Zhao Y., Lau C.L. Ex vivo lung perfusion: current achievements and future directions. Transplantation. 2021;105:979–985. doi: 10.1097/TP.0000000000003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruttens D., Martens A., Ordies S., Verleden S.E., Neyrinck A.P., Vos R., et al. Short- and long-term outcomes after lung transplantation from circulatory-dead donors: a single-center experience. Transplantation. 2017;101:2691–2694. doi: 10.1097/TP.0000000000001678. [DOI] [PubMed] [Google Scholar]
- 12.Van Raemdonck D., Keshavjee S., Levvey B., Cherikh W.S., Snell G., Erasmus M., et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant. 2019;38:1235–1245. doi: 10.1016/j.healun.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Villavicencio M.A., Axtell A.L., Spencer P.J., Heng E.E., Kilmarx S., Dalpozzal N., et al. Lung transplantation from donation after circulatory death: United States and single-center experience. Ann Thorac Surg. 2018;106:1619–1627. doi: 10.1016/j.athoracsur.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Cypel M., Yeung J., Donahoe L., Yasufuku K., Wang A., Pietroski R., et al. Outcomes of lung transplantation at a Canadian center using donors declined in the United States. J Thorac Cardiovasc Surg. 2022;164:1661–1668.e1. doi: 10.1016/j.jtcvs.2021.11.098. [DOI] [PubMed] [Google Scholar]
- 15.Urban M., Castleberry A.W., Markin N.W., Chacon M.M., Strah H.M., Um J.Y., et al. Successful lung transplantation with graft recovered after thoracoabdominal normothermic perfusion from donor after circulatory death. Am J Transplant. 2022;22:294–298. doi: 10.1111/ajt.16806. [DOI] [PubMed] [Google Scholar]
- 16.D'Alessandro D.A., Wolfe S.B., Osho A.A., Drezek K., Prario M.N., Rabi S.A., et al. Hemodynamic and clinical performance of hearts donated after circulatory death. J Am Coll Cardiol. 2022;80:1314–1326. doi: 10.1016/j.jacc.2022.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman J.R., McMaster W.G., Rali A.S., Rahaman Z., Balsara K., Absi T., et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant. 2021;40:1408–1418. doi: 10.1016/j.healun.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Cypel M., Levvey B., Van Raemdonck D., Erasmus M., Dark J., Mason D., et al. Lung transplantation using controlled donation after circulatory death donors: trials and tribulations. J Heart Lung Transplant. 2016;35:146–147. doi: 10.1016/j.healun.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Siddique A., Urban M., Strah H., Herber K., Morrow L., Loor G., et al. Controlled DCD lung transplantation: circumventing imagined and real barriers-time for an international taskforce? J Heart Lung Transplant. 2022;41:1198–1203. doi: 10.1016/j.healun.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Saxena P., Zimmet A.D., Snell G., Levvey B., Marasco S.F., McGiffin D.C. Procurement of lungs for transplantation following donation after circulatory death: the Alfred technique. J Surg Res. 2014;192:642–646. doi: 10.1016/j.jss.2014.07.063. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z., Gerull W.D., Gauthier J.M., Meyers B.F., Kozower B.D., Patterson G.A., et al. Shipping lungs greater distances increases costs without cutting waitlist mortality. Ann Thorac Surg. 2020;110:1691–1697. doi: 10.1016/j.athoracsur.2020.04.086. [DOI] [PubMed] [Google Scholar]
- 22.Costa J., Shah L., Robbins H., Raza K., Sreekandth S., Arcasoy S., et al. Use of lung allografts from donation after cardiac death donors: a single-center experience. Ann Thorac Surg. 2018;105:271–278. doi: 10.1016/j.athoracsur.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Puri V., Hachem R.R., Frye C.C., Harrison M.S., Semenkovich T.R., Lynch J.P., et al. Unintended consequences of changes to lung allocation policy. Am J Transplant. 2019;19:2164–2167. doi: 10.1111/ajt.15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Just released: newsletter transplant 2019–European Directorate for the Quality of Medicines & HealthCare–EDQM. European Directorate for the Quality of Medicines & HealthCare. https://www.edqm.eu/en/-/just-released-newsletter-transplant-2019
- 25.Bobba C.M., Whitson B.A., Henn M.C., Mokadam N.A., Keller B.C., Rosenheck J., et al. Trends in donation after circulatory death in lung transplantation in the United States: impact of era. Transpl Int. 2022;35 doi: 10.3389/ti.2022.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palleschi A., Rosso L., Musso V., Rimessi A., Bonitta G., Nosotti M. Lung transplantation from donation after controlled cardiocirculatory death. Systematic review and meta-analysis. Transplant Rev (Orlando) 2020;34 doi: 10.1016/j.trre.2019.100513. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J., Chen B., Liao H., Wang Z., Lyu M., Man S., et al. The comparable efficacy of lung donation after circulatory death and brain death: a systematic review and meta-analysis. Transplantation. 2019;103:2624–2633. doi: 10.1097/TP.0000000000002888. [DOI] [PubMed] [Google Scholar]