Abstract
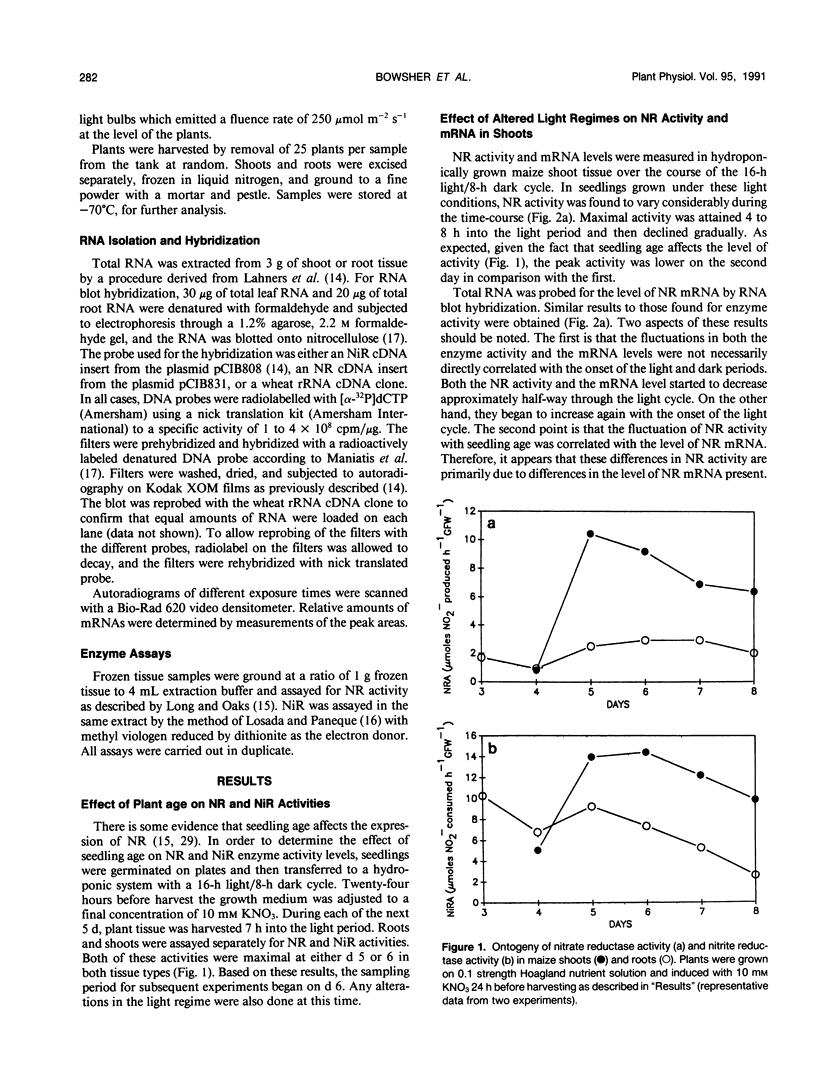
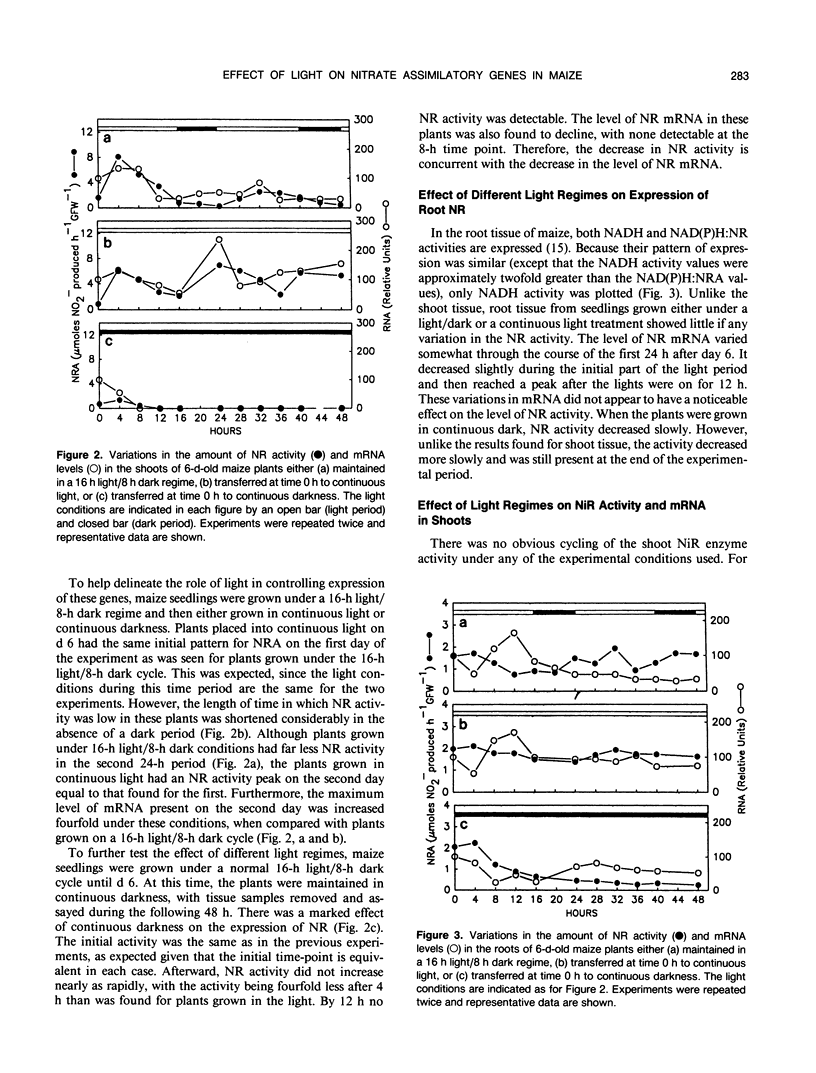
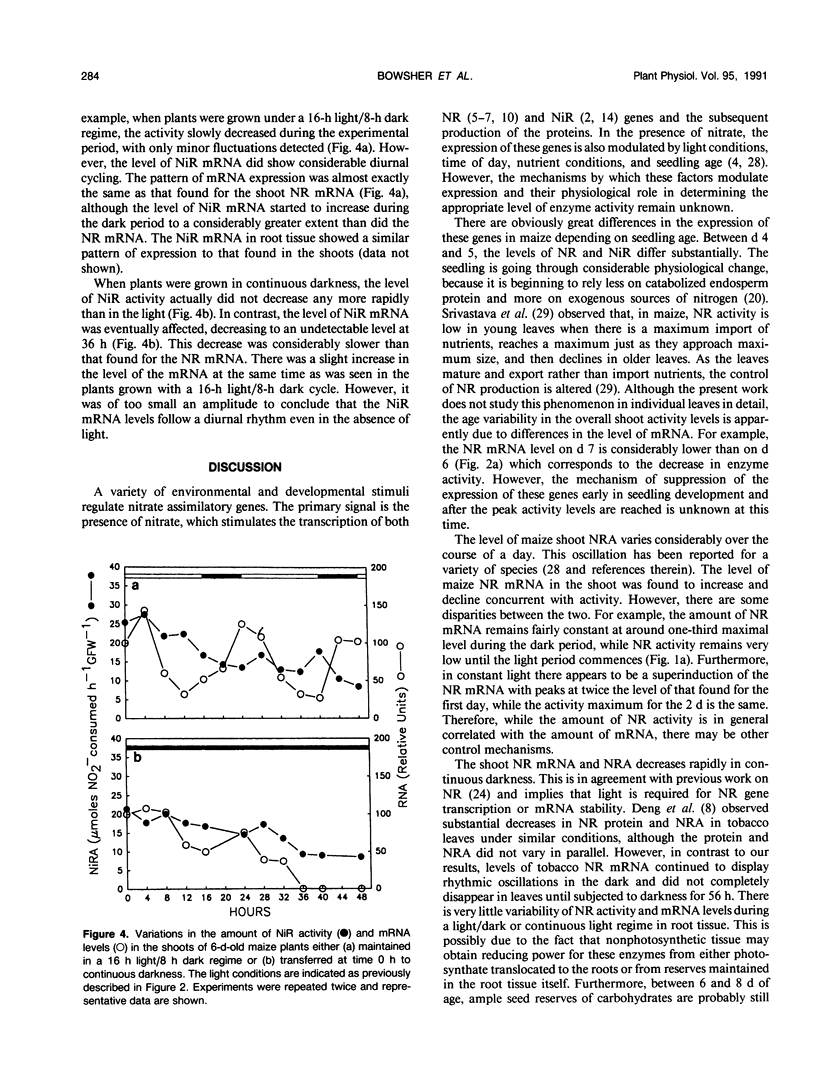
The level of nitrate reductase (NR) and nitrite reductase (NiR) varied in both shoot and root tissue from nitrate-fed Zea mays L. grown under a 16-hour light/8-hour dark regime over a 10-day period postgermination, with peak activity occurring in days 5 to 6. To study the effect of different light regimes on NR and NiR enzyme activity and mRNA levels, 6-day-old plants were grown in the presence of continuous KNO3 (10 millimolar). Both shoot NRA and mRNA varied considerably, peaking 4 to 8 hours into the light period. Upon transferring plants to continuous light, the amplitude of the peaks increased, and the peaks moved closer together. In continuous darkness, no NR mRNA or NR enzyme activity could be detected by 8 hours and 12 hours, respectively. In either a light/dark or continuous light regime, root NRA and mRNA did not vary substantially. However, when plants were placed in continuous darkness, both declined steadily in the roots, although some remained after 48 hours. Although there was no obvious cycling of NiR enzyme activity in shoot tissue, changes in mRNA mimicked those seen for NR mRNA. The expression of NR and NiR genes is affected by the light regime adopted, but light does not have a direct effect on the expression of these genes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back E., Burkhart W., Moyer M., Privalle L., Rothstein S. Isolation of cDNA clones coding for spinach nitrite reductase: complete sequence and nitrate induction. Mol Gen Genet. 1988 Apr;212(1):20–26. doi: 10.1007/BF00322440. [DOI] [PubMed] [Google Scholar]
- Calza R, Huttner E, Vincentz M, Rouzé P, Galangau F, Vaucheret H, Chérel I, Meyer C, Kronenberger J, Caboche M. Cloning of DNA fragments complementary to tobacco nitrate reductase mRNA and encoding epitopes common to the nitrate reductases from higher plants. Mol Gen Genet. 1987 Oct;209(3):552–562. doi: 10.1007/BF00331162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. L., Dewdney J., Kleinhofs A., Goodman H. M. Cloning and nitrate induction of nitrate reductase mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6825–6828. doi: 10.1073/pnas.83.18.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Campbell W. H., Davis R. W. Nitrate reductase from squash: cDNA cloning and nitrate regulation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8073–8076. doi: 10.1073/pnas.83.21.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galangau F., Daniel-Vedele F., Moureaux T., Dorbe M. F., Leydecker M. T., Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988 Oct;88(2):383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Hoffman N. E., Ko K., Scolnik P. A., Cashmore A. R. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988 Dec 1;7(12):3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C., Beevers L. Synthesis and degradation of nitrite reductase in pea leaves. Plant Physiol. 1984 May;75(1):251–252. doi: 10.1104/pp.75.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Sheard R. W. Nitrate reductase activity: phytochrome mediation of induction in etiolated peas. Nat New Biol. 1972 Aug 16;238(85):221–222. doi: 10.1038/newbio238221a0. [DOI] [PubMed] [Google Scholar]
- Lahners K., Kramer V., Back E., Privalle L., Rothstein S. Molecular cloning of complementary DNA encoding maize nitrite reductase: molecular analysis and nitrate induction. Plant Physiol. 1988 Nov;88(3):741–746. doi: 10.1104/pp.88.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D. M., Oaks A. Stabilization of nitrate reductase in maize roots by chymostatin. Plant Physiol. 1990 Jul;93(3):846–850. doi: 10.1104/pp.93.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Grimm B., Ottersbach P., Kloppstech K. Circadian Control of the Accumulation of mRNAs for Light- and Heat-Inducible Chloroplast Proteins in Pea (Pisum sativum L.). Plant Physiol. 1988 Sep;88(1):21–25. doi: 10.1104/pp.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen H., Bogorad L. Diurnal and Circadian Rhythms in the Accumulation and Synthesis of mRNA for the Light-Harvesting Chlorophyll a/b-Binding Protein in Tobacco. Plant Physiol. 1988 Dec;88(4):1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmler J. L., Campbell W. H. Regulation of Corn Leaf Nitrate Reductase : II. Synthesis and Turnover of the Enzyme's Activity and Protein. Plant Physiol. 1986 Feb;80(2):442–447. doi: 10.1104/pp.80.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Mackown C. T., Volk R. J. Effects of Altered Carbohydrate Availability on Whole-Plant Assimilation of NO(3). Plant Physiol. 1989 Feb;89(2):457–463. doi: 10.1104/pp.89.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. A., Kuo T. M., Kleinhofs A., Warner R. L., Oaks A. Synthesis and degradation of barley nitrate reductase. Plant Physiol. 1983 Aug;72(4):949–952. doi: 10.1104/pp.72.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]


