Abstract
Objective
The study objective was to evaluate the midterm outcome of thoracic endovascular aortic repair compared with open repair in patients with descending thoracic aortic aneurysm.
Methods
From August 1993 to February 2023, 499 patients with descending thoracic aortic aneurysms underwent open repair (n = 221) or thoracic endovascular aortic repair (n = 278). Of these, 120 matched pairs were identified using propensity score matching based on age, sex, chronic lung disease, stroke, coronary artery disease, diabetes, ejection fraction, dialysis, peripheral vascular disease, prior cardiac surgery, connective tissue disease, and chronic dissection. Primary outcomes were postoperative paralysis, operative mortality, reoperation, and midterm survival.
Results
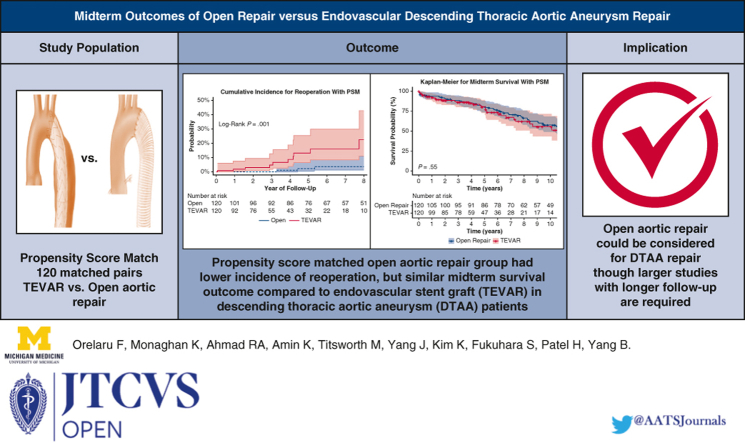
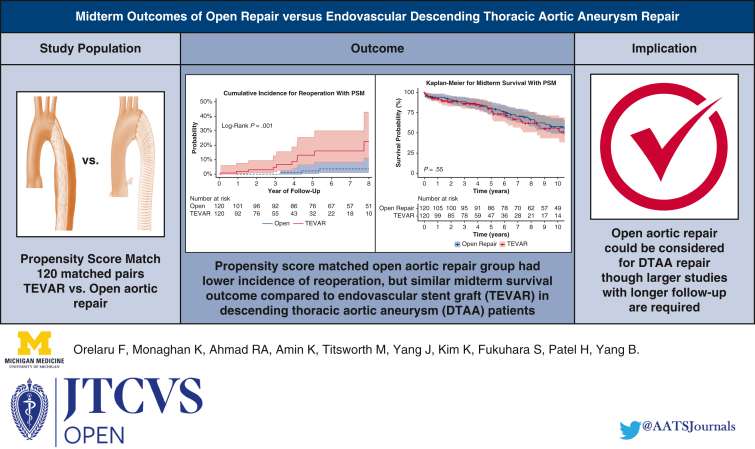
After matching, the preoperative demographics and comorbidities were balanced in both groups. Intraoperatively, open repair had a lower temperature (18 °C vs 36 °C) and more patients required blood products (66% vs 8%), P < .001. Postoperatively, patients undergoing thoracic endovascular aortic repair had fewer strokes (2.5% vs 9.2%; P = .03), less dialysis (0% vs 3.3%; P = .04), and shorter length of stay (5 days vs 12 days, P < .001), but similar lower-extremity paralysis (2.5% vs 2.5%, P = 1.00) compared with open repair. Furthermore, thoracic endovascular aortic repair had higher 7-year incidence of first reoperation (16.1% vs 3.6%, P < .001) but similar operative mortality (0.8% vs 4.2%; P = .10) and 10-year survival outcome (56%; 95% CI, 43-72 vs 58%; 95% CI, 49-68; P = .55) compared with open aortic repair. The hazard ratio was 0.93 (P = .78) for thoracic endovascular aortic repair for midterm mortality and 6.87 (P < .001) for reoperation.
Conclusions
Open repair could be the first option for patients with descending thoracic aortic aneurysms who were surgical candidates.
Key Words: descending thoracic aneurysm, endovascular stent grafts, midterm survival, open aortic repair
Graphical Abstract
Open thoracic aneurysm repair had similar survival compared with endovascular repair.
Central Message.
Endovascular repair had better perioperative outcomes compared with open repair of DTAA. Open repair had lower reoperation and similar midterm survival.
Perspective.
Our findings showed that TEVAR had better perioperative outcomes, but significantly higher reoperation rate compared with open aortic repair in patients with DTAAs. We identified a similar midterm survival after open aortic repair compared with TEVAR, although a larger sample size study with longer follow-up is needed.
See Discussion on page 36.
Historically, open aortic repair is the standard care for managing descending thoracic aortic aneurysm (DTAA).1,2 Because of high mortality (up to 44%) and morbidity in high-risk patients,2, 3, 4 many surgeons began to consider alternative management strategies such as thoracic endovascular aortic repair (TEVAR) in high-risk patients.5, 6, 7 Despite the controversial outcomes of TEVAR studies, recent guidelines further recommend TEVAR for low- and moderate-risk patients with DTAA as the first option.8, 9, 10, 11, 12 Therefore, this study aims to evaluate the midterm outcome of TEVAR compared with open repair in patients with DTAA. We hypothesized that patients with DTAA who underwent open aortic repair had a better surgical outcome including operative mortality, reoperation, and midterm survival compared with TEVAR.
Material and Methods
This study was approved by the Institutional Review Board at Michigan Medicine (HUM00133791, December 3, 2017). A waiver of informed consent was obtained and follows the Health Insurance Portability and Accountability Act regulations.
Data Collection
Data from August 1993 to February 2023 were obtained from the Society of Thoracic Surgeons Data Warehouse in the department of Cardiac Surgery at the University of Michigan. All patients with DTAA due to degenerative diseases or chronic aortic dissections who underwent TEVAR or open aortic repair using aortic clamping (AC) or hypothermic circulatory arrest (HCA) were enrolled in the database at the University of Michigan. Descending thoracic aorta was defined as an aorta segment between left subclavian artery and celiac artery (zones 3-5). Patients with thoracoabdominal aortic aneurysms repair, acute type A or B aortic dissection, aortic rupture, mycotic aneurysm or pseudoaneurysm, or traumatic aortic injury were excluded from the study.
Midterm survival data were obtained from the National Death Index through December 2018, the Michigan Death Index through May 2023,13 and medical chart review. Postoperative paralysis was defined as the inability to move the lower extremities after descending thoracic aneurysm repair.
Patient Selection
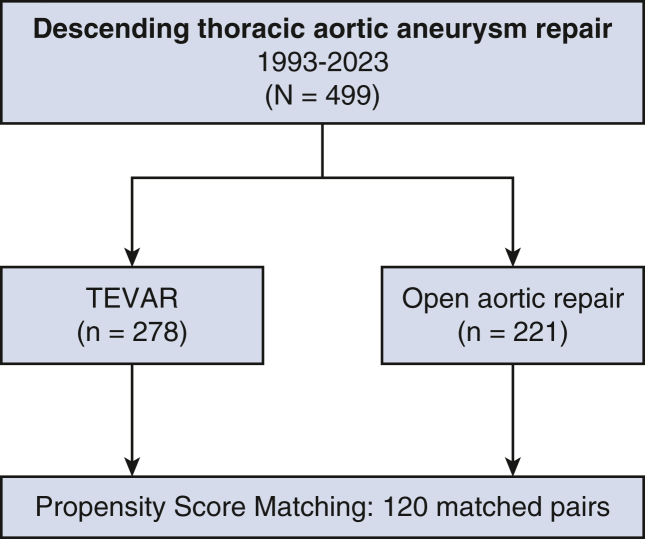
From August 1993 to February 2023, 499 patients with DTAA who had degenerative disease (n = 288) or chronic dissection (n = 211) underwent open repair (n = 221) or TEVAR (n = 278) at our institution. Of these, 120 matched pairs were identified using propensity score matching based on age, sex, chronic lung disease, stroke, coronary artery disease, diabetes, ejection fraction, dialysis, peripheral vascular disease, prior cardiac surgery, connective tissue disease, and chronic dissection (Figure 1). We enrolled patients who underwent open repair for DTAA from August 1993 to February 2023 and TEVAR from January 2008 to February 2023. Indications for DTAA repair were symptomatic (chest or back discomfort) aortic aneurysm, asymptomatic aortic aneurysm 6 cm or more, aortic growth rate 0.5 cm or more per year, or presence of saccular aneurysm. The feasibility for TEVAR was determined on the basis of the quality of access vessels and DTAA anatomy including proximal and distal landing zones, and proximity to critical branch vessels.14 Open aortic repair was performed by AC with mild hypothermia or under HCA alone (18 °C). HCA was preferred in patients with DTAA with aneurysm due to chronic aortic dissection, inability to clamp the proximal aorta, aortic arch aneurysm (>4 cm), and connective tissue disease. AC was favored in patients with DTAA with no arch or proximal descending thoracic aneurysm and in patients with classic or frozen elephant trunk without an endoleak and who required a replacement.15
Figure 1.
CONSORT diagram of selection and distribution of study population. TEVAR, Thoracic endovascular aortic repair.
For the propensity score matching, we performed a 1:1 matching within 0.20 (20%) standard deviation of the logit scale of the propensity score as the caliper widths. Standardized difference less than 0.1 was an acceptable balance of the covariates between the matched groups (Table 1). Using the propensity-matched cohort, we further analyze the perioperative outcomes, cumulative incidence of reoperation, and midterm survival.
Table 1.
Standard mean difference of the propensity score–matched population
| Demographics | Before propensity score matching (%) |
After propensity score matching (%) |
||||
|---|---|---|---|---|---|---|
| Open repair (221) | TEVAR (278) | SD (%) | Open repair (120) | TEVAR (120) | SD (%) | |
| Age, y | 59.21 | 67.62 | 72.78 | 63.96 | 63.11 | 7.55 |
| Sex | ||||||
| Male | 72.85 | 55.75 | 36.19 | 65.83 | 64.16 | 3.48 |
| Female | 27.14 | 44.24 | 36.19 | 34.16 | 35.83 | 3.48 |
| CLD | 22.22 | 38.04 | 34.93 | 31.67 | 35.00 | 7.05 |
| Stroke | 9.95 | 12.23 | 7.24 | 11.67 | 13.33 | 5.02 |
| CAD | 24.89 | 15.47 | 23.58 | 25.00 | 17.50 | 18.33 |
| Diabetes | 11.31 | 17.27 | 17.04 | 13.33 | 12.50 | 2.47 |
| Prior cardiac surgery | 48.86 | 32.01 | 34.79 | 40.00 | 40.83 | 1.69 |
| CTD | 7.24 | 3.24 | 18.00 | 4.16 | 4.16 | 0 |
| Ejection fraction | 58.24 | 60.35 | 10.79 | 59.04 | 59.40 | 3.70 |
| Dialysis | 2.26 | 3.24 | 5.95 | 1.67 | 1.67 | 0 |
| PAD | 30.77 | 57.55 | 55.9 | 40.00 | 45.83 | 11.76 |
| Chronic dissection | 53.85 | 33.09 | 42.72 | 43.33 | 45.83 | 5.01 |
SD less than 10% was an acceptable balance of the covariates between the matched groups. TEVAR, Thoracic endovascular aortic repair; SD, standard deviation; CLD, chronic lung disease; CAD, coronary artery disease; CTD, connective tissue disease; PAD, peripheral arterial disease.
Technique for Thoracic Endovascular Aortic Repair
Our approach for TEVAR has been extensively described in our previous study.14 Endograft sizing was performed using spiral computed tomography with 3-dimensional reconstruction, angiography, and intravascular ultrasound. Percutaneous vascular access was used for angiograms and an open-vessel exposure for endograft delivery. The final position and subsequent deployment of TEVAR device were guided by angiographic landmarks. Completion aortography was routinely performed and any identifiable endoleak (type I or type III) were corrected by repeat balloon dilatation of stent graft or additional coverage. Patients who underwent endograft stenting of long aortic segments (≥20 cm) or had a history of abdominal aortic aneurysm repair underwent lumbar drain placement for 24 to 48 hours. Spinal perfusion pressures after TEVAR were maintained at a mean arterial pressure of 90 to 100 mm Hg (if no lumbar drain) or 80 mm Hg or greater (if a lumbar drain was placed).14
Technique for Open Descending Thoracic Aortic Aneurysm Repair
As discussed in our previous articles,15,16 a left posterior lateral incision with 2 separate thoracotomies in the fourth and seventh intercostal space or eighth intercostal was made for most DTAA repair. Intercostal arteries (T9-T12) in diseased distal thoracic aorta were reimplanted. All patients had a lumbar drain placement before thoracotomy. In AC repair, distal perfusion was attained through cannulation of the left femoral or iliac artery or nonaneurysmal distal aorta. Cardiopulmonary bypass or left heart bypass was used. HCA was used for proximal anastomosis in patients with DTAA with chronic dissection, connective tissue disorder, arch aneurysm greater than 4 cm, or inability to clamp proximal aorta. Patients were cooled to 18 °C during HCA through aortic arch cannulation or left subclavian artery cannulation. Left ventricle venting was performed if mild or less aortic insufficiency was present.
Statistical Analysis
Data were presented as median (25%, 75%) for continuous data and n (%) for categorical data. Univariate analyses were performed using Wilcoxon rank-sum tests for continuous data and chi-square tests for categorical data. Kolmogorov-Smirnov test was used to check for normality of continuous data. Cox proportional hazard regression models were used to calculate the hazard ratio (HR) for midterm mortality in the propensity score–matched cohort, adjusting for age, male sex, dialysis, chronic lung disease, chronic dissection, history of myocardial infarction, and TEVAR. The HR for reoperation was adjusted for age, sex, chronic dissection, connective tissue disease, and TEVAR. Variables for propensity score matching and Cox model were chosen on the basis of their clinical relevance and literature findings.17 Kaplan–Meier method with log-rank testing was used to calculate midterm survival outcome. Gray's test was used to evaluate statistical difference in the cumulative incidence of reoperation between the 2 groups, adjusting for death as a competing factor. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc).
Results
Preoperative Demographics Data
After the propensity score match, compared with the open repair group, the TEVAR group had fewer patients who were current smokers (18% vs 59%, P < .001), similar coronary artery disease, and higher rate of prior myocardial infarction. All other comorbidities were well balanced between groups (Table 2).
Table 2.
Demographics and preoperative comorbidities of propensity score–matched cohort
| Demographics | Open repair (n = 120) | TEVAR (n = 120) | P value |
|---|---|---|---|
| Age, y | 65 (58, 72) | 64 (54, 73) | .59 |
| Male sex | 79 (66) | 77 (64) | .79 |
| BSA (m2) | 2.0 (1.9, 2.2) | 2.1 (1.9, 2.2) | .85 |
| BMI (kg/m2) | 29 (25, 32) | 29 (26, 34) | .38 |
| Hypertension | 106 (88) | 109 (91) | .53 |
| Diabetes | 16 (13) | 15 (13) | .85 |
| Coronary artery disease | 30 (25) | 21 (18) | .16 |
| History of MI | 7 (5.8) | 28 (23) | <.001 |
| Smoking status | |||
| Never | 28 (27) | 47 (39) | .05 |
| Former | 15 (14) | 51 (43) | <.001 |
| Current | 61 (59) | 22 (18) | <.001 |
| Renal failure | 20 (17) | 27 (23) | .25 |
| Preoperative dialysis | 2 (1.7) | 2 (1.7) | 1 |
| COPD | 38 (32) | 42 (35) | .58 |
| History of CVA | 14 (12) | 16 (13) | .70 |
| Cardiogenic shock | 1 (0.8) | 0 (0) | .32 |
| Peripheral vascular diseases | 48 (40) | 55 (46) | .36 |
| Ejection fraction (%) | 60 (55, 65) | 60 (55, 65) | .31 |
| Preoperative creatinine (mg/dL) | 1.0 (0.9, 1.3) | 1.0 (0.8, 1.3) | .28 |
| Previous cardiac surgery | 48 (40) | 49 (41) | .90 |
| Prior aortic repair | 48 (40) | 50 (42) | .79 |
| Chronic dissection | 52 (43) | 55 (46) | .70 |
| Connective tissue disorder | 5 (4.2) | 5 (4.2) | 1 |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data. P value indicates the difference between Open repair and TEVAR group. Significant P values less than or equal to .05, were embolden. TEVAR, Thoracic endovascular aortic repair; BSA, body surface area; BMI, body mass index; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident.
Intraoperative and Postoperative Outcomes
Compared with open repair, TEVAR was performed at higher body temperature (36 °C vs 18 °C, P < .001) and fewer patients required blood products (8% vs 66%, P < .001). The median cardiopulmonary bypass time in the open repair group was 212 minutes, and crossclamp time was 65 minutes (Table 3). In the open aortic repair group, 76.7% (92/120) underwent HCA, and 23.3% (28/120) had AC. In the TEVAR group, 42.5% (51/120) of patients underwent left subclavian artery bypass, and 17.5% (21/120) had debranching of head vessels. Postoperatively, the TEVAR group had fewer patients with stroke (2.5% vs 9.2%, P = .03), prolonged ventilation (2.5% vs 29%, P < .001), renal failure requiring dialysis (0% vs 3.3%, P = .04), gastrointestinal complications (0% vs 9.2%, P < .001), atrial fibrillation (5.8% vs 21%, P < .001), and shorter total length of stay (5 days vs 12 days, P < .001) compared with the open repair group. Both TEVAR and open repair had similar lower-extremity paralysis (2.5% vs 2.5%, P = 1.0). Finally, the operative mortality was low and similar between the TEVAR group and open aortic repair group (0.8% vs 4.2%; P = .10; Table 3). Among the 5 patients who died in the open aortic repair group, causes of death include stroke, pulseless electrical activity, respiratory failure, ischemic brain infarct/cerebral edema, and unknown in 1 patient who died 2 days after discharge to rehabilitation. In the TEVAR group, only 1 patient died 2 weeks after discharge to rehabilitation, and the cause of death was unknown.
Table 3.
Intraoperative and postoperative outcomes of propensity score–matched cohort
| Demographics | Open repair (n = 120) | TEVAR (n = 120) | P value |
|---|---|---|---|
| Lowest temperature (Celsius) | 18 (18, 21) | 36 (35, 36) | <.001 |
| CPB time (min) | 212 (135, 239) | - | - |
| Crossclamp time (min) | 65 (43, 83) | - | - |
| AC | 28 (23.3) | - | - |
| HCA | 92 (76.7) | - | - |
| Left heart bypass | 19 (16) | - | |
| Full CPB | 101 (84) | - | |
| Left subclavian artery bypass | - | 51 (42.5) | |
| Head vessel debranching | - | 21 (17.5) | |
| Intraoperative blood product use | 79 (66) | 9 (7.5) | <.001 |
| Reoperation for bleeding | 2 (1.7) | 0 (0) | .16 |
| Lower-extremity paralysis | 3 (2.5) | 3 (2.5) | 1 |
| Permanent stroke | 11 (9.2) | 3 (2.5) | .03 |
| Prolonged ventilation | 35 (29) | 3 (2.5) | <.001 |
| Pneumonia | 20 (16) | 1 (0.8) | <.001 |
| ∗Acute renal failure | 5 (4.2) | 0 (0) | .02 |
| Dialysis | 4 (3.3) | 0 (0) | .04 |
| Gastrointestinal complications | 11 (9.2) | 0 (0) | <.001 |
| Atrial fibrillation | 25 (21) | 7 (5.8) | <.001 |
| Endoleak | 6 (5) | 43 (36) | <.001 |
| Postoperative length of stay (d) | 10 (7, 19) | 4 (3, 6) | <.001 |
| Total length of stay (d) | 12 (7, 21) | 5 (3, 8) | <.001 |
| †Operative mortality | 5 (4.2) | 1 (0.8) | .10 |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data. P value indicates the difference between Open repair and TEVAR groups. Significant P values less than or equal to .05, were embolden. TEVAR, Thoracic endovascular aortic repair; CPB, cardiopulmonary bypass; AC, aortic clamping; HCA, hypothermic circulatory arrest; ICU, intensive care unit.
Acute renal failure defined using the STS definition: (1) an increase in serum creatinine level 3× greater than baseline, or serum creatinine level ≥4 mg/dL, with an acute increase being at least 0.5 mg/dL or (2) a new requirement for dialysis postoperatively.
Operative mortality includes 30-day mortality or in-hospital mortality.
Midterm Outcomes
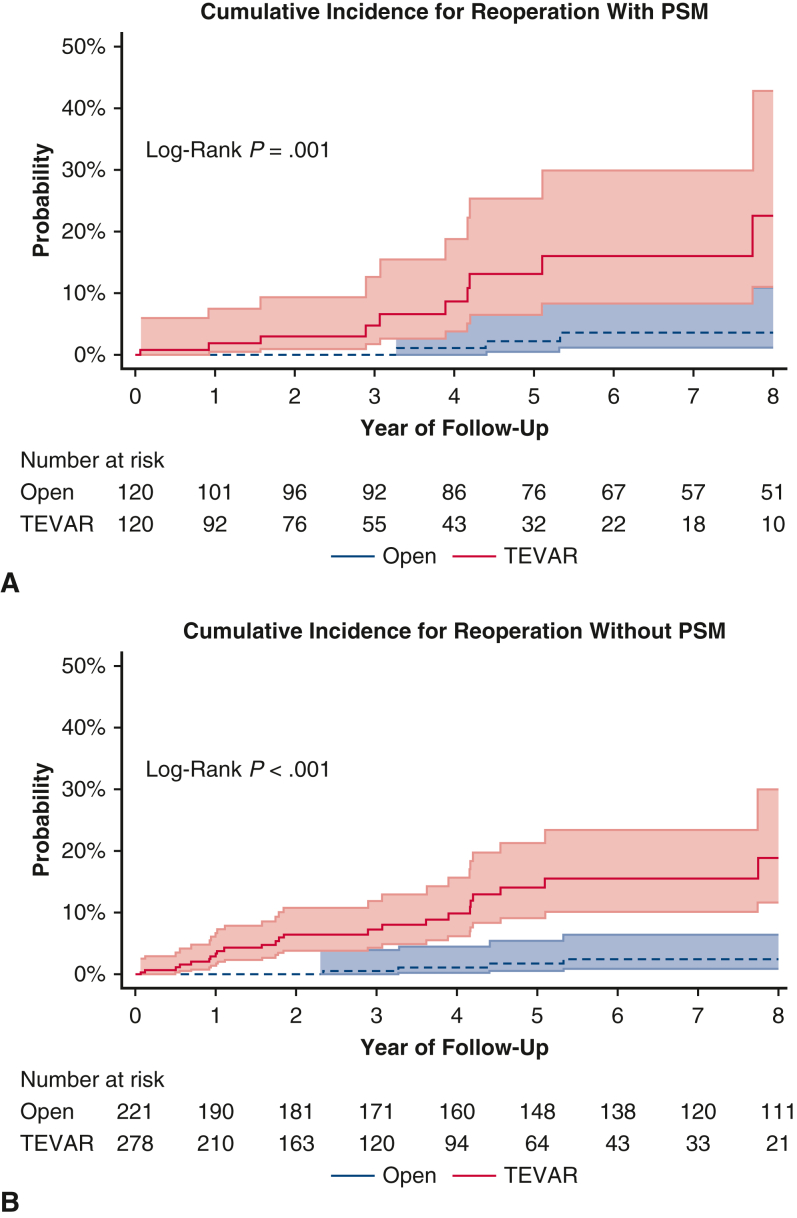
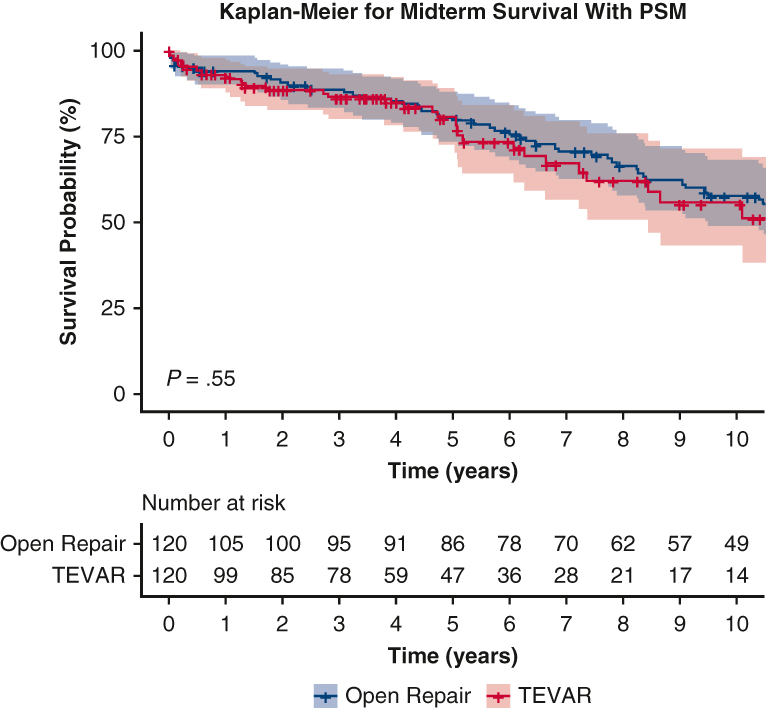
The 7-year cumulative incidence of first reoperation was higher in the matched TEVAR group compared with the open aortic repair group (16.1%; 95% CI, 8-30 vs 3.6%; 95% CI, 1-11; P < .001) (Figure 2). Likewise, the 7-year cumulative incidence of first reoperation in the unmatched TEVAR group was significantly higher compared with the open aortic repair group (15.6%; 95% CI, 10.2-23.4 vs 2.5%; 95% CI, 0.9-6.5; P < .001). The reintervention rate was 0.5% per patient-year in the open aortic repair group and 2.8% per patient-year in the TEVAR group. Causes of reoperation in both the TEVAR and Open repair groups included growth of DTAA or endoleak (Table E1). The median follow-up time for survival in the propensity score–matched open repair group was 8.3 years (interquartile range, 4.3-13.8) and 3.9 years in the TEVAR group (interquartile range, 1.7-6.6). There was 100% completeness to follow-up with the end of study date in May 2023. The 10-year survival was 58% (95% CI, 49-68) in the open aortic repair group compared with 56% (95% CI, 43-72) in the TEVAR group (P = .55; Figure 3, A). In the unmatched cohort, TEVAR had a significantly lower 10-year survival outcome compared with open aortic repair: 44% (95% CI, 35-55) versus 67% (95% CI, 60-74) (P < .0001, Figure 3, B). Furthermore, The HR of TEVAR for late mortality was 0.93 (95% CI, 0.57-1.52; P = .78) and reoperation (HR, 6.87; 95% CI, 2.56-18.45; P < .001). Age was a significant risk factor for midterm mortality (HR, 1.06; 95% CI, 1.04-1.09; P < .001), as well as chronic lung disease (HR, 1.78; 95% CI, 1.17-2.72; P = .008) (Table 4). The model fit for the regression model of midterm mortality was 0.66 ± 0.03, and for reoperation, it was 0.80 ± 0.06. The preoperative, intraoperative, and postoperative outcomes of unmatched patients in both TEVAR and open aortic repair groups are summarized in Tables E2 and E3.
Figure 2.
A, The 7-year cumulative incidence of first reoperation in the propensity score–matched cohort was higher in the TEVAR group compared with the open aortic repair group 16.1% (95% CI, 8-30) versus 3.6% (95% CI, 1-11); P < .001. B, The 7-year cumulative incidence of first reoperation in the unmatched TEVAR group was significantly higher compared with the open aortic repair group 15.6% (95% CI, 10.2-23.4) versus 2.5% (95% CI, 0.9-6.5); P < .001. PSM, Propensity score match; TEVAR, thoracic endovascular aortic repair.
Figure 3.
A, Kaplan–Meier analysis showed the midterm survival was similar among propensity score–matched patients with DTAA who underwent TEVAR compared with open repair; 10-year: 56% (95% CI, 43-72) versus 58% (95% CI, 49-68), P = .55. B, Kaplan–Meier analysis in unmatched cohort showed that midterm survival was lower in patients with DTAA who underwent TEVAR compared with open repair; 10-year: 44% (95% CI, 35-55) versus 67% (95% CI, 60-74), P < .0001. PSM, Propensity score match; TEVAR, thoracic endovascular aortic repair.
Table 4.
Multivariable regression model of risk factors for midterm mortality and reoperation in the propensity score–matched cohorts
| Demographics | Hazard ratio (95% CI) | P value |
|---|---|---|
| Midterm mortality | ||
| TEVAR | 0.93 (0.57-1.52) | .78 |
| Sex, male | 1.02 (0.67-1.57) | .92 |
| Age | 1.06 (1.04-1.09) | <.001 |
| Chronic lung disease | 1.78 (1.17-2.72) | .008 |
| Chronic dissection | 1.03 (0.67-1.59) | .89 |
| Dialysis | 2.26 (0.53-9.67) | .27 |
| Prior myocardial infarction | 1.36 (0.66-2.80) | .41 |
| Reoperation | ||
| TEVAR | 6.87 (2.56-18.5) | <.001 |
| Sex, male | 0.98 (0.46-2.07) | .96 |
| Age | 0.99 (0.96-1.03) | .57 |
| Chronic dissection | 3.56 (0.48-26.6) | .22 |
| Connective tissue disorder | 0.56 (0.07-4.46) | .58 |
Significant P values less than or equal to .05, were embolden. TEVAR, Thoracic endovascular aortic repair.
Discussion
In this study, we found that the TEVAR group had better perioperative outcomes, but similar midterm survival outcome and higher cumulative incidence of reoperation compared with open aortic repair in propensity score–matched patients with DTAA. The midterm outcomes are summarized in the Graphical Abstract (Figure 4).
Figure 4.
Summary of the study showing propensity score–matched TEVAR group had a higher cumulative incidence of first reoperation compared with open aortic repair group: 16.1% (95% CI, 8-30) versus 3.6% (95% CI, 1-11); P < .001. TEVAR had similar midterm survival outcome compared with open aortic repair group, 10-year: 56% (95% CI, 43-72) versus 58% (95% CI, 49-68), P = .55. PSM, Propensity score match; TEVAR, thoracic endovascular aortic repair.
As expected, and in line with reported literature,18 the TEVAR group had better perioperative outcomes, including blood transfusion, strokes, respiratory complications, and the total length of stay compared with open aortic repair. The operative mortality was not significant, which could be due to relatively small sample size. Those findings were not surprising because TEVAR was a smaller operation with a minimally invasive approach.
The majority of available literature highlights the short-term surgical outcome of TEVAR versus open aortic repair in patients with DTAA.12,19,20 However, data are scarce on the mid- and long-term outcomes of TEVAR in low- or high-risk patients with DTAA. In this study, we found that in the unmatched cohorts, the TEVAR group had a 23% lower 10-year survival outcome compared with the open repair (P < .0001) but in the propensity score match, both groups had similar survival outcome (56% vs 58%, P = .55). Similar to our study, the study by Goodney and colleagues10 reported a better 5-year survival outcome after open repair in DTAA patients compared with TEVAR (72% vs 62%). In their study, the operative mortality was significantly lower in TEVAR (6.1% vs 7.1%, P = .07), although most open repair patients regained the survival advantage of TEVAR within the first year of surgery.10 Likewise, Tong and colleagues21 show in their study on thoracic or thoracoabdominal aortic aneurysm repair that after propensity score matching, the TEVAR group had poorer 10-year survival compared with open repair. These findings underscored the notion that TEVAR may not be the ideal approach for patients with DTAA who were surgical candidates, contrary to recent guidelines suggesting TEVAR as the first choice for patients with DTAA.8, 9, 10, 11, 12
Additionally, 3 patients in both the TEVAR and open aortic groups (2.5%) had postoperative lower-extremity paralysis. The Zenith TX2 trial, in contrast to our study, shows that patients undergoing TEVAR had a lower rate of paraplegia compared with open repair (1.3% vs 5.7%, P = .07), but TEVAR resulted in more paraparesis (4.4% vs 0%, P = .10).19 They defined paraparesis as partial weakness with preserved ability to walk, whereas we defined paralysis as the inability to move lower extremities after DTAA repair. It is possible that the lower rate of paralysis in our open repair group was due to intercostal artery reimplantation, which is not possible during TEVAR. Also, to prevent paralysis in both groups, we routinely place lumbar drains before thoracotomy in open repairs or in TEVAR with endograft stenting of long aortic segments (≥20 cm) or patients with a history of abdominal aortic aneurysm repair.
Finally, the 7-year cumulative incidence of first reoperation and reintervention rate per patient-year were 4 to 5 times higher in the TEVAR group compared with the open repair group: 16% versus 4%, <.002 and 2.8% versus 0.5%, respectively. Those findings indicated that not only 4 times more patients had reintervention in the TEVAR group, but also the total interventions were 5 times higher in the TEVAR group because many patients in the TEVAR group had more than 1 reintervention. Causes of reoperation in the TEVAR group were endoleak and residual DTAA growth. The literature shows that the short-term incidence of endoleak and DTAA growth after repair in patients with TEVAR is approximately 15% and 7.1%, respectively.12,18,19 A high incidence of reoperation due to endoleak or residual DTAA growth could make TEVAR less preferable for patients who were surgical candidates due to need for reintervention, more imaging follow-up, resource use, and poorer quality of life. Gillen and colleagues22 report that the in-hospital costs of TEVAR repair is significantly higher compared with open aortic repair in patients with DTAA ($52,008 vs $37,172, P = .001). Likewise, Karimi and colleagues23 show that during a 2-year follow-up among patients with DTAA who survived to discharge, TEVAR repair had twice the mean number of clinic follow-up and imaging surveillance, and higher direct cost of rehospitalization (>$22,345.56) compared with open aortic repair. Last, using the Short Form Health Survey, Dick and colleagues24 report that TEVAR had lower, but nonsignificant, overall physical health score (81 vs 94, P = .57) and higher mental health score (93 vs 88, P = .86) compared with open aortic repair.
Study Limitations
Our study is limited by a single-center observational retrospective study. Patients were not randomized to treatment group, that is, patients considered for TEVAR could have been poor surgical candidates, and there could have been selection bias. Also, we performed propensity score matching based on preoperative demographics to better compare the TEVAR and open repair group; however, this unintentionally created a smaller study size and could result in type II error. In addition, the lack of significant difference in the operative mortality between the TEVAR group and the open repair group could have been due to low sample size. Finally, although this was a large-volume aortic center, more patients underwent TEVAR in the unmatched cohort, highlighting a national trend toward TEVAR in DTAA repair since Food and Drug Administration approval in 2005. Increased use of TEVAR could be a potential source of bias as more surgeons became proficient in TEVAR with subsequent improved outcomes compared with open repair. We need to reconsider open aortic repair as a valid option for patients with DTAA in the contemporary era.
Conclusions
Our study findings showed that open aortic repair in patients with DTAA had similar midterm survival, but a significantly lower rate of reinterventions compared with TEVAR. Open repair could be considered in younger patients with DTAA who were surgical candidates. However, a larger sample size study with longer follow-up is needed to better elucidate the DTAA repair technique.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/midterm-outcomes-of-open-repair-versus-endovascular-descending-thoracic-aortic-aneurysm-repair.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
B.Y. is supported by the National Heart, Lung, and Blood Institute of National Institutes of HealthK08HL130614, R01HL141891, and R01HL151776, Phil Jenkins and Darlene & Stephen J. Szatmari Funds.
Date and number of Institutional Review Board Approval: HUM00133791; December 3, 2017. A waiver of informed consent was obtained.
F.O. and K.M. contributed equally to this article.
Appendix 1
Table E1.
Indications for reoperation
| Patients | TEVAR | Open aortic repair |
|---|---|---|
| 1 | Residual distal thoracic aorta growth 6 cm just above the diaphragm | Degenerated residual distal thoracic and proximal abdomen aorta aneurysm of 6 cm |
| 2 | Growth of proximal thoracic landing zone due to type 1A endoleak | Aneurysmal degeneration in the middle portion of the graft due to intercostal artery patch growth |
| 3 | Symptomatic type 1A endoleak | Persistent growth of the residual distal thoracic aorta |
| 4 | Type 1A endoleak secondary to stent graft induced entry tear | |
| 5 | Aneurysmal degeneration of the untreated TEVAR segment | |
| 6 | Degenerative aneurysm distal to the stent with rapid size growth | |
| 7 | Degenerated and rapid growth of distal thoracic aorta down to the level of the thoracoabdominal junction | |
| 8 | Type 1B endoleak and growth of distal thoracic aorta | |
| 9 | Aneurysm of distal half of the descending thoracic aorta | |
| 10 | Type 1A endoleak |
TEVAR, Thoracic endovascular aortic repair.
Table E2.
Demographics and preoperative comorbidities of entire cohort
| Demographics | Open repair (n = 221) | TEVAR (n = 278) | P value |
|---|---|---|---|
| Age, y | 60 (51, 69) | 70 (61, 76) | <.001 |
| Male sex | 161 (73) | 155 (56) | <.001 |
| BSA (m2) | 2.1 (1.9, 2.2) | 2.0 (1.8, 2.1) | <.001 |
| BMI (kg/m2) | 28 (25, 33) | 28 (25, 32) | .38 |
| Hypertension | 188 (85) | 247 (89) | .21 |
| Diabetes | 25 (11) | 48 (17) | .06 |
| Coronary artery disease | 55 (25) | 43 (16) | .008 |
| Smoking status | |||
| Never | 63 (34) | 95 (35) | .71 |
| Former | 23 (12) | 128 (47) | <.001 |
| Current | 102 (54) | 47 (17) | <.001 |
| Preoperative dialysis | 5 (2.3) | 9 (3.2) | .51 |
| COPD | 48 (2) | 105 (38) | <.001 |
| History of CVA | 22 (10) | 34 (12) | .42 |
| History of MI | 13 (5.9) | 68 (25) | <.001 |
| Cardiogenic shock | 1 (0.5) | 0 (0) | .26 |
| Peripheral vascular diseases | 68 (31) | 160 (58) | <.001 |
| Ejection fraction (%) | 60 (55, 65) | 60 (55, 65) | <.001 |
| Preoperative creatinine (mg/dL) | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.3) | .23 |
| Previous cardiac surgery | 108 (49) | 89 (32) | <.001 |
| Chronic dissection | 119 (54) | 92 (33) | <.001 |
| Connective tissue disorder | 16 (7.2) | 9 (3.2) | .04 |
Significant P values less than or equal to .05, were embolden. TEVAR, Thoracic endovascular aortic repair; BSA, body surface area; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cardiovascular accident; MI, myocardial infarction.
Table E3.
Intraoperative and postoperative outcomes of entire cohort
| Demographics | Open repair (n = 221) | TEVAR (n = 278) | P value |
|---|---|---|---|
| Lowest temperature (Celsius) | 18 (18, 18) | 36 (35, 36) | <.001 |
| CPB time (min) | 202 (135, 237) | - | - |
| Crossclamp time (min) | 62 (37, 84) | - | - |
| Intraoperative blood product use | 144 (65) | 23 (8.3) | <.001 |
| Reoperation for bleeding | 8 (3.6) | 0 (0) | .001 |
| Lower-extremity paralysis | 4 (1.8) | 4 (1.4) | .74 |
| Permanent stroke | 15 (6.8) | 9 (3.2) | .07 |
| Prolonged ventilation | 50 (23) | 10 (3.6) | <.001 |
| Pneumonia | 24 (11) | 5 (1.8) | <.001 |
| ∗Acute renal failure | 7 (3.2) | 1 (0.4) | .01 |
| Dialysis | 5 (2.3) | 0 (0) | .01 |
| Gastrointestinal complications | 14 (6.3) | 6 (2.2) | .02 |
| Atrial fibrillation | 38 (17) | 22 (7.9) | .001 |
| Postoperative length of stay (d) | 10 (7, 15) | 4 (3, 7) | <.001 |
| Total length of stay (d) | 10 (7, 17) | 5 (3, 8) | <.001 |
| †Operative mortality | 7 (3.2) | 5 (1.8) | .32 |
Significant P values less than or equal to .05, were embolden. TEVAR, Thoracic endovascular aortic repair; CPB, cardiopulmonary bypass.
Acute renal failure defined using the STS definition: (1) an increase in serum creatinine level 3× greater than baseline, or serum creatinine level ≥4 mg/dL, with an acute increase being at least 0.5 mg/dL or (2) a new requirement for dialysis postoperatively.
Operative mortality includes 30-day mortality or in-hospital mortality.
References
- 1.Coselli J.S., LeMaire S.A., Miller C.C., III, Schmittling Z.C., Köksoy C., Pagan J., et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg. 2000;69:409–414. doi: 10.1016/s0003-4975(99)01478-2. [DOI] [PubMed] [Google Scholar]
- 2.Kouchoukos N.T., Dougenis D. Surgery of the thoracic aorta. N Engl J Med. 1997;336:1876–1888. doi: 10.1056/NEJM199706263362606. [DOI] [PubMed] [Google Scholar]
- 3.Svensson L.G., Crawford E.S., Hess K.R., Coselli J.S., Safi H.J. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–368. discussion 368-70. [PubMed] [Google Scholar]
- 4.Safi H.J., Estrera A.L., Miller C.C., Huynh T.T., Porat E.E., Azizzadeh A., et al. Evolution of risk for neurologic deficit after descending and thoracoabdominal aortic repair. Ann Thorac Surg. 2005;80:2173–2179. doi: 10.1016/j.athoracsur.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 5.Dake M.D., Miller D.C., Semba C.P., Mitchell R.S., Walker P.J., Liddell R.P. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331:1729–1734. doi: 10.1056/NEJM199412293312601. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell R.S., Dake M.D., Semba C.P., Fogarty T.J., Zarins C.K., Liddel R.P., et al. Endovascular stent-graft repair of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 1996;111:1054–1062. doi: 10.1016/s0022-5223(96)70382-3. [DOI] [PubMed] [Google Scholar]
- 7.Scali S.T., Goodney P.P., Walsh D.B., Travis L.L., Nolan B.W., Goodman D.C., et al. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53:1499–1505. doi: 10.1016/j.jvs.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiratzka L.F., Bakris G.L., Beckman J.A., Bersin R.M., Carr V.F., Casey D.E., Jr., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/sir/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Upchurch G.R., Escobar G.A., Azizzadeh A., Beck A.W., Conrad M.F., Matsumura J.S., et al. Society for Vascular Surgery Clinical Practice Guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J Vasc Surg. 2021;73:55S–83S. doi: 10.1016/j.jvs.2020.05.076. [DOI] [PubMed] [Google Scholar]
- 10.Goodney P.P., Travis L., Lucas F.L., Fillinger M.F., Goodman D.C., Cronenwett J.L., et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124:2661–2669. doi: 10.1161/CIRCULATIONAHA.111.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demers P., Miller D.C., Mitchell R.S., Kee S.T., Sze D., Razavi M.K., et al. Midterm results of endovascular repair of descending thoracic aortic aneurysms with first generation stent grafts. J Thorac Cardiovasc Surg. 2004;127:664–673. doi: 10.1016/j.jtcvs.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Bavaria J.E., Appoo J.J., Makaroun M.S., Verter J., Yu Z.F., Mitchell R.S., Gore TAG Investigators Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–377. doi: 10.1016/j.jtcvs.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention; National Center for Health Statistics National Death Index. https://www.cdc.gov/nchs/ndi/index.htm
- 14.Patel H.J., Shillingford M.S., Williams D.M., Upchurch G.R., Dasika N.L., Prager R.L., et al. Survival benefit of endovascular descending thoracic aortic repair for the high-risk patient. Ann Thorac Surg. 2007;83:1628–1633. doi: 10.1016/j.athoracsur.2006.12.070. [DOI] [PubMed] [Google Scholar]
- 15.Patel H.J., Shillingford M.S., Mihalik S., Proctor M.C., Deeb G.M. Resection of the descending thoracic aorta: outcomes after use of hypothermic circulatory arrest. Ann Thorac Surg. 2006;82:90–95. doi: 10.1016/j.athoracsur.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 16.Clemence J., Malik A., Farhat L., Wu X., Kim K., Patel H., et al. Cryoablation of intercostal nerves decreased narcotic usage after thoracic or thoracoabdominal aortic aneurysm repair. Semin Thorac Cardiovasc Surg. 2020;32:404–412. doi: 10.1053/j.semtcvs.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopaldas R.R., Huh J., Dao T.K., LeMaire S.A., Chu D., Bakaeen F.G., et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg. 2010;140:1001–1010. doi: 10.1016/j.jtcvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Orandi B.J., Dimick J.B., Deeb G.M., Patel H.J., Upchurch G.R., Jr. A population-based analysis of endovascular versus open thoracic aortic aneurysm repair. J Vasc Surg. 2009;49:1112–1116. doi: 10.1016/j.jvs.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Fairman R.M., Criado F., Farber M., Kwolek C., Mehta M., White R., et al. Pivotal results of the Medtronic vascular talent thoracic stent graft system: the VALOR trial. J Vasc Surg. 2008;48:546–554. doi: 10.1016/j.jvs.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura J.S., Cambria R.P., Dake M.D., Moore R.D., Svensson L.G., Snyder S., TX2 Clinical Trial Investigators International controlled clinical trial of thoracic endovascular aneurysm repair with the zenith tx2 endovascular graft: 1-year results. J Vasc Surg. 2008;47:247–257. doi: 10.1016/j.jvs.2007.10.032. discussion 257. [DOI] [PubMed] [Google Scholar]
- 21.Tong M.Z., Eagleton M.J., Roselli E.E., Blackstone E.H., Xiang F., Ibrahim M., et al. Outcomes of open versus endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2022;113:1144–1152. doi: 10.1016/j.athoracsur.2021.04.100. [DOI] [PubMed] [Google Scholar]
- 22.Gillen J.R., Schaheen B.W., Yount K.W., Cherry K.J., Kern J.A., Kron I.L., et al. Cost analysis of endovascular versus open repair in the treatment of thoracic aortic aneurysms. J Vasc Surg. 2015;61:596–603. doi: 10.1016/j.jvs.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karimi A., Walker K.L., Martin T.D., Hess P.J., Klodell C.T., Feezor R.J., et al. Midterm cost and effectiveness of thoracic endovascular aortic repair versus open repair. Ann Thorac Surg. 2012;93:473–479. doi: 10.1016/j.athoracsur.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Dick F., Hinder D., Immer F.F. Outcome and quality of life after surgical and endovascular treatment of descending aortic lesions. J Vasc Surg. 2009;49:1627. doi: 10.1016/j.athoracsur.2008.01.027. [DOI] [PubMed] [Google Scholar]