Abstract
Objective
The choice to operate on moderate tricuspid regurgitation (TR) during mitral surgery is challenging owing to limited mid-term data. We assess whether concomitant tricuspid operations improve mid-term quality of life, morbidity, or mortality.
Methods
An institutional database identified mitral surgery recipients with moderate TR at the time of surgery from 2010 to 2019. Patients were stratified by the presence of a concomitant tricuspid operation. Quality of life at the last follow-up was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ-12). Morbidity was compared using the χ2 test, Mann-Whitney U test, and Student t test. Survival was analyzed with Kaplan-Meier estimation.
Results
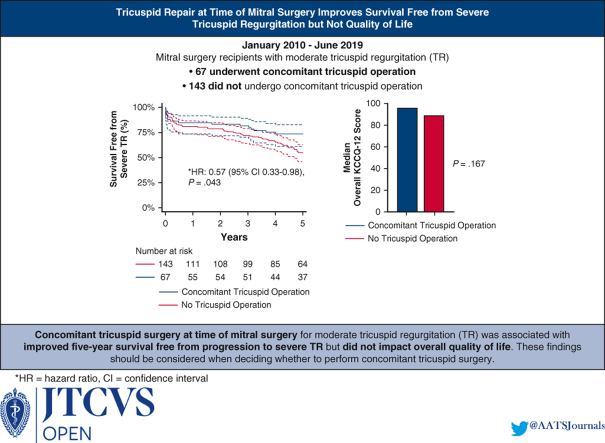
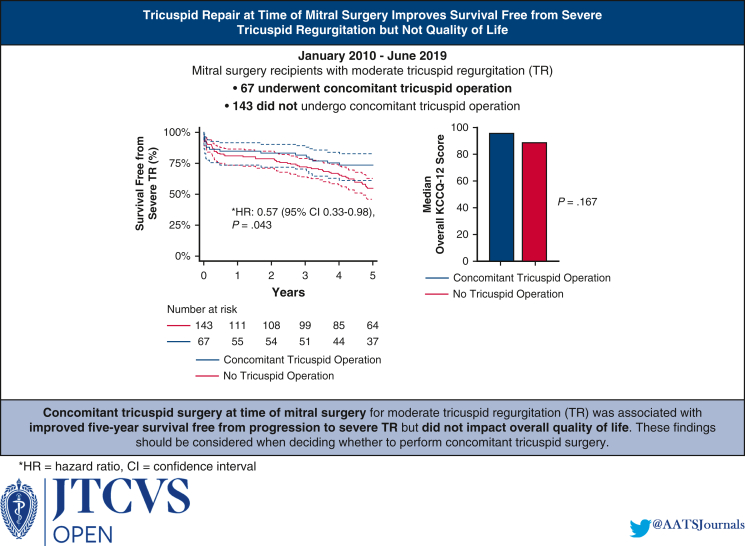
Of 210 mitral surgery recipients, 67 (31.9%) underwent concomitant tricuspid surgery. The concomitant tricuspid surgery cohort had greater preoperative dialysis use (10.5% vs 3.5%; P = .043) but similar age, New York Heart Association class, and cardiac surgery history relative to the nonconcomitant cohort (P > .05 for all). The concomitant tricuspid surgery cohort had a longer cardiopulmonary bypass time (144 minutes vs 122 minutes; P = .005) but a similar rate of mitral repair (P = .220). Postoperative KCCQ-12 scores reflected high quality of life in both cohorts (95.1 vs 89.1; P = .167). The concomitant tricuspid surgery cohort trended toward a higher perioperative pacemaker placement rate (22.8% vs 12.7%; P = .088) but were less likely to develop severe TR (0.0% vs 13.0%; P = .004). Overall survival was comparable between the 2 cohorts at 1 year (84.9% vs 81.6%; P = .628) and 5 years (73.5% vs 57.9%; P = .078). Five-year survival free from severe TR was higher in the concomitant cohort (73.5% vs 54.3%; P = .032).
Conclusions
Concomitant tricuspid surgery for moderate TR is associated with increased 5-year survival free from severe TR but not with increased quality of life.
Key Words: mitral surgery, tricuspid regurgitation, quality of life
Graphical abstract
Survival free from severe tricuspid regurgitation in mitral surgery recipients.
Central Message.
Concomitant tricuspid surgery for moderate tricuspid regurgitation is associated with longer 5-year survival free from severe tricuspid regurgitation but not with improved mid-term quality of life.
Perspective.
The choice to perform tricuspid surgery for moderate tricuspid regurgitation (TR) at the time of mitral surgery requires an individualized assessment of the procedure's risks and benefits. Our study assessed the impact of concomitant tricuspid surgery on mid-term quality of life and found no association, despite showing an association between tricuspid surgery and survival free from severe TR.
Moderate or worse tricuspid regurgitation (TR) is found in 23% to 30% of patients undergoing mitral surgery and may develop in up to 25% of patients who had less than moderate TR preoperatively.1, 2, 3 Moderate or-worse TR is independently associated with mortality, heart failure hospitalization, and heart failure symptoms.4, 5, 6 Although tricuspid surgery is standard of care for patients with severe and/or symptomatic TR, its use in patients with moderate TR at the time of left-sided valve surgery is controversial.7,8
In the United States, roughly 31% of patients with moderate TR at time of mitral surgery undergo tricuspid repair.9 Variation in management is related in part to a dearth of data on mid-term and long-term outcomes. A recent Cardiothoracic Surgery Network (CTSN) randomized trial assessing concomitant tricuspid surgery at time of mitral surgery demonstrated reduced TR progression, increased heart block, and no survival benefit at 2 years in the concomitant tricuspid surgery group.10 This study did not characterize the impact of concomitant tricuspid surgery on mid- and long-term morbidity, mortality, and quality of life. Additionally, the trial assessed patients with both moderate and less than moderate TR when perhaps only those with moderate TR are expected to benefit substantially from a tricuspid operation. Finally, the CTSN trial focused strictly on patients with degenerative mitral disease, potentially limiting the applicability of its conclusions to patients with other mitral etiologies. The mid-term risk-benefit profile of concomitant tricuspid surgery in all mitral surgery patients with moderate TR remains unclear.
This study aimed to characterize the effects of concomitant tricuspid surgery on mid-term morbidity, mortality, and quality of life in patients with moderate TR at time of mitral surgery.
Methods
Data Sources and Patient Population
A single institution's Society of Thoracic Surgeons (STS) database was used to identify all adult (age ≥18 years) patients with moderate TR who underwent mitral surgery between January 1, 2010, through June 13, 2019. TR severity was determined from transesophageal echocardiography performed immediately prior to surgery. Transesophageal echocardiography was used rather than transthoracic echocardiography, as the latter were performed for a wide variety of indications and patient clinical status, were obtained at different times preoperatively, and were less consistently available. Only patients with moderate TR as opposed to mild to moderate or moderate to severe were included. Patients with endocarditis were excluded.
The institutional STS database is maintained prospectively and updated retrospectively with follow-up data from an electronic medical record review. Mortality data are supplemented and confirmed with National Death Index records. Data on long-term complications were obtained via thorough a review of electronic medical records, including records generated outside of the health system at which patients underwent mitral surgery. Quality of life data were generated via a phone interview offered to each living patient at last follow-up.
The interview included the Kansas City Cardiomyopathy Questionnaire (KCCQ-12) and 2 additional questions: “Knowing what you know now, would you have your mitral surgery done again?” and “Is there anything else you want to share about your surgery experience?” Interviews were conducted by 4 investigators who were not involved in patient care. The KCCQ-12 was administered because of its high reliability, sensitivity to clinical change, and common use in trials assessing TR interventions.11, 12, 13 The KCCQ-12 was used here solely for the purpose of research.
Surgical Technique
Mitral valves were repaired rather than replaced whenever feasible. Mitral repair typically entailed ring annuloplasty with leaflet resection and/or chordal reconstruction. The choice of valve prosthesis and the decision to perform a concomitant tricuspid procedure were determined by the clinical care team. Tricuspid valves were repaired whenever feasible using rigid annuloplasty, at the surgeon's discretion.
Statistical Analysis
Patients were stratified into 2 cohorts by the presence of concomitant tricuspid surgery. Baseline characteristics, as well as postoperative morbidity, quality of life, and mortality data are reported for all patients. Morbidity outcomes include stroke, heart block necessitating permanent pacemaker placement, and readmission for heart failure. Quality of life outcomes included overall KCCQ-12 score, scores of the 4 KCCQ-12 domains (physical limitation, symptom frequency, quality of life, and social limitation), Likert scale responses to a question regarding whether they would undergo mitral surgery again, and a thematic analysis of patients' answers to a free response question about their overall surgical experience. Overall KCCQ-12 score and its domain scores are scored from 0 to 100, with scores of 75 to 100 representing “good to excellent” quality of life.11 Mortality outcomes included actuarial in-hospital mortality and Kaplan-Meier survival estimates at 1, 2, and 5 years.
Categorical variables are expressed as count (frequency), and continuous variables are expressed as median (interquartile range). Comparisons between cohorts were performed using the χ2 test for categorical variables with group sample size >5, the Fisher exact test for categorical variables with sample size ≤5, the unpaired 2-sample Student t-tests for parametrically distributed continuous variables, and the Kruskal-Wallis test for nonparametrically distributed continuous variables. Parametricity was assessed for each continuous variable using the Shapiro-Wilk test. Overall survival and survival free from severe TR were assessed with Kaplan-Meier estimation and compared between cohorts using a log-rank test.
Cox regression was used to determine hazards for 5-year mortality and for a composite of 5-year mortality and progression to severe TR. Univariable regression analysis was performed with the following independent variables: concomitant tricuspid operation, advanced age, male sex, diabetes, chronic lung disease, dialysis dependence, peripheral arterial disease, cerebrovascular disease, history of previous cardiac intervention, New York Heart Association (NYHA) class III or IV, preoperative inotrope use within 48 hours of surgery, systolic pulmonary artery pressure, urgent or emergent operation status, presence of concomitant coronary artery bypass grafting, and concomitant thoracic aortic surgery. Multivariable regression models were generated from the listed covariates using backward stepwise regression with a cutoff of P < .20. Missing data were excluded from analysis rather than imputed. All statistical analyses were performed using STATA/MP 17.0 software (StataCorp).
Ethics
This study was deemed by the University of Pennsylvania institutional review board to be not human subject research (protocol 850803; approved March 4, 2022). The need for patient written informed consent was waived. Verbal consent was obtained prior to administration of the quality of life survey.
Results
Of 8186 patients in our institution's mitral surgery database, 210 met our inclusion criteria. Among the mitral surgery recipients with moderate TR who underwent surgery between 2010 and 2019, 67 (31.9%) underwent concomitant tricuspid surgery and 143 (68.1%) did not.
Baseline Characteristics
The median age of the included patients was 70 years (Table 1). The majority (n = 123; 58.6%) were female, had degenerative mitral disease (n = 114; 54.3%), and had functional TR (n = 182; 86.7%). The concomitant tricuspid surgery cohort had a higher rate of preoperative dialysis requirement compared with the non-tricuspid surgery cohort (10.5% vs 3.5%; P = .043). The 2 cohorts did not differ with respect to mitral or tricuspid disease etiology, ejection fraction, NYHA classification, or history of previous cardiac interventions (P > .05 for all). Missingness for all baseline characteristics was ≤1.0% except for pulmonary artery systolic pressure (Table E1).
Table 1.
Baseline characteristics of mitral surgery recipients with moderate tricuspid regurgitation
| Variable | Overall (N = 210) | No TV operation (N = 143) | Concomitant TV operation (N = 67) | P value |
|---|---|---|---|---|
| Age median (IQR) | 70 (62-78) | 69 (63-78) | 73 (61-78) | .866 |
| Female sex, n (%) | 123 (58.6) | 89 (62.2) | 34 (50.8) | .115 |
| BMI, kg/m2, median (IQR) | 26.4 (23.4-29.6) | 26.2 (23.5-30.1) | 26.4 (22.7-29.4) | .734 |
| Race, n (%) | .723 | |||
| White | 165 (78.6) | 113 (79.0) | 52 (77.6) | |
| Black | 28 (13.3) | 18 (12.6) | 10 (14.9) | |
| Mitral etiology, n (%) | .103 | |||
| Degenerative | 114 (54.3) | 71 (49.7) | 43 (64.2) | |
| Failed repair or replacement | 40 (19.1) | 29 (20.3) | 11 (16.4) | |
| Rheumatic | 21 (10.0) | 15 (10.5) | 6 (9.0) | |
| Ischemic | 16 (7.6) | 14 (9.8) | 2 (3.0) | |
| Mitral regurgitation, n (%) | ||||
| ≥Moderate | 182 (86.7) | 124 (86.7) | 58 (86.6) | .977 |
| Severe | 116 (55.2) | 83 (58.0) | 33 (49.3) | .233 |
| Mitral stenosis, n (%) | 59 (28.1) | 42 (29.4) | 17 (25.4) | .548 |
| Tricuspid etiology | .130 | |||
| Functional | 182 (86.7) | 127 (88.8) | 55 (82.1) | |
| Rheumatic | 11 (5.2) | 5 (3.5) | 6 (9.0) | |
| Failed repair or replacement | 6 (2.9) | 4 (2.8) | 2 (3.0) | |
| LVEF, %, median (IQR) | 59.0 (48.0-65.0) | 60.0 (47.5-65.0) | 58.0 (50.0-65.0) | .900 |
| PASP, mm Hg, median (IQR) | 51.5 (42.0-65.0) | 52.0 (43.0-66.0) | 51.0 (41.0-59.5) | .189 |
| NYHA class III or IV, n (%) | 141 (67.8) | 100 (70.9) | 41 (61.2) | .161 |
| Comorbidities, n (%) | ||||
| Hypertension | 162 (77.4) | 112 (78.3) | 50 (74.6) | .552 |
| Atrial fibrillation | 137 (65.2) | 94 (65.7) | 43 (64.2) | .825 |
| Chronic lung disease | 47 (22.4) | 30 (21.0) | 17 (25.4) | .476 |
| Diabetes | 47 (22.4) | 29 (20.3) | 18 (26.9) | .286 |
| Dialysis | 12 (5.7) | 5 (3.5) | 7 (10.5) | .043 |
| Prior cardiac intervention | 115 (54.8) | 82 (57.3) | 33 (49.3) | .272 |
| Prior mitral surgery | 40 (19.1) | 29 (20.3) | 11 (16.4) | .507 |
| Prior TV surgery | 6 (2.9) | 4 (2.8) | 2 (3.0) | .939 |
| Prior CABG | 19 (9.1) | 15 (10.5) | 4 (6.0) | .287 |
| Prior PCI | 40 (19.1) | 30 (20.1) | 10 (14.9) | .298 |
| Prior PPM | 35 (16.7) | 25 (17.5) | 10 (14.9) | .643 |
| Laboratory values, median (IQR) | ||||
| Creatinine, mg/dL | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) | .519 |
| WBC count, 109/L | 6.9 (5.8-8.7) | 7.0 (6.0-8.6) | 6.6 (5.6-8.7) | .444 |
| Hematocrit, % | 38.0 (33.0-41.0) | 38.0 (34.0-41.0) | 37.0 (32.0-40.0) | .126 |
Bold type denotes P < .05. TV, Tricuspid valve; IQR, interquartile range; BMI, body mass index; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; NYHA, New York Heart Association; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; PPM, permanent pacemaker placement; WBC, white blood cell.
Operative Characteristics
Nearly one-half of all mitral surgery recipients underwent mitral repair (n = 103; 49.1%) and one-third (n = 70; 33.3%) underwent bioprosthetic mitral replacement (Table 2). Non-tricuspid concomitant procedures were common, with roughly one-quarter of all patients undergoing a Maze procedure and/or aortic valve surgery (n = 56 and 50, respectively). The vast majority of patients with a concomitant tricuspid operation (n = 64; 95.5%) underwent tricuspid repair. As expected, the concomitant tricuspid cohort had longer cardiopulmonary bypass times (144 minutes vs 122 minutes; P = .005) and cross-clamp times (108 minutes vs 90 minutes; P = .012). Rates of mitral repair, non-tricuspid concomitant procedures, and a right thoracotomy approach did not differ between the 2 cohorts (P > .05 for all). Missingness for all operative characteristics was <1.0% (Table E2).
Table 2.
Operative characteristics of mitral surgery recipients with moderate tricuspid regurgitation
| Variable | Overall | No TV operation | Concomitant TV operation | P value |
|---|---|---|---|---|
| Urgent or emergent, n (%) | 57 (27.1) | 35 (24.5) | 22 (32.8) | .204 |
| Mitral operation, n (%) | ||||
| Repair | 103 (49.1) | 66 (46.2) | 37 (55.2) | .220 |
| Bioprosthetic replacement | 70 (33.3) | 51 (35.7) | 19 (28.4) | .295 |
| Mechanical replacement | 37 (17.6) | 26 (18.2) | 11 (16.4) | .754 |
| Tricuspid operation, n (%) | ||||
| Repair | 64 (30.5) | - | 64 (95.5) | - |
| Bioprosthetic replacement | 2 (1.0) | - | 2 (3.0) | - |
| Mechanical replacement | 1 (0.5) | - | 1 (1.5) | - |
| CPB time, min, median (IQR) | 129.0 (97.0-178.0) | 122.0 (88.0-174.0) | 144.0 (112.0-109.0) | .005 |
| Cross-clamp time, min, median (IQR) | 98.0 (69.0-132.0) | 90.0 (62.0-131.0) | 108.0 (81.0-137.0) | .012 |
| Right thoracotomy, n (%) | 33 (15.7) | 25 (17.5) | 8 (11.0) | .304 |
| Robot-assisted, n (%) | 3 (1.4) | 2 (1.4) | 1 (1.5) | .957 |
| Non-TV concomitant procedure, n (%) | ||||
| Maze | 56 (26.7) | 36 (25.2) | 20 (29.9) | .475 |
| Aortic valve surgery | 50 (23.8) | 35 (24.5) | 15 (22.4) | .741 |
| CABG | 35 (16.7) | 24 (16.8) | 11 (16.4) | .947 |
| IABP | 22 (10.5) | 12 (8.4) | 10 (14.9) | .150 |
| Thoracic aortic procedure | 7 (3.3) | 4 (2.8) | 3 (4.5) | .682 |
| ECMO | 3 (1.4) | 2 (1.4) | 1 (1.5) | .957 |
| VAD | 1 (0.5) | 1 (0.7) | 0 (0.0) | .493 |
Bold type denotes P < .05. TV, Tricuspid valve; CPB, cardiopulmonary bypass; IQR, interquartile range; CABG, coronary artery bypass graft; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device.
In the patients who underwent mitral repair, the most common techniques used were ring annuloplasty (n = 96; 95.1%), triangular resection (n = 21; 20.4%), and artificial chordae placement (n = 11; 10.7%). The median mitral ring/band size was 28. Among patients who underwent tricuspid repair, the most frequently used technique was ring annuloplasty (n = 58; 92.1%). The median tricuspid ring/band size was 30. Among all patients who underwent concomitant tricuspid surgery, tricuspid surgery was intended in only 38 (56.7%), as determined by an explicit mention in surgeon's preoperative notes.
The mitral surgeries were performed by a total of 18 surgeons. The 5 surgeons who performed the highest volume of surgeries in this study accounted for roughly 75% of the study's operations. Among these 5 highest-volume surgeons, the rate of concomitant tricuspid operation for moderate TR ranged from 8.3% to 50.0%.
Morbidity
The median overall duration of follow-up for all included patients was 57.9 months and did not differ between the cohorts (68.0 months for the concomitant tricuspid surgery cohort vs 56.4 months for the non-tricuspid surgery cohort; P = .665) (Table 3). The 2 cohorts had similar in-hospital outcomes with comparable hospital length of stay (9.0 days vs 9.0 days; P = .668), stroke rate (1.5% vs 2.1%; P = .765), and rate of discharge to home (64.2% vs 63.6%; P = .939). The concomitant tricuspid surgery cohort trended toward a higher rate of new permanent pacemaker placement during index admission (22.8% vs 12.7%; P = .088).
Table 3.
Morbidity, mortality, and quality of life of mitral surgery recipients with moderate tricuspid regurgitation
| Variable | Overall | No TV operation | Concomitant TV operation | P value |
|---|---|---|---|---|
| Morbidity | ||||
| In-hospital | ||||
| Hospital LOS, d, median (IQR) | 9.0 (7.0-14.0) | 9.0 (7.0-134.0) | 9.0 (7.0-13.0) | .668 |
| Initial ICU LOS, h, median (IQR) | 72.4 (43.3-121.0) | 72.0 (41.0-120.0) | 76.8 (48.0-142.9) | .587 |
| Initial ventilator time, h, median (IQR) | 15.1 (7.9-24.5) | 15.1 (8.0-24.0) | 15.0 (7.0-47.1) | .981 |
| New PPM, n (%) | 28 (16.0) | 15 (12.7) | 13 (22.8) | .088 |
| Reoperation for bleeding, n (%) | 9 (4.3) | 8 (5.6) | 1 (1.5) | .171 |
| Stroke, n (%) | 4 (1.9) | 3 (2.1) | 1 (1.5) | .765 |
| Discharged home, n (%) | 134 (63.8) | 91 (63.6) | 43 (64.2) | .939 |
| Overall | ||||
| Follow-up time, mo, median (IQR) | 57.9 (27.6-97.3) | 56.4 (25.2-99.0) | 68.0 (36.4-97.3) | .665 |
| Stroke, n (%) | 15 (7.1) | 12 (8.4) | 3 (4.5) | .305 |
| Gastrointestinal bleed, n (%) | 22 (10.5) | 15 (10.5) | 7 (10.5) | .993 |
| New PPM, n (%) | 43 (24.6) | 27 (22.9) | 16 (28.1) | .455 |
| ≥1 heart failure readmission, n (%) | 35 (18.1) | 25 (18.8) | 10 (16.7) | .722 |
| New AF postdischarge, n (%) | 18 (27.7) | 12 (27.3) | 6 (28.6) | .913 |
| Ventricular arrythmia necessitating defibrillation or cardioversion, n (%) | 4 (1.0) | 2 (1.4) | 2 (3.0) | .433 |
| Supraventricular arrythmia necessitating cardioversion, n (%) | 32 (15.2) | 19 (13.3) | 13 (19.4) | .250 |
| Tricuspid intervention, n (%) | 3 (1.4) | 1 (0.7) | 2 (3.0) | .193 |
| Mitral reintervention, n (%) | 4 (1.9) | 3 (2.1) | 1 (1.5) | .765 |
| Latest echocardiogram, n (%) | ||||
| LVEF, %, median (IQR) | 57.5 (49.0-62.5) | 57.5 (50.0-62.5) | 55.0 (45.0-62.5) | .539 |
| ≥Moderate TR, n (%) | 24 (15.7) | 24 (24.0) | 0 (0.0) | <.001 |
| Severe TR, n (%) | 13 (8.5) | 13 (13.0) | 0 (0.0) | .004 |
| Mortality | ||||
| Death before discharge, n (%) | 17 (8.1) | 10 (7.0) | 7 (10.5) | .392 |
| 1-y survival estimate, median (IQR) | 82.7 (76.8-87.2) | 81.6 (74.2-87.1) | 84.9 (73.7-91.6) | .628 |
| 2-y survival estimate, median (IQR) | 80.7 (74.6-85.4) | 79.4 (71.8-85.2) | 83.3 (71.9-90.4) | .569 |
| 5-y survival estimate | 62.7 (55.5-69.1) | 57.9 (49.0-65.8) | 73.5 (60.9-82.7) | .078 |
| Quality of life | ||||
| KCCQ-12 overall score, median (IQR) | 92.4 (76.2-97.9) | 89.1 (71.9-95.8) | 95.1 (80.2-100) | .167 |
| Physical limitation domain, median (IQR) | 100.0 (83.3-100) | 100.0 (83.3-100.0) | 100.0 (87.5-100.0) | .314 |
| Symptom frequency domain, median (IQR) | 91.7 (75.0-100.0) | 91.7 (70.8-100.0) | 95.8 (83.3-100.0) | .126 |
| Quality of life domain, median (IQR) | 87.5 (75.0-100.0) | 87.5 (75.0-100.0) | 87.5 (75.0-100.0) | .445 |
| Social limitation domain, median (IQR) | 100.0 (83.3-100.0) | 100.0 (83.3-100.0) | 100.0 (100.0-100.0) | .435 |
Bold type denotes P < .05. TV, Tricuspid valve; LOS, length of stay; IQR, interquartile range; ICU, intensive care unit; PPM, permanent pacemaker; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; TR, tricuspid regurgitation; KCCQ, Kansas City Cardiomyopathy Questionnaire.
During the full follow-up period, the 2 cohorts did not differ in rates of stroke (4.5% vs 8.4%; P = .305), new pacemaker placement (28.1% vs 22.9%; P = .455), or the need for at least 1 heart failure readmission (16.7% vs 18.8%; P = .722). Both cohorts had few mitral reinterventions (1.0% vs 2.1%; P = .765) and few new tricuspid interventions after the index surgery (3.0% vs 0.7%; P = .193). The concomitant tricuspid surgery cohort had a lower rate of severe TR at the last follow-up (0.0% vs 13.0%; P = .003). Missingness for all morbidity variables was <1.0% (Table E3).
Mortality and Survival Free from Severe TR
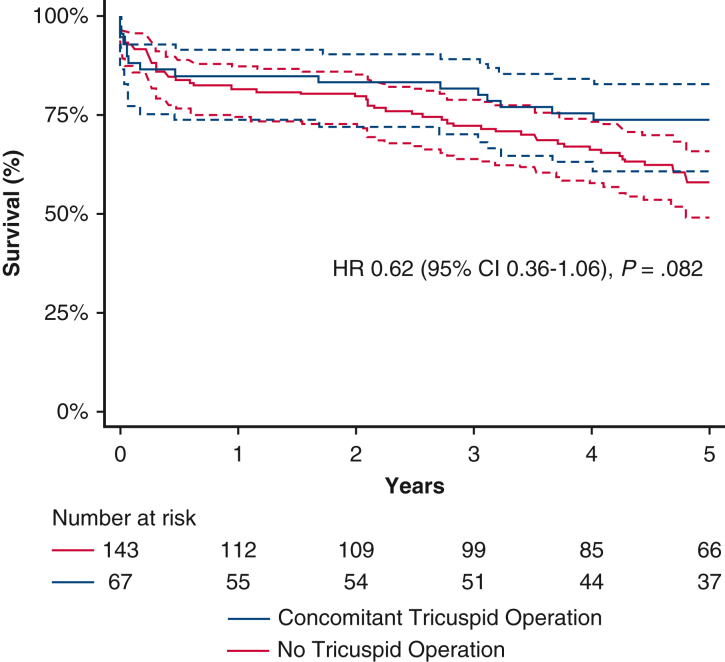
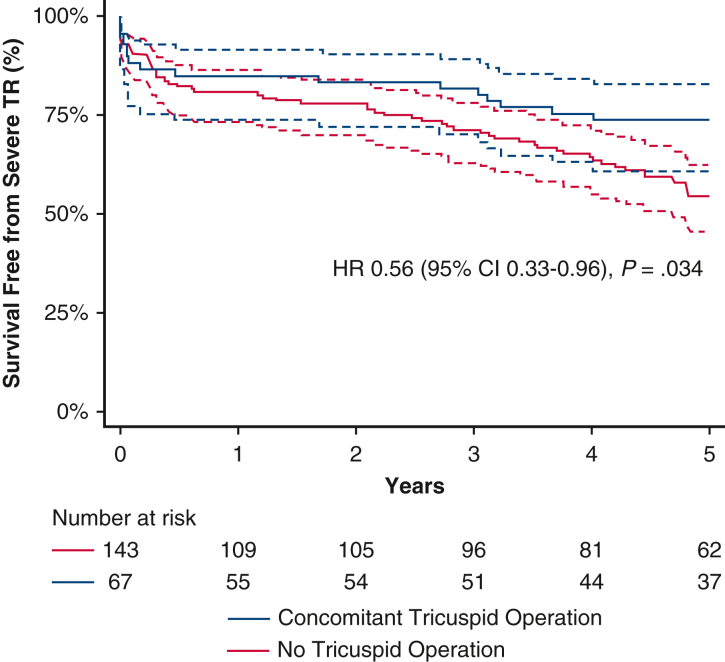
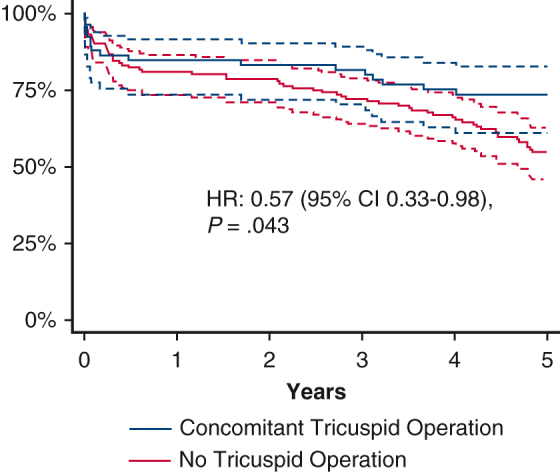
In-hospital mortality was 8.1% among all included patients and did not differ between the 2 cohorts (10.5% vs 7.0%; P = .392). The 2 cohorts had comparable survivals at 1 year (84.9% vs 81.6%; P = .628) and 2 years (83.3% vs 79.4%; P = .569). The concomitant tricuspid surgery cohort trended toward higher 5-year survival (73.5% vs 57.9%; P = .078) (Figure 1) and had higher survival free from severe TR at 5 years (73.5% vs 54.3%; P = .032) (Figure 2).
Figure 1.
Kaplan-Meier survival estimate of mitral surgery recipients with moderate tricuspid regurgitation, stratified by the presence of concomitant tricuspid operation. 95% CIs are displayed with dashed lines. HR, Hazard ratio; CI, confidence interval.
Figure 2.
Kaplan-Meier estimate of survival free of progression to severe tricuspid regurgitation among mitral surgery recipients with moderate tricuspid regurgitation, stratified by the presence of concomitant tricuspid surgery. 95% CIs are indicated by the dashed lines. TR, Tricuspid regurgitation; HR, hazard ratio; CI, confidence interval.
On univariable Cox regression, the concomitant tricuspid operation had a hazard ratio (HR) for 5-year mortality of 0.62 (95% confidence interval [CI], 0.36-1.06; P = .082); however, on univariable Cox regression for 5-year mortality free from severe TR, the HR was 0.56 (95% CI, 0.33-0.96; P = .034). Multivariable regression for 5-year mortality free from severe TR showed an HR of 0.45 (95% CI, 0.23-0.89; P = .023). The other risk factors for 5-year mortality free from severe TR on multivariable regression were advanced age, decreasing ejection fraction, need for dialysis, peripheral arterial disease, and use of inotropes within 48 hours of surgery (P < .05 for all).
Quality of Life
Of the 112 patients alive at last follow-up, 69 (61.2%) participated in a phone interview that entailed completing the KCCQ-12 survey and discussing their surgical experience. Among these patients, the interview participation rate was comparably high in the concomitant tricuspid surgery cohort (n = 26; 60.5%) and non-tricuspid surgery cohort (n = 43; 62.3%). The median overall KCCQ-12 score among all survey respondents was high at 92.4 (interquartile range, 76.2-97.9) and was comparable in the concomitant tricuspid surgery cohort and the non-tricuspid surgery cohort (95.1 vs 89.1; P = .167). Both cohorts had similar scores in the 4 KCCQ-12 domains of physical limitation, symptom frequency, quality of life, and social limitation (P > .05 for all).
Among all survey respondents, 56 (82.4%) reported that they would be “extremely likely” to undergo mitral surgery again, knowing what they know now. Eight (11.8%) responded that they would be “quite a bit likely” to have their surgery again, 3 (4.4%) responded that they would be “moderately likely,” and 1 (1.5%) responded that they would be “not at all likely.” The concomitant tricuspid surgery cohort was no more likely than the non-tricuspid surgery cohort to report being “extremely likely” to undergo the operation again (76.9% vs 85.7%; P = .355).
Thematic analysis of free responses to question about patients' overall surgical experience revealed the following common themes among all patients: gratitude for care/happiness with overall outcome (n = 19), decreased quality of life/ability to perform activities of daily living after surgery (n = 5), increased quality of life/ability to perform activities of daily living after surgery (n = 4), and frustration with complications (n = 3).
Discussion
This retrospective study assessing the management of moderate TR at time of mitral surgery demonstrated that concomitant tricuspid surgery was associated with increased 5-year survival free of progression to severe TR. Concomitant tricuspid surgery recipients showed a trend toward increased 5-year survival despite perhaps being sicker at baseline, but with an increased (albeit not statistically significant) risk of early postoperative need for permanent pacemaker placement. Concomitant tricuspid surgery was not associated with increased mid-term quality of life, which was high regardless of whether patients underwent concomitant tricuspid surgery.
Our present study is the first to assess the effect of concomitant tricuspid surgery on mid-term quality of life among mitral surgery recipients with moderate TR. Our findings regarding quality of life are consistent with those of the recent CTSN randomized trial, which found that concomitant tricuspid surgery at time of mitral surgery did not impact quality of life at 2 years.10 Unlike the CTSN trial, our study included only patients with moderate TR—who represented approximately one-third of the CTSN trial patients—and assessed quality of life at roughly 5 years postoperatively. Also unlike in the CTSN trial, we focused on a more medically and surgically complex population, with 68% of patients having NYHA class III or IV symptoms, 46% having non-degenerative mitral disease etiologies and 27% presenting for urgent or emergent operations. While our conclusions regarding quality of life must be tempered by our modest survey response rate, our findings may inform surgical management and mid-term prognostication for complex mitral surgery patients with moderate TR.
It is surprising that concomitant tricuspid surgery did not affect quality of life, given that it reduced progression to severe TR, which itself is associated with significant heart failure symptoms and impaired functional outcomes.6 Explanations for this discrepancy may include lower survey response rates among those with worse disease and confounding variables that counteract the quality of life gains afforded by reducing TR, such as increased renal disease in the concomitant tricuspid surgery cohort. It is also possible that following resolution of mitral disease, residual TR does not have a significant impact on quality of life. Large randomized studies are needed to validate the finding that concomitant tricuspid surgery has no impact on mid-term quality of life in patients with moderate TR.
Our finding that concomitant tricuspid surgery improves 5-year survival free of severe TR is perhaps more intuitive. Tricuspid surgery for moderate TR not only should mitigate TR progression but also may reverse right ventricular adverse remodeling and reduce the sequelae of right heart failure.14, 15, 16 Whether these effects can improve long-term survival for tricuspid surgery recipients remains unknown. Our study showed a trend toward increased 5-year survival in tricuspid surgery recipients (73.5% vs 57.9%; P = .078), but it was underpowered to demonstrate a significant difference between groups. A recent meta-analysis assessing the effects of concomitant tricuspid surgery at time of left-sided valve surgery for either severe TR or mild-to-moderate TR with coexistent tricuspid annular dilation or right heart failure found that concomitant tricuspid surgery decreased cardiac-related mortality at a 6-year follow-up.17 These findings conflict with the results of a recent propensity-matched nationwide registry study that showed no 5-year survival benefit from concomitant tricuspid surgery at the time of mitral surgery.18 These discordant results are likely explained in part by differences in patient selection. Our study focused exclusively on patients with moderate TR, under the hypothesis that they would be more likely to benefit from concomitant tricuspid surgery than those with less than moderate TR. It is likely that some patients with moderate TR are more likely than others to benefit from tricuspid surgery. Patients with risk factors for TR progression after mitral surgery—such as surgical atrial fibrillation ablation, increased indexed tricuspid annular diameter, and ischemic or rheumatic heart disease—perhaps are the best candidates for concomitant tricuspid repair and should be separately evaluated in further studies.19,20 Further studies also may benefit from stratifying patients by more specific TR etiologies, such as atrial functional TR versus nonatrial functional TR, as these distinctions likely affect prognosis.21
Our finding of a high postoperative permanent pacemaker placement rate in both treatment groups may be explained by the high rate of mitral valve replacement in our study, as well as advanced patient age, ischemic heart disease, and concomitant aortic valve and coronary artery bypass graft surgeries, all known risk factors for permanent pacemaker placement.22 Although there was a trend toward an increased need for early postoperative permanent pacemaker placement in the concomitant tricuspid surgery group, this difference in the need for pacemakers vanishes when comparing groups over the entire postoperative period. This suggests that the increased risk of injury to the conduction system during tricuspid surgery should have less influence on surgical decision making in a patient population that is already at high risk of eventually requiring a pacemaker.
Although the data presented herein should inform a surgeon's decision to manage moderate TR at time of mitral surgery, it is worth noting that TR management is evolving. The TRILUMINATE randomized trial recently demonstrated that tricuspid edge-to-edge repair for patients with severe, symptomatic TR reduces TR severity and improves quality of life at 1 year but does not impact survival or heart failure hospitalization rates relative to medical therapy.12 Other studies assessing transcatheter tricuspid replacement and annular reconstruction implants have demonstrated safety and modest reduction of TR severity.23,24 It is possible that as transcatheter interventions improve, it will be reasonable to defer concomitant tricuspid surgery for a subset of patients with moderate TR, high surgical risk, and anatomy amenable to transcatheter intervention. Further studies may be necessary to compare the outcomes of delayed transcatheter management for moderate TR versus upfront surgical management.
Our study's limitations are attributable to its retrospective design, modest sample size, and focus on a single institution. Determinations of TR severity were subject to the judgment of individual echocardiographers rather than indexed to volume status in a standardized fashion and thus potentially systematically underestimated owing to the effects of general anesthesia. Other echocardiographic features that may influence the decision to intervene on the tricuspid valve, such as tricuspid annulus size, were not reported because of inconsistent inclusion in medical records. The decision to operate on tricuspid valves was made on a case-by-case basis and might have been subject to surgeon bias. Long-term complication rates might have been significantly underestimated from patients following up at institutions that do not share electronic medical records with the institution at which their mitral surgery was performed. Modest sample size may have led to type II errors when comparing group baseline characteristics, such as rates of ischemic and severe mitral regurgitation, and outcomes such as the need for a permanent pacemaker and stroke. Additionally, overall perioperative mortality at our institution was high because of patient complexity, which might have limited our ability to isolate the influence of tricuspid intervention on long-term survival.
Overall, our findings lead us to conclude that concomitant tricuspid surgery for moderate TR at the time of mitral repair may improve 5-year survival free of progression to severe TR without a significant impact on quality of life (Figure 3). Surgeons should consider these mid-term data while tailoring their decision to perform concomitant tricuspid surgery to each patient's individual risk-benefit profile.
Figure 3.
Graphical abstract summarizing the study methods, results, and implications. HR, Hazard ratio; CI, confidence interval.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/management-of-moderate-tricuspid-regurgitation-during-mitral-surgery-does-concomitant-tricuspid-surgery-impact-quality-of-life.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
Table E1.
Missingness for Table 1
| Variable | Overall, n (%) | No TV operation, n (%) | Concomitant TV operation, n (%) |
|---|---|---|---|
| Age | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Female sex | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BMI (kg/m2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Race | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mitral etiology | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mitral regurgitation | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mitral stenosis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tricuspid etiology | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| LVEF (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PASP (mm Hg) | 36 (17.1) | 25 (17.5) | 11 (16.4) |
| NYHA class 3 or 4 | 2 (1.0) | 2 (1.4) | 0 (0.0) |
| Comorbidities | |||
| Hypertension | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Atrial fibrillation | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chronic lung disease | 1 (0.5) | 1 (0.7) | 0 (0.0) |
| Diabetes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dialysis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior cardiac intervention | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior mitral surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior TV surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior CABG | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior PCI | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior PPM | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Laboratory values | |||
| Creatinine (mg/dL) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| WBC count (109/L) | 1 (0.5) | 1 (0.7) | 0 (0.0) |
| Hematocrit (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
TV, Tricuspid valve; BMI, body mass index; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; NYHA, New York Heart Association; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; PPM, permanent pacemaker placement; WBC, white blood cell.
Table E2.
Missingness for Table 2
| Variable | Overall, n (%) | No TV operation, n (%) | Concomitant TV operation, n (%) |
|---|---|---|---|
| Urgent or emergent | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mitral operation | |||
| Repair | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bioprosthetic replacement | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mechanical replacement | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tricuspid operation | |||
| Repair | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bioprosthetic replacement | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mechanical replacement | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CPB time (min) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cross-clamp time (min) | 1 (0.5) | 0 (0.0) | 1 (1.5) |
| Right thoracotomy | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Robot-assisted | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-tricuspid concomitant procedures | |||
| Maze | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aortic valve surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CABG | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IABP | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Thoracic aortic procedure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ECMO | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| VAD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
TV, Tricuspid valve; CPB, cardiopulmonary bypass; CABG, coronary artery bypass graft; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device.
Table E3.
Missingness for Table 3
| Variable | Overall, n (%) | No TV operation, n (%) | Concomitant TV operation, n (%) |
|---|---|---|---|
| Morbidity | |||
| In-hospital | |||
| Hospital LOS (d) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Initial ICU LOS (h) | 1 (0.5) | 0 (0.0) | 1 (1.5) |
| Initial ventilator time (h) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| New PPM | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Reoperation for bleeding | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stroke | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discharged home | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Overall | |||
| Follow-up (mo) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stroke | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal bleed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| New PPM | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥1 heart failure readmission | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| New AF postdischarge | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ventricular arrythmia necessitating defibrillation or cardioversion | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Supraventricular arrythmia necessitating cardioversion | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tricuspid intervention | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mitral reintervention | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Latest echocardiogram | |||
| LVEF (%) | 50 (23.8) | 38 (26.6) | 12 (17.9) |
| ≥ Moderate TR | 54 (25.8) | 41 (28.7) | 13 (19.4) |
| Severe TR | 57 (27.1) | 43 (30.1) | 14 (20.9) |
| Mortality | |||
| Death before discharge | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1-y survival estimate | N/A | N/A | N/A |
| 4-y survival estimate | N/A | N/A | N/A |
| Quality of life | |||
| KCCQ-12 overall score | 142 (67.6) | 101 (70.6) | 41 (61.1) |
| Physical limitation domain | 152 (72.4) | 107 (74.8) | 45 (67.2) |
| Symptom frequency domain | 141 (67.1) | 100 (69.9) | 41 (61.1) |
| Quality of life domain | 141 (67.1) | 100 (69.9) | 41 (61.1) |
| Social limitation domain | 143 (68.1) | 101 (70.6) | 42 (62.7) |
TV, Tricuspid valve; LOS, length of stay; ICU, intensive care unit; PPM, permanent pacemaker; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; TR, tricuspid regurgitation; KCCQ, Kansas City Cardiomyopathy Questionnaire.
References
- 1.De Bonis M., Lapenna E., Sorrentino F., La Canna G., Grimaldi A., Maisano F., et al. Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg. 2008;33:600–606. doi: 10.1016/j.ejcts.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Matsunaga A., Duran C.M. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. 2005;112:I453–I457. doi: 10.1161/CIRCULATIONAHA.104.524421. [DOI] [PubMed] [Google Scholar]
- 3.Kwak J.J., Kim Y.J., Kim M.K., Kim H.K., Park J.S., Kim K.H., et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008;155:732–737. doi: 10.1016/j.ahj.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Wang N., Fulcher J., Abeysuriya N., McGrady M., Wilcox I., Celermajer D., et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. 2019;40:476–484. doi: 10.1093/eurheartj/ehy641. [DOI] [PubMed] [Google Scholar]
- 5.Messika-Zeitoun D., Verta P., Gregson J., Pocock S.J., Boero I., Feldman T.E., et al. Impact of tricuspid regurgitation on survival in patients with heart failure: a large electronic health record patient-level database analysis. Eur J Heart Fail. 2020;22:1803–1813. doi: 10.1002/ejhf.1830. [DOI] [PubMed] [Google Scholar]
- 6.Bartko P.E., Arfsten H., Frey M.K., Heitzinger G., Pavo N., Cho A., et al. Natural history of functional tricuspid regurgitation: implications of quantitative Doppler assessment. JACC Cardiovasc Imaging. 2019;12:389–397. doi: 10.1016/j.jcmg.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., III, Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 8.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 9.Badhwar V., Rankin J.S., He M., Jacobs J.P., Furnary A.P., Fazzalari F.L., et al. Performing concomitant tricuspid valve repair at the time of mitral valve operations is not associated with increased operative mortality. Ann Thorac Surg. 2017;103:587–593. doi: 10.1016/j.athoracsur.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Gammie J.S., Chu M.W.A., Falk V., Overbey J.R., Moskowitz A.J., Gillinov M., et al. Concomitant tricuspid repair in patients with degenerative mitral regurgitation. N Engl J Med. 2022;386:327–339. doi: 10.1056/NEJMoa2115961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spertus J.A., Jones P.G., Sandhu A.T., Arnold S.V. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542. [DOI] [PubMed] [Google Scholar]
- 12.Sorajja P., Whisenant B., Hamid N., Naik H., Makkar R., Tadros P., et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388:1833–1842. doi: 10.1056/NEJMoa2300525. [DOI] [PubMed] [Google Scholar]
- 13.Kodali S., Hahn R.T., George I., Davidson C.J., Narang A., Zahr F., et al. Transfemoral tricuspid valve replacement in patients with tricuspid regurgitation: TRISCEND study 30-day results. JACC Cardiovasc Interv. 2022;15:471–480. doi: 10.1016/j.jcin.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Patlolla S.H., Schaff H.V., Greason K.L., Pochettino A., Daly R.C., Frye R.L., et al. Early right ventricular reverse remodeling predicts survival after isolated tricuspid valve surgery. Ann Thorac Surg. 2021;112:1402–1409. doi: 10.1016/j.athoracsur.2021.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand P.B., Koppers G., Verbrugge F.H., Mullens W., Vandervoort P., Dion R., et al. Tricuspid annuloplasty concomitant with mitral valve surgery: effects on right ventricular remodeling. J Thorac Cardiovasc Surg. 2014;147:1256–1264. doi: 10.1016/j.jtcvs.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Benedetto U., Melina G., Angeloni E., Refice S., Roscitano A., Comito C., et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg. 2012;143:632–638. doi: 10.1016/j.jtcvs.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Pagnesi M., Montalto C., Mangieri A., Agricola E., Puri R., Chiarito M., et al. Tricuspid annuloplasty versus a conservative approach in patients with functional tricuspid regurgitation undergoing left-sided heart valve surgery: a study-level meta-analysis. Int J Cardiol. 2017;240:138–144. doi: 10.1016/j.ijcard.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Olsthoorn J.R., Heuts S., Houterman S., Roefs M., Maessen J.G., Nia P.S. Cardiothoracic Surgery Registration Committee of the Netherlands Heart Registration. Does concomitant tricuspid valve surgery increase the risks of minimally invasive mitral valve surgery? A multicentre comparison based on data from The Netherlands Heart Registration. J Card Surg. 2022;37:4362–4370. doi: 10.1111/jocs.17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand P.B., Overbey J.R., Zeng X., Levine R.A., Ailawadi G., Acker M.A., et al. Progression of tricuspid regurgitation after surgery for ischemic mitral regurgitation. J Am Coll Cardiol. 2021;77:713–724. doi: 10.1016/j.jacc.2020.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsi V., Raftopoulos L., Aggeli C., Vlasseros I., Felekos I., Tousoulis D., et al. Tricuspid regurgitation after successful mitral valve surgery. Interact Cardiovasc Thorac Surg. 2012;15:102–108. doi: 10.1093/icvts/ivs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlotter F., Dietz M.F., Stolz L., Kresoja K.P., Besler C., Sannino A., et al. Atrial functional tricuspid regurgitation: novel definition and impact on prognosis. Circ Cardiovasc Interv. 2022;15:e011958. doi: 10.1161/CIRCINTERVENTIONS.122.011958. [DOI] [PubMed] [Google Scholar]
- 22.Helmers M.R., Shin M., Iyengar A., Arguelles G.R., Mays J., Han J.J., et al. Permanent pacemaker implantation following mitral valve surgery: a retrospective cohort study of risk factors and long-term outcomes. Eur J Cardiothorac Surg. 2021;60:140–147. doi: 10.1093/ejcts/ezab091. [DOI] [PubMed] [Google Scholar]
- 23.Fam N.P., von Bardeleben R.S., Hensey M., Kodali S.K., Smith R.L., Hausleiter J., et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501–511. doi: 10.1016/j.jcin.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Gray W.A., Abramson S.V., Lim S., Fowler D., Smith R.L., Grayburn P.A., et al. 1-Year outcomes of cardioband tricuspid valve reconstruction system early feasibility study. JACC Cardiovasc Interv. 2022;15:1921–1932. doi: 10.1016/j.jcin.2022.07.006. [DOI] [PubMed] [Google Scholar]