Abstract
Objective
Although regurgitant mitral valves can be repaired through surgical or transcatheter approaches, contemporary comparative outcomes are limited with the impact of residual and recurrent mitral regurgitation (MR) on clinical outcomes being poorly defined. We hypothesized that moderate (2+) or greater residual or recurrent (RR) MR—regardless of type of repair—predicts worse clinical outcomes.
Methods
Our institutional experience of 660 consecutive patients undergoing mitral valve repair (2015-2021) consisting of 393 surgical mitral valve repair (SMVr) and 267 transcatheter edge-to-edge mitral valve repair (TEER) was studied. The echocardiographic impact of RRMR (2+) following both SMVr and TEER on death and reintervention was evaluated.
Results
Patients averaged 67.8 ± 14.2 years (SMVr = 63.8 ± 13.3 vs 73.6 ± 13.6, P < .0001) and 62.1% were male. Baseline clinical and demographic data were vastly different between the 2 groups. Residual or recurrent 2+ or greater MR developed in 25% (n = 68) of patients who received TEER compared with 6% (n = 25) of SMVr (P < .0001). Reintervention (9.3% vs 2.4%, P = .002) and death (37.9% vs 10.4%, P < .0001) rates at 3-years were greater among the TEER group versus SMVr group. Given the heterogeneity in baseline characteristics and difference in survival, each cohort was analyzed separately, stratified by RRMR, using multivariable modeling to identify predictors of repeat reintervention and death. There were too few events of RRMR in the SMVr cohort for evaluation. For the TEER subgroups, we observed greater long-term mortality, but not reintervention among those with RRMR., Hypertension was the strongest predictor of death and obesity was for reintervention.
Conclusions
Patients undergoing SMVr and TEER are vastly different with respect to baseline patient characteristics and clinical outcomes, with patients who undergo TEER being much greater risk with poorer prognosis. Moderate or greater RRMR predicted worse long-term survival but not reintervention among patients who received TEER. Given the difference in survival among patients with RRMR following TEER, care must be taken to ensure that patients entering clinical trials and receiving TEER should have a high probability of achieving mild or less MR as seen in contemporary surgical results.
Key Words: mitral valve, mitral valve repair, surgical, transcatheter
We observed increased mortality in the older and sicker TEER cohort.
Central Message.
TEER patients were much older and sicker than surgical patients and did not tolerate recurrent or residual mitral regurgitation.
Perspective.
Residual and recurrent mitral regurgitation following transcatheter and surgical mitral valve repair is more common in older, sicker patients who undergo transcatheter repair. This is important for designing clinical trials comparing surgical and transcatheter repairs, as residual and recurrent mitral regurgitation predicted mortality in transcatheter patients.
See Discussion on page 207.
It is an exciting time to treat patients with mitral regurgitation (MR), as there are both excellent surgical and transcatheter options. Transcatheter edge-to-edge repair (TEER) has expanded patient access to corrective mitral valve (MV) procedures for patients who have historically been too frail to undergo mitral valve surgery. As with most transcatheter procedures, TEER was initially indicated for patients who were prohibitively high risk for conventional surgery. With positive results in the early high-risk trials,1, 2, 3, 4 randomized controlled trials are now underway in patients across the surgical-risk spectrum.5,6
In the surgical literature, residual and recurrent MR (RRMR) predicts worse clinical outcomes, but whether this applies to TEER is less well established.7 We hypothesized that RRMR, regardless of type of repair, would predict worse clinical outcomes.
Methods
The primary aim of this study is to evaluate the association of moderate (2+) or greater RRMR, regardless of the type of MV repair, to adverse clinical outcomes. When the data were collected, we observed that the groups were widely disparate with respect to clinical profiles and risk, preventing meaningful head-to-head comparisons. We, therefore, analyzed each cohort separately for the end points. Patients undergoing surgical mitral valve repair (SMVr) or TEER at an Intermountain Healthcare Hospital from 2015 to 2021 were studied. Intermountain Healthcare is a nonprofit, integrated health care system that included 24 hospitals, 215 clinics, and an affiliated health insurance company in Utah, Idaho, and Nevada at the time of the study. Intermountain Healthcare has an extensive and long-standing (>25 years) integrated electronic medical records system. This study was approved by the Intermountain Healthcare institutional review board (#1007205, approval date: December 27, 2022).
Baseline demographics and clinical characteristics of study patients were collected at the time of MV repair. Demographics and clinical characteristics were obtained from electronic medical records using International Classification of Diseases (ICD) codes and previous test results (see Table 1 for list). Transthoracic or transesophageal echocardiography results were obtained before MV repair and at different time points following the procedure and included left ventricular ejection fraction (LVEF), left ventricular internal diameter end diastole (LVIDd), left ventricular internal diameter end systole, left atrial size, and MR severity. RRMR was defined as 2+ or greater MR on postoperative transthoracic or transesophageal echocardiography.
Table 1.
Baseline characteristics overall and stratified by type of mitral valve procedure received
| Overall, n = 660 | SMVr, n = 393 | TEER, n = 267 | P value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 67.8 ± 14.2 | 63.8 ± 13.3 | 73.6 ± 13.6 | <.0001 |
| Sex (male) | 62.1% | 62.3% | 61.8% | .89 |
| Hypertension | 79.7% | 75.3% | 86.1% | <.0001 |
| Hyperlipidemia | 71.5% | 68.7% | 75.7% | .05 |
| Diabetes | 29.8% | 25.7% | 36.0% | .005 |
| Depression history | 28.5% | 27.0% | 30.7% | .30 |
| Previous myocardial infarction | 15.8% | 14.2% | 18.0% | .20 |
| Coronary artery disease | 67.0% | 60.6% | 76.4% | <.0001 |
| Heart failure | 7.8% | 61.6% | 86.9% | <.0001 |
| Previous percutaneous coronary intervention | 11.8% | 7.9% | 17.6% | <.0001 |
| Previous coronary artery bypass graft | 14.1% | 18.1% | 8.2% | <.0001 |
| Previous stroke | 9.1% | 7.9% | 10.9% | .19 |
| Previous transient ischemic attack | 9.5% | 7.1% | 13.1% | .01 |
| Atrial fibrillation history | 59.8% | 53.7% | 68.9% | <.0001 |
| Atrial flutter history | 23.9% | 20.4% | 29.2% | .009 |
| Implantable cardioverter defibrillator | 7.7% | 2.8% | 15.0% | <.0001 |
| Pacemaker | 10.5% | 5.9% | 17.2% | <.0001 |
| Previous valve surgery | 56.2% | 65.1% | 43.1% | <.0001 |
| Body mass index, kg/m2 | ||||
| Mean ± SD | 27.2 ± 5.5 | 27.4 ± 5.0 | 26.8 ± 6.2 | .20 |
| Category | .002 | |||
| <25.0 | 37.1% | 31.8% | 44.9% | |
| 25-29.9 | 37.3% | 41.7% | 30.7% | |
| ≥30 | 25.6% | 26.5% | 24.3% | |
| STS predicted risk of mortality score (%), n = 226 | 1.9 ± 3.2 (median, 1.0) | 1.8 ± 3.2 (median, 1; n = 222) | 4.3 ± 2.1 (median, 4.5; n = 4) | .009 |
| LVEF closest before mitral procedure | ||||
| Number of patients | 635 | 378 | 257 | |
| Days, median (range) | 23.0 (1.0, 3660.0) | 28.0 (11.0, 2272.0) | 15.5 (1.0, 3660.0) | <.0001 |
| LVEF | 53.4 ± 13.8 | 56.9 ± 11.2 | 49.1 ± 15.8 | <.0001 |
| LVIDd closest before mitral procedure | ||||
| Number of patients | 608 | 356 | 252 | |
| Days, median (range) | 31.5 (1.0, 3660.0) | 32.0 (1.0, 2952.0) | 30.5 (1.0, 3660.0) | .97 |
| LVIDd | 5.2 ± 0.9 | 5.1 ± 0.8 | 5.3 ± 1.0 | .12 |
| LVIDs closest prior to mitral procedure | ||||
| Number of patients | 604 | 353 | 251 | |
| Days, median (range) | 32.0 (1.0, 4293.0) | 33.0 (1.0, 2853.0) | 31.0 (1.0, 4293.0) | .20 |
| LVIDs | 5.2 ± 0.9 | 5.1 ± 0.8 | 5.3 ± 1.0 | .90 |
| LA size closest before mitral procedure, n = 465 | ||||
| Number of patients | 465 | 261 | 204 | |
| Days, median (range) | 42.0 (1.0, 4767.0) | 42.0 (1.0, 4767.0) | 40.5 (1.0, 4293.0) | .60 |
| Categories | <.0001 | |||
| 0 | 11.8% | 14.2% | 8.8% | |
| 1.0 | 18.1% | 22.6% | 12.3% | |
| 1.5 | 15.1% | 19.2% | 28.6% | |
| 2.0 | 16.8% | 15.3% | 18.6% | |
| 2.5 | 6.9% | 8.0% | 5.4% | |
| 3.0 | 31.4% | 20.7% | 45.1% |
SMVr, Surgical mitral valve repair; TEER, transcatheter edge-to-edge mitral valve repair; SD, standard deviation; STS, Society of Thoracic Surgeons; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter end diastole; LVIDs, left ventricular internal diameter end systole; LA left atrial.
Contemporary surgical techniques with guiding principles of resection/neochords for unsupported segments, cleft closure, and various types of annuloplasty rings/bands were used. For the TEER cohort, this analysis includes both the MitraClip device (Abbott Laboratories) and the PASCAL device (Edwards Lifesciences). In our practice, we overwhelmingly treat functional MR with TEER and degenerative MR with surgery reserving TEER in degenerative MR only for patients with prohibitive surgical risk.
The primary end point was the impact of RRMR on the clinical outcomes of death and the need for MV reintervention. Death was determined from the state of Utah death certificates and electronic medical records. MV reintervention was determined from electronic medical records using ICD, Ninth Revision, codes 35.02, 35.12, 35.23, and 35.24; and ICD, Tenth Revision, codes 02_G∗ (where _ is any one character and ∗ is anything [zero or more characters]). The Student t test and the χ2 statistic were used to examine baseline and clinical characteristic differences for continuous and discrete variables, respectively, between SMVr and TEER groups and by MR severity. The Mann–Whitney U test was used to determine significant differences between continuous, non-normally distributed variables. The χ2 statistic was used to determine significant differences in frequencies of the outcomes at 3-years and long-term. The Kaplan–Meier survival estimate and log-rank P-value were used for graphical evaluation and to assess initial associations to the outcomes. Multivariable Cox hazard regression was used to estimate hazard ratios, 95% confidence intervals, and P values (SPSS, version 29; IBM Corp). Models used forced variable entry with final models retaining significant (P < .05) and confounding covariables. The proportional hazards assumption was evaluated and met.
Results
A total of 660 consecutive patients underwent either SMVr (n = 393) or TEER (n = 267). Baseline clinical characteristics were profoundly different between the SMVr and TEER cohorts with respect to age, severity of cardiac disease, and medical complexity (Table 1). Specifically, compared with patients who underwent SMVr, the patients who underwent TEER were older and had more severe cardiovascular disease burden, as evidenced by greater proportions of hypertension, hyperlipidemia, diabetes, atrial fibrillation, and a diagnosis of heart failure. The TEER cohort also had more patients with a history of previous percutaneous coronary intervention, permanent pacemaker, and atrial implantable cardioverter defibrillator placement, but fewer patients with a previous history of coronary artery bypass graft surgery and previous valve surgery. Patients who underwent TEER had a lower body mass index. From an echocardiographic standpoint, at baseline patients who underwent TEER had worse cardiac function as evidenced by lower LVEF% (49% vs 57% P < .001) and larger ventricles, LVIDd (5.3 cm vs 5.1 cm, P = .03) (Table 1). Echo follow-up demonstrated significant reverse remodeling of LV structure and function with average % change improvements (median) in LVEF; +7.3% (0) for SMVr and +15.6% (+7.7), LVIDd; −13.6% (−14.3) for SMVr and −7.4% (−7.8); and left ventricular internal diameter end systole −39.9% (−41.2) and −32.4% (−34), respectively (P < .001 for all comparisons) (Table 2). No patients who underwent TEER had a concomitant procedure performed at the time of mitral repair. However, there were a total of 33 patients who received SMVr and had 1 or more concomitant procedures (atrial fibrillation ablation, coronary artery bypass surgery, or other valve surgery) performed at the time of MV repair surgery.
Table 2.
Echocardiogram changes, reported as mean ± standard deviation (median), stratified by type of mitral valve procedure
| Overall | SMVr | TEER | P value | |
|---|---|---|---|---|
| LVEF (baseline to greatest follow-up measurement after procedure) | ||||
| Number of patients | 561 | 329 | 232 | |
| Absolute change | 2.1 ± 11.8 (1.0) | 0.44 ± 12.4 (0) | 4.4 ± 10.5 (4.0) | <.0001 |
| Percent change | 10.7 ± 57.1 (2.2) | 7.3 ± 63.5 (0) | 15.6 ± 46.1 (7.7) | <.0001 |
| LVIDd (baseline to lowest follow-up measurement after procedure) | ||||
| Number of patients | 551 | 317 | 234 | |
| Absolute change | −0.6 ± 0.7 (−0.6) | −0.7 ± 0.7 (−0.7) | −0.4 ± 0.8 (−0.4) | <.0001 |
| Percent change | −11.0 ± 14.1 (−11.6) | −13.6 ± 13.0 (−14.3) | −7.4 ± 14.9 (−7.8) | <.0001 |
| LVIDs (baseline to lowest follow-up measurement after procedure) | ||||
| Number of patients | 546 | 313 | 233 | |
| Absolute change | −1.9 ± 0.8 (−1.9) | −2.1 ± 0.7 (−2.1) | −1.7 ± 0.9 (−1.7) | <.0001 |
| Percent change | −36.7 ± 14.7 (−38.4) | −39.9 ± 12.5 (−41.2) | −32.4 ± 16.2 (−34.3) | <.0001 |
| Mitral valve regurgitation severity (greatest within 1-y after procedure) | ||||
| Severity category | 660 | 393 | 267 | <.0001 |
| 0 | 10.9% (72) | 15.3% (60) | 4.5% (12) | |
| 0.25 | 2.6% (17) | 3.1% (12) | 1.9% (5) | |
| 0.5 | 32.4% (214) | 45.5% (179) | 13.1% (35) | |
| 1.0 | 25.6% (169) | 23.2% (91) | 29.2% (78) | |
| 1.5 | 14.4% (95) | 6.6% (26) | 25.8% (69) | |
| 2.0 | 10.6% (70) | 5.1% (20) | 18.7% (50) | |
| 2.5 | 1.7% (11) | 0.5% (2) | 3.4% (9) | |
| 3.0 | 1.8% (12) | 0.8% (3) | 3.4% (9) | |
| 4.0 | 0% (0/0) | 0% (0/0) | 0% (0/0) | |
| Mitral valve stenosis severity (greatest follow-up measurement after procedure) | ||||
| Severity category | N = 490 | n = 292 | n = 198 | <.0001 |
| 0 | 13.9% | 14.0% | 13.6% | |
| 1.0+ | 51.6% | 58.6% | 41.4% | |
| 2.0+ | 30.6% | 25.7% | 37.9% | |
| 3.0+ | 3.9% | 1.7% | 7.1% | |
| 4.0 | 0% | 0% | 0% |
Baseline is the measurement that is taken closest, but before the repair procedure. SMVr, Surgical mitral valve repair; TEER, transcatheter edge-to-edge mitral valve repair; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter end diastole; LVIDs, left ventricular internal diameter end systole.
Survival
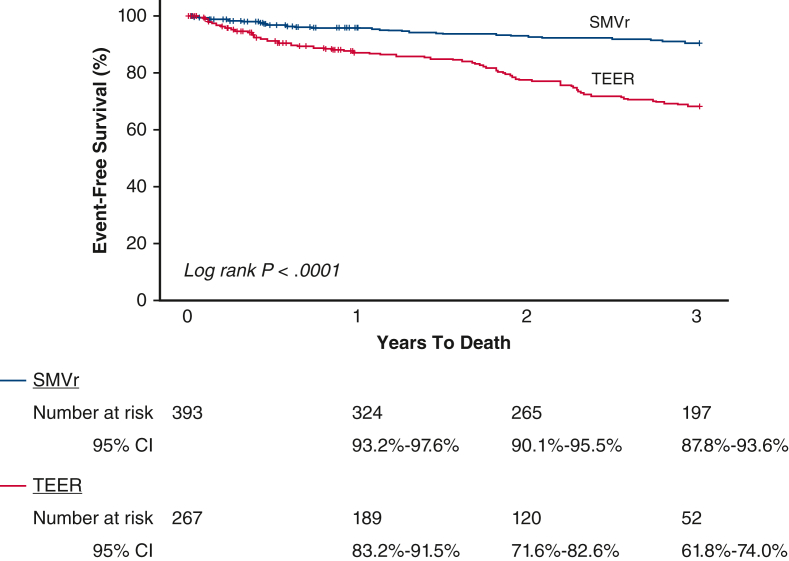
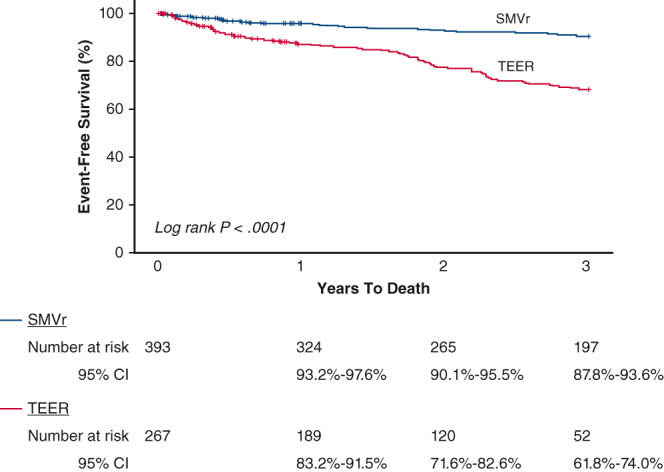
Average length of follow-up was 1120 ± 682 days (median, 1098 days) for the SMVr group and 698 ± 474 days (median, 660 days) for the TEER group (P < .001). However, the time to death among those who died was not significantly different between the groups: SMVr: 745 ± 596 days (median, 659 days) versus TEER: 572 ± 421 days (median, 619 days), P = .19. As expected, the much sicker TEER cohort had more deaths at 3 years (37.9% vs 10.4%, P < .0001) and long-term (30.0% vs 12.0%, P < .0001) compared with the SMVr group, which persisted after adjustment by baseline risk factors (Table 3, Figure 1).
Table 3.
Mortality and mitral valve reintervention at 3 years and long-term stratified mitral valve procedure
| Overall | SMVr | TEER | P value | |
|---|---|---|---|---|
| Death | ||||
| 3-y | 20.3% (91) | 10.4% (30) | 37.9% (61) | <.0001 |
| Long-term | 19.2% (127) | 12.0% (47) | 30.0% (80) | <.0001 |
| Average days of follow-up | 949.1 ± 641.9 (median, 842.5) | 1119.9 ± 682.2 (median, 1098) | 697.6 ± 473.8 (median, 660) | <.0001 |
| Average days to death | 636.1 ± 497.6 (median, 624) | 744.7 ± 596.1 (median, 659) | 572.2 ± 420.6 (median, 618.5) | .19 |
| Adjusted HR (95% CI), P value | 3-y: HR, 3.55 (2.33-5.43), P < .0001 | Long-term: HR, 2.74 (1.80-4.16), P < .0001 | ||
| Follow-up mitral valve intervention | ||||
| 3-y | 4.9% (22) | 2.4% (7) | 9.3% (15) | .002 |
| Long-term | 4.5% (30) | 2.5% (10) | 7.5% (20) | .003 |
| Average days to follow-up mitral intervention | 315.6 ± 473.6 (median, 145) | 600.1 ± 713.2 (median, 388.5) | 173.3 ± 193.8 (median, 119.5) | .04 |
| Adjusted HR (95% CI), P value | 3-y: HR, 5.08 (2.17-11.18), P < .0001 | Long-term: HR, 4.73 (2.08-10.74), P < .0001 | ||
Outcome frequencies are presented as % (n). A total of 449 patients had the opportunity for 3-years of follow-up (SMVr, n = 288; TEER, n = 161). SMVr, Surgical mitral valve repair; TEER, transcatheter edge-to-edge mitral valve repair; HR, hazard ratio; CI, confidence interval.
Figure 1.
Kaplan–Meier survival estimates and log-rank P value for long-term death stratified by MV repair method. MV, Mitral valve; SMVr, surgical mitral valve repair; CI, confidence interval; TEER, transcatheter edge-to-edge mitral valve repair.
Durability
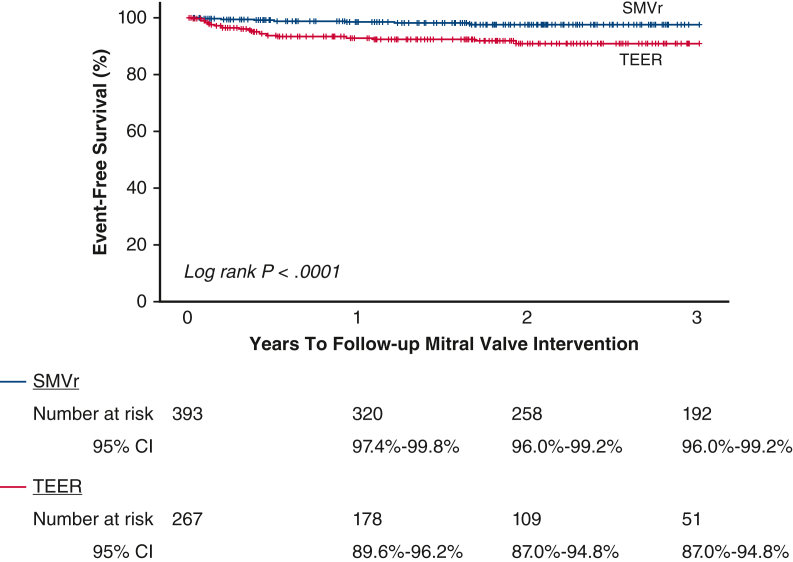
The longer follow-up for the SMVr cohort provides more “days at risk” for repeat MV reintervention; however, the TEER had significantly more MV reinterventions compared with SMVr at 3 years (9.3% vs 2.4%, P = .002) and long-term (7.5% vs 2.5%, P = .003) (Table 3). Of those 7.5% (n = 20) patients who received TEER with follow-up MV interventions, 12 were surgical MV replacements. The median time to MV reintervention was significantly shorter among patients who received TEER (120 days) compared with patients who received SMVr (389 days). Kaplan–Meier survival curve for long-term MV reintervention is shown in Figure 2. Even after multivariable adjustment by baseline risk factors, TEER had a significantly (∼5-fold) increased risk of MV reintervention (Table 3).
Figure 2.
Kaplan–Meier survival estimates and log-rank P value for long-term MV reintervention stratified by MV repair method. MV, Mitral valve; SMVr, surgical mitral valve repair; CI, confidence interval; TEER, transcatheter edge-to-edge mitral valve repair.
Comparisons
In our practice, we overwhelmingly treat functional MR with TEER and degenerative MR with surgery, reserving TEER in degenerative MR only for patients with prohibitive surgical risk. Given the large differences between the 2 cohorts, the SMVr and TEER groups were not directly compared. Rather, each cohort was analyzed for predictors of the end points and described to follow.
Patients with SMVr
Of the 393 patients with SMVr, a total of 25 (6.4%) of patients developed RRMR during the 1 year following their MV repair procedure. Baseline characteristics stratified by RRMR are shown in Table E1. Characteristics between those who developed RRMR and those did not were statistically similar. However, there were some notable differences, namely in sex, history of depression, and previous implantable cardioverter defibrillator, which differences most likely did not achieve significance due to power. Echocardiogram changes are shown in Table E2.
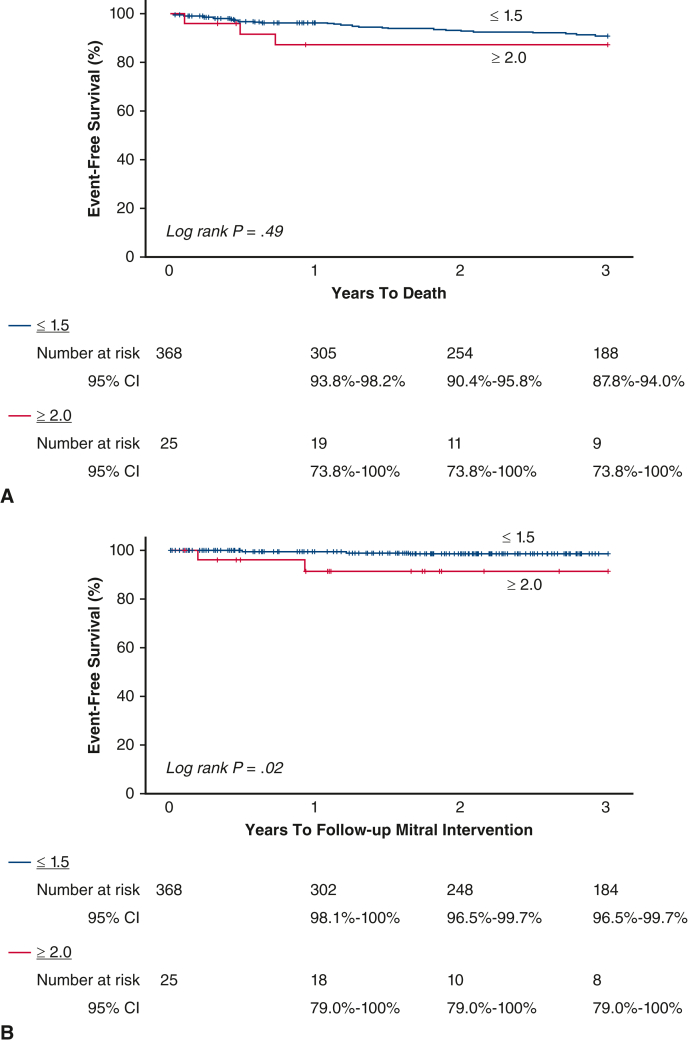
For the outcomes of death and MV reintervention, those without RRMR had lower frequencies of 3-year death and MV reintervention, but not long-term death (Table E3). Although statistical significance was not achieved, meaningful clinical differences were observed. Kaplan–Meier survival estimates and the log rank P value for long-term death MV reintervention are shown in Figure E1, A and B, respectively. Due to the low event rate, regression analysis was unable to be performed.
Figure E1.
Kaplan–Meier survival estimates and log-rank P value for long-term (A) death and (B) MV reintervention stratified by MR ≥2.0 versus ≤1.5 among SMVr. MV, Mitral valve; MR, mitral regurgitation; SMVr, surgical mitral valve repair; CI, confidence interval.
Patients with TEER
Of the 267 patients who underwent TEER, a total of 68 (25.5%) developed RRMR during the 1-year following their MV repair procedure, which is significantly more than the SMVr group (P < .0001). Patients were similar in age (Table E4), but patients with RRMR less often had hyperlipidemia, diabetes, a previous stroke, and had a lower body mass index. Echocardiogram changes are shown in Table E5.
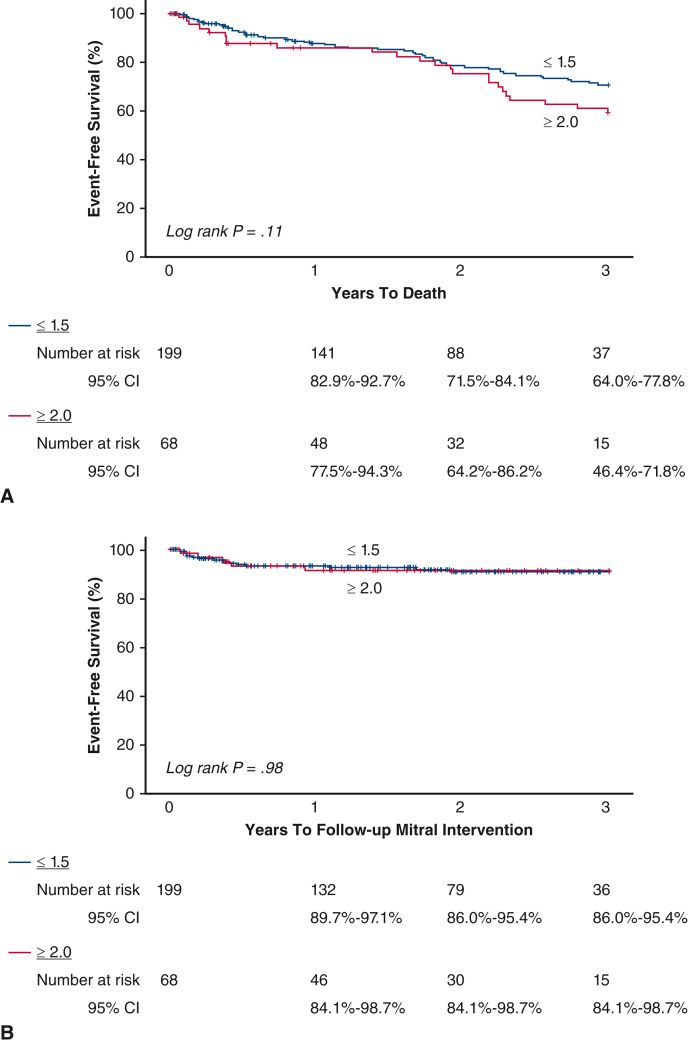
Only long-term death was significantly different between those with and without RRMR, with patients who had RRMR dying more frequently: 42.6% versus 25.6%, P = .008. After adjustment by baseline risk factors, clinical meaningful associations persisted, although not statistically significant (Table E6). Hypertension was the strongest predictor of death (hazard ratio, 5.97 [1.46, 24.35], P = .01). There was no difference in risk between the groups for MV reintervention (Table E6). Increased body mass index was the strongest risk factor associated with MV reintervention. Kaplan–Meier survival estimates and the log rank P-value for long-term death MV reintervention are shown in Figure E2, A and B, respectively.
Figure E2.
Kaplan–Meier survival estimates and log-rank P value for long-term (A) death and (B) MV reintervention stratified by MR ≥2.0 versus ≤1.5 among TEER. MV, Mitral valve; MR, mitral regurgitation; TEER, transcatheter edge-to-edge mitral valve repair; CI, confidence interval.
Discussion
This study was performed to better understand the impact of residual and recurrent MR on clinical outcomes in the context of ongoing randomized clinical trials being designed and conducted in lower risk patients. The main finding of this study is that 2+ or greater residual and recurrent MV independently predicted mortality in patients receiving TEER suggesting the necessity of selecting patients with equal postprocedural probability of eliminating MR and durability for randomized clinical trials.
Other important findings include that in current clinical practice, patients receiving TEER and SMVr are widely different with respect to patient clinical characteristics, risk, and life expectancy, with patients receiving TEER much more medically complex. This makes head-to-head comparisons from this type of analysis statistically challenging. With respect to another meaningful end point, reverse LV remodeling, we observed positive reverse remodeling of LV structure and function following MV repair regardless of repair type.
Given the dramatic differences in patient characteristics, overall survival and need for MV reintervention for the TEER group was worse for patients with TEER at both intermediate and long-term follow-up, but whether than can be extrapolated to lower-risk cohorts is unknown. Further studies are needed to determine whether it is appropriate to lump ≥2+ together or if moderate MR behaves differently than ≥3+ MR, which it likely does.8
Finally, given the mortality differences in patients with RRMR observed in this study, randomized controlled trials should be designed to enroll patients with equal probability of success, to avoid residual 2+ or greater MR, is essential to maintain ethical equipoise necessary for randomization until the ramifications of moderate MR are more fully elucidated.9, 10, 11, 12, 13
The main limitations of the present analysis include the single-system experience and the retrospective nature of this study design, which does not allow proper head-to-head analysis, given the striking differences between the groups with respect to age, pathophysiology (functional vs degenerative MR), and medical risk/life expectancy. Although various statistical methods exist for comparisons between disparate groups, ie, propensity matching, we felt that the extent of the differences, and the profiles of patients receiving each therapy (primary vs secondary MR) were too great for meaningful statistical comparisons. To avoid this type of forced comparison, we, therefore, analyzed each group separately. Ultimately, however, it will require randomized, controlled, clinical trials to balance these factors to provide answers. Another limitation of the study is that, because it is observational, patients were treated at the best clinical judgment of their clinician and therefore ascertainment of tests were not done at specified intervals and so comparisons could not be made at specific timepoints. There is also the possibility that not all follow-up events were captured. Intermountain provides health care to two-thirds of Utah, and specialty care is usually always maintained unless patients move or change insurance requiring a change in provider or in the case of death, is a non-Utah resident and dies outside of an Intermountain facility. Since diagnoses and procedures were identified by ICD codes, there is the possibility of misclassification or missed diagnoses.
Conclusions
We conclude that patients receiving TEER and SMVr are very different from one another with patients who undergo TEER being much older, sicker, and with shorter life expectancy. We also conclude recurrent and residual MR independently predicts mortality, but not repeat mitral valve reintervention in patients who undergo TEER. As randomized clinical trials study younger and healthier patients, it is essential that only patients with equal probability of success (addressing residual MR) are enrolled.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/recurrent-or-residual-mitral-regurgitation-predicts-death-in-transcatheter-mitral-valve-repair.

Conflict of Interest Statement
Dr Whisenant consults for Edwards Lifesciences and Abbott Vascular. Dr McKellar consults for Abbott Vascular. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This study was funded by internal funds from Intermountain Heart Institute and Intermountain Medical Center.
Supplementary Data
Recurrent and residual mitral regurgitation. Video available at: https://www.jtcvs.org/article/S2666-2736(23)00337-6/fulltext.
Appendix E1
Table E1.
Baseline characteristics for patients undergoing SMVr stratified by follow-up MR ≤1.5 versus ≥2.0 within 1 year of procedure
| Overall | MR ≤1.5, n = 368 | MR ≥2.0, n = 25 | P value | |
|---|---|---|---|---|
| Age, y | 63.8 ± 13.3 | 63.7 ± 13.3 | 65.7 ± 13.4 | .46 |
| Sex (male) | 62.3% | 63.6% | 44.0% | .05 |
| Hypertension | 75.3% | 75.0% | 80.0% | .81 |
| Hyperlipidemia | 68.7% | 68.5% | 72.0% | .71 |
| Diabetes | 25.7% | 25.3% | 32.0% | .46 |
| Depression history | 27.0% | 28.0% | 12.0% | .10 |
| Previous myocardial infarction | 14.2% | 13.9% | 20.0% | .38 |
| Coronary artery disease | 60.6% | 60.6% | 60.0% | .95 |
| Heart failure | 61.6% | 60.9% | 72.0% | .27 |
| Previous percutaneous coronary intervention | 7.9% | 8.2% | 4.0% | .71 |
| Previous coronary artery bypass graft | 18.1% | 18.5% | 12.0% | .59 |
| Previous stroke | 7.9% | 8.2% | 4.0% | .71 |
| Previous transient ischemic attack | 7.1% | 7.3% | 4.0% | 1.00 |
| Atrial fibrillation history | 53.7% | 53.0% | 64.0% | .29 |
| Atrial flutter history | 20.4% | 19.8% | 28.0% | .33 |
| Implantable cardioverter defibrillator | 2.8% | 2.4% | 8.0% | .15 |
| Pacemaker | 5.9% | 5.7% | 8.0% | .65 |
| Body mass index, kg/m2 | ||||
| Mean ± SD | 27.4 ± 5.0 | 27.4 ± 5.0 | 27.6 ± 4.3 | .96 |
| Category | .86 | |||
| <25.0 | 31.8% | 32.3% | 24.0% | |
| 25-29.9 | 41.7% | 41.3% | 48.0% | |
| ≥30 | 26.5% | 26.4% | 28.0% | |
| STS predicted risk of mortality score (%), n = 222 | 1.8 ± 3.2 | 1.8 ± 3.2 (n = 211) | 2.3 ± 2.5 (n = 11) | .62 |
| LVEF closest before mitral procedure | ||||
| Number of patients | 378 | 353 | 25 | |
| Days, median (range) | 28 (1, 2272) | 28 (1, 2272) | 24 (1, 1734) | .86 |
| EF | 56.9 ± 11.2 | 57.0 ± 11.2 | 55.8 ± 10.9 | .62 |
| LVIDd closest prior to mitral procedure | ||||
| Number of patients | 356 | 334 | 22 | |
| Days, median (range) | 32 (1, 2952) | 32 (1, 2952) | 35 (1, 624) | .24 |
| LVIDd | 5.1 ± 0.8 | 5.1 ± 0.8 | 5.2 ± 0.7 | .56 |
| Number of patients | 354 | 331 | 23 | |
| Days, median (range) | 33 (1, 2853) | 33 (1, 2853) | 39 (1, 624) | .27 |
| LA size closest before mitral procedure | ||||
| Number of patients | 262 | 242 | 20 | |
| Days, median (range) | 42 (1, 4767) | 41.5 (1, 4767) | 120.5 (1, 2714) | .06 |
| Categories | .20 | |||
| 0 | 14.2% | 13.7% | 20.0% | |
| 1.0 | 22.6% | 23.2% | 15.0% | |
| 1.5 | 19.2% | 18.7% | 25.0% | |
| 2.0 | 15.3% | 14.1% | 30.0% | |
| 2.5 | 8.0% | 8.3% | 5.0% | |
| 3.0 | 20.7% | 22.0% | 5.0% | |
| Right ventricular size closest before mitral procedure | ||||
| Number of patients | 352 | 330 | 22 | |
| Categories | .15 | |||
| Normal to mild | 87.8% | 88.5% | 77.3% | |
| Moderate | 10.2% | 9.4% | 22.7% | |
| Severe | 2.0% | 2.1% | 0% | |
| Right ventricular function closest before mitral procedure | ||||
| Number of patients | 350 | 328 | 22 | |
| Categories | .74 | |||
| Normal to mild | 74.0% | 74.1% | 72.7% | |
| Mild to moderate or reduced | 22.6% | 22.6% | 22.7% | |
| Severely reduced | 3.4% | 3.4% | 4.5% | |
| Tricuspid regurgitation closest before mitral procedure | ||||
| Number of patients | 352 | 330 | 222 | |
| Categories | .04 | |||
| None, trace, or mild | 79.3% | 80.6% | 59.1% | |
| Mild-to-moderate or moderate | 15.6% | 14.5% | 31.8% | |
| Moderate-to-severe or severe | 5.1% | 4.8% | 9.1% |
MR, Mitral regurgitation; SD, standard deviation; STS, Society of Thoracic Surgeons; LVEF, left ventricular ejection fraction; EF, ejection fraction; LVIDd, left ventricular internal diameter end diastole; LA, left atrial; SMVr, surgical mitral valve repair.
Table E2.
Echocardiogram changes among patients who received SMVr, reported as mean ± standard deviation (median), stratified by MR ≤1.5 versus ≥2.0 within 1 year of procedure
| Overall | MR ≤1.5 | MR ≥2.0 | P value | |
|---|---|---|---|---|
| LVEF (baseline to greatest follow-up measurement) | ||||
| Number of patients | 329 | 309 | 20 | |
| Absolute change | 0.44 ± 12.4 (0) | 0.6 ± 12.4 (0) | −1.8 ± 11.1 (−3.5) | .41 |
| Percent change | 7.3 ± 63.5 (0) | 7.9 ± 65.2 (0) | −2.7 ± 26.8 (−5.2) | .51 |
| LVIDd (baseline to lowest follow-up measurement) | ||||
| Number of patients | 317 | 299 | 18 | |
| Absolute change | −0.7 ± 0.7 (−0.7) | −0.7 ± 0.7 (−0.7) | −0.5 ± 0.6 (−0.6) | .20 |
| Percent change | −13.6 ± 13.0 (−14.3) | −13.9 ± 13.0 (−14.5) | −8.9 ± 12.5 (−10.7) | .26 |
| Mitral valve regurgitation severity (highest within 1 y of procedure) | ||||
| Severity category | – | |||
| 2.0+ | 88.0% (22/25) | – | 88.0% (22/25) | |
| 3.0+ | 12.0% (3/25) | – | 12.0% (3/25) | |
| 4.0 | 0% (0/0) | – | 0% (0/0) | |
| Mitral valve stenosis severity (greatest follow-up measurement) | ||||
| Severity category | n = 292 | n = 274 | n = 18 | .46 |
| 0 | 14.0% | 13.5% | 22.2% | |
| 1+ | 58.6% | 59.5% | 44.4% | |
| 2.0+ | 25.7% | 25.2% | 33.3% | |
| 3.0+ | 1.7% | 1.8% | 0% | |
| 4.0 | 0% | 0% | 0% |
MR, Mitral regurgitation; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter end diastole; SMVr, surgical mitral valve repair.
Table E3.
Mortality and mitral valve reintervention at 3 years and long-term among patients who underwent SMVr stratified MR ≤1.5 and MR ≥2.0
| Overall | MR ≤1.5 | MR ≥2.0 | P value | |
|---|---|---|---|---|
| Death | ||||
| 3-y | 10.4% (30) | 10.0% (27) | 17.6% (3) | .26 |
| Long-term | 12.0% (47) | 12.0% (44) | 12.0% (3) | 1.00 |
| Average days of follow-up | 1119.9 ± 682.2 (median, 1098) | 1126.7 ± 672.0 (median, 1085) | 934.3 ± 802.9 (median, 638.0) | .45 |
| Average days to death | 744.7 ± 596.1 (median, 659) | 784.7 ± 595.0 (median, 709) | 159.0 ± 114.5 (median, 176) | .10 |
| Adjusted HR (95% CI), P value | 3-y∗ | Long-term∗ | ||
| Follow-up mitral valve intervention | ||||
| 3-y | 2.4% (7) | 1.8% (5) | 11.8% (2) | .06 |
| Long-term | 2.5% (10) | 2.2% (8) | 8.0% (2) | .13 |
| Average days to follow-up mitral intervention | 600.1 ± 713.2 (median, 388.5) | 699.5 ± 769.7 (median, 202.5) | 202.5 ± 188.8 (median, 202.5) | .40 |
| Adjusted HR (95% CI), P value | 3-y∗ | Long-term∗ | ||
Outcome frequencies are presented as % (n). A total of 288 patients had the opportunity for 3-years of follow-up (MR ≤1.5, n = 271; MR ≥2.0, n = 17). MV, Mitral valve; HR, hazard ratio; CI, confidence interval; SMVr, surgical mitral valve repair.
Not enough events to evaluate.
Table E4.
Baseline characteristics for patients who underwent TEER stratified by follow-up MR ≤1.5 versus ≥2.0 within 1 year of procedure
| Overall | MR ≤1.5, n = 199 | MR ≥2.0, n = 68 | P value | |
|---|---|---|---|---|
| Age, y | 73.6 ± 13.6 | 73.7 ± 12.7 | 73.1 ± 16.0 | .76 |
| Sex (male) | 61.8% | 60.8% | 64.7% | .57 |
| Hypertension | 86.1% | 87.4% | 82.4% | .30 |
| Hyperlipidemia | 75.7% | 78.9% | 66.2% | .04 |
| Diabetes | 36.0% | 40.7% | 22.1% | .006 |
| Depression history | 30.7% | 33.2% | 23.5% | .14 |
| Previous myocardial infarction | 18.0% | 17.1% | 20.6% | .52 |
| Coronary artery disease | 76.4% | 76.9% | 75.0% | .75 |
| Heart failure | 86.9% | 87.4% | 85.3% | .65 |
| Previous percutaneous coronary intervention | 17.6% | 18.6% | 14.7% | .47 |
| Previous coronary artery bypass graft | 8.2% | 9.0% | 5.9% | .61 |
| Previous stroke | 10.9% | 14.1% | 1.5% | .003 |
| Previous transient ischemic attack | 13.1% | 14.6% | 8.8% | .23 |
| Atrial fibrillation history | 68.9% | 68.3% | 70.6% | .73 |
| Atrial flutter history | 29.2% | 29.6% | 27.9% | .79 |
| Implantable cardioverter defibrillator | 15.0% | 14.1% | 17.6% | .48 |
| Pacemaker | 17.2% | 18.6% | 13.2% | .31 |
| Body mass index, kg/m2 | ||||
| Mean ± SD | 26.8 ± 6.2 | 27.6 ± 6.4 | 24.8 ± 4.9 | .001 |
| Category | .007 | |||
| <25.0 | 44.9% | 39.7% | 60.3% | |
| 25-29.9 | 30.7% | 32.2% | 26.5% | |
| ≥30 | 24.3% | 28.1% | 13.2% | |
| STS predicted risk of mortality score (%), n = 4 | 4.3 ± 2.1 | 4.7 ± 2.3 (n = 3) | 3.0 (n = 1) | .60 |
| LVEF closest before mitral procedure | ||||
| Number of patients | 257 | 194 | 63 | |
| Days, median (range) | 15.5 (1, 3660) | 18 (1, 2275) | 13 (1, 3660) | .19 |
| EF | 49.1 ± 15.8 | 49.6 ± 15.8 | 47.6 ± 15.7 | .38 |
| LVIDd closest before mitral procedure | ||||
| Number of patients | 252 | 190 | 62 | |
| Days, median (range) | 30.5 (1, 3660) | 33 (1, 3256) | 22 (1, 3660) | .01 |
| LVIDd | 5.3 ± 1.0 | 5.3 ± 1.0 | 5.4 ± 1.2 | .27 |
| LA size closest prior to mitral procedure | ||||
| Number of patients | 204 | 159 | 45 | |
| Days, median (range) | 40.5 (1, 4293) | 39 (1, 4160) | 43 (1, 4293) | .99 |
| Categories | .13 | |||
| 0 | 8.8% | 9.4% | 6.7% | |
| 1.0 | 12.3% | 10.1% | 20.0% | |
| 1.5 | 9.8% | 9.4% | 11.1% | |
| 2.0 | 18.6% | 18.9% | 17.8% | |
| 2.5 | 5.4% | 3.8% | 11.1% | |
| 3.0 | 45.1% | 48.4% | 33.3% | |
| Right ventricular size closest before mitral procedure | ||||
| Number of patients | 248 | 187 | 61 | |
| Categories | .52 | |||
| Normal to mild | 73.4% | 74.3% | 70.5% | |
| Moderate | 19.8% | 18.2% | 24.6% | |
| Severe | 6.9% | 7.5% | 4.9% | |
| Right ventricular function closest prior to mitral procedure | ||||
| Number of patients | 248 | 187 | 61 | |
| Categories | .70 | |||
| Normal to mild | 61.0% | 57.4% | 60.1% | |
| Mild to moderate or reduced | 38.0% | 41.0% | 38.7% | |
| Severely reduced | 1.1% | 1.6% | 1.2% | |
| Tricuspid regurgitation closest before mitral procedure | ||||
| Number of patients | 248 | 187 | 61 | |
| Categories | .11 | |||
| None, trace, or mild | 52.4% | 52.9% | 50.8% | |
| Mild-to-moderate or moderate | 35.5% | 37.4% | 29.5% | |
| Moderate-to-severe or severe | 12.1% | 9.6% | 19.7% |
MR, Mitral regurgitation; SD, standard deviation; STS, Society of Thoracic Surgeons; LVEF, left ventricular ejection fraction; EF, ejection fraction; LVIDd, left ventricular internal diameter end diastole; LA, left atrial; TEER, transcatheter edge-to-edge mitral valve repair.
Table E5.
Echocardiogram changes among patients who underwent TEER, reported as mean ± standard deviation (median), stratified by MR ≤1.5 versus ≥2.0 within 1 year of procedure
| Overall | MR ≤1.5 | MR ≥2.0 | P value | |
|---|---|---|---|---|
| LVEF (baseline to greatest follow-up measurement) | ||||
| Number of patients | 232 | 174 | 58 | |
| Absolute change | 4.4 ± 10.5 (4.0) | 4.7 ± 11.3 (4.0) | 3.3 ± 7.5 (5.0) | .89 |
| Percent change | 15.6 ± 46.1 (7.7) | 17.5 ± 52.0 (6.7) | 9.9 ± 19.0 (9.2) | .66 |
| LVIDd (baseline to lowest follow-up measurement) | ||||
| Number of patients | 234 | 178 | 56 | |
| Absolute change | −0.4 ± 0.8 (−0.4) | −0.4 ± 0.8 (−0.4) | −0.4 ± 0.7 (−0.5) | .46 |
| Percent change | −7.4 ± 14.9 (−7.8) | −7.6 ± 14.1 (−7.7) | −6.8 ± 17.4 (−8.8) | .55 |
| Mitral valve regurgitation severity (greatest within 1 year of procedure) | ||||
| Severity category | – | |||
| 2.0+ | 86.8% (59/68) | – | 86.8% (59/68) | |
| 3.0+ | 13.2% (9/68) | – | 13.2% (9/68) | |
| 4.0 | 0% (0/0) | – | 0% (0/0) | |
| Mitral valve stenosis severity (greatest follow-up measurement) | ||||
| Severity category | N = 198 | n = 149 | n = 49 | .03 |
| 0 | 13.6% | 10.7% | 22.4% | |
| 1+ | 41.4% | 42.3% | 38.8% | |
| 2.0+ | 37.9% | 37.6% | 38.8% | |
| 3.0+ | 7.1% | 9.4% | 0% | |
| 4.0 | 0% | 0% | 0% |
MR, Mitral regurgitation; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter end diastole; TEER, transcatheter edge-to-edge mitral valve repair.
Table E6.
Mortality and mitral valve reintervention at 3 years and long-term among patients who received TEER stratified MR ≤1.5 and MR ≥2.0
| Overall | MR ≤1.5 | MR ≥2.0 | P value | |
|---|---|---|---|---|
| Death | ||||
| 3-y | 37.9% (61) | 35.3% (41) | 44.4% (20) | .37 |
| Long-term | 30.0% (80) | 25.6% (51) | 42.6% (29) | .008 |
| Average days of follow-up | 697.6 ± 478.1 (median, 662) | 696.7 ± 473.8 (median, 660) | 700.4 ± 494.0 (median, 681.5) | .98 |
| Average days to death | 572.2 ± 420.6 (median, 618.5) | 502.2 ± 351.8 (median, 519) | 695.3 ± 503.6 (median, 707) | .14 |
| Adjusted HR (95% CI), P-value | 3-y: HR, 1.63 (0.99-2.68), P = .05 | Long-term: HR, 1.47 (0.92-2.34), P = .11 | ||
| Follow-up mitral valve intervention | ||||
| 3-y | 9.3% (15) | 10.3% (12) | 6.7% (3) | .35 |
| Long-term | 7.5% (20) | 7.5% (15) | 7.4% (5) | 1.00 |
| Average days to follow-up mitral intervention | 173.3 ± 193.8 (median, 119.5) | 183.2 ± 215.6 (median, 108.0) | 143.6 ± 119.0 (median, 131.0) | 1.00 |
| Adjusted HR (95% CI), P-value | 3-y: HR, 1.05 (0.35-3.18), P = .93 | Long-term: HR, 1.06 (0.35-3.22), P = .92 | ||
Outcome frequencies are presented as % (n). A total of 161 patients had the opportunity for 3 years of follow-up (MR ≤1.5, n = 116; MR ≥2.0, n = 45). MR, Mitral regurgitation; HR, hazard ratio; CI, confidence interval; TEER, transcatheter edge-to-edge mitral valve repair.
References
- 1.Feldman T., Kar S., Rinaldi M., Fail P., Hermiller J., Smalling R., et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair study) cohort. J Am Coll Cardiol. 2009;54:686–694. doi: 10.1016/j.jacc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 2.Feldman T., Foster E., Glower D.D., Kar S., Rinaldi M.J., Fail P.S., et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 3.Stone G.W., Lindenfeld J., Abraham W.T., Kar S., Lim D.S., Mishell J.M., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 4.Obadia J.-F., Messika-Zeitoun D., Leurent G., Iung B., Bonnet G., Piriou N., et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy P.M., Whisenant B., Asgar A.W., Ailawadi G., Hermiller J., Williams M., et al. Percutaneous MitraClip device or surgical mitral valve repair with primary mitral regurgitation who are candidates for surgery: design and rationale for the REPAIR MR trial. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.027504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Percutaneous or surgical mitral valve repair (PRIMARY trial) https://clinicaltrials.gov/ct2/show/NCT05051033
- 7.Suri R.M., Schaff H.V., Dearani J.D., Sundt T.M., Daly R.C., Mullany C.J., et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg. 2006;82:819–826. doi: 10.1016/j.athoracsur.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi S., Orban M., Stolz L., Karam N., Praz F., Kalbacher D., et al. Impact of residual mitral regurgitation on survival after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Interv. 2021;14:1243–1253. doi: 10.1016/j.jcin.2021.03.050. [DOI] [PubMed] [Google Scholar]
- 9.Anwer L.A., Dearani J.A., Daly R.C., Stulak J.M., Schaff H.V., Nguyen A., et al. Degenerative mitral regurgitation after nonmitral cardiac surgery: MitraClip versus surgical reconstruction. Ann Thorac Surg. 2019;107:725–731. doi: 10.1016/j.athoracsur.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Puls M., Lubos E., Boekstegers P., von Bardeleben R.S., Ouarrak T., Butter C., et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. 2016;37:703–712. doi: 10.1093/eurheartj/ehv627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capodanno D., Adomo M., Barbanti M., Giannini C., Laudisa M.L., Cannata S., et al. Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J. 2015;170:187–195. doi: 10.1016/j.ahj.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Paranskaya L., D’Ancona G., Bodzag-Turan I., Akin I., Kische S., Turan G.R., et al. Residual mitral regurgitation after percutaneous mitral valve repair with the MitraClip system is a risk factor for adverse one-year outcome. Catheter Cardiovasc Interv. 2013;81:609–617. doi: 10.1002/ccd.24586. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura A., Kavsur R., Spieker M., Iliadis C., Goto T., Öztürk C., et al. Recurrent mitral valve regurgitation after MitraClip: predictive factors, morphology, and clinical implication. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.121.010895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recurrent and residual mitral regurgitation. Video available at: https://www.jtcvs.org/article/S2666-2736(23)00337-6/fulltext.