Abstract
Expression of the prpBCDE operon of Salmonella typhimurium LT2 required (i) the synthesis of propionyl-coenzyme A (CoA) by the PrpE protein or the acetyl-CoA-synthesizing systems of the cell and (ii) the synthesis of 2-methylcitrate from propionyl-CoA and oxaloacetate by the PrpC protein. We propose that either 2-methylcitrate or a derivative of it signals the presence of propionate in the environment. This as yet unidentified signal is thought to serve as a coregulator of the activity of PrpR, the member of the sigma-54 family of transcriptional activators needed for activation of prpBCDE transcription. The CobB protein was also required for expression of the prpBCDE operon, but its role is less well understood. Expression of the prpBCDE operon in cobB mutants was restored to wild-type levels upon induction of the propanediol utilization (pdu) operon by 1,2-propanediol. This effect did not require catabolism of 1,2-propanediol, suggesting that a Pdu protein, not a catabolite of 1,2-propanediol, was responsible for the observed effect. We explain the existence of these redundant functions in terms of metabolic pathway integration. In an environment with 1,2-propanediol as the sole carbon and energy source, expression of the prpBCDE operon is ensured by the Pdu protein that has CobB-like activity. Since synthesis of this Pdu protein depends on the availability of 1,2-propanediol, the cell solves the problem faced in an environment devoid of 1,2-propanediol where propionate is the sole carbon and energy source by having cobB located outside of the pdu operon and its expression independent of 1,2-propanediol. At present, it is unclear how the CobB and Pdu proteins affect prpBCDE expression.
Understanding metabolic pathway integration is a central issue in cell physiology. Learning more about this important aspect of cell function requires that we uncover and dissect the strategies used by the cell to ensure the coordinated and timely synthesis or degradation of metabolites.
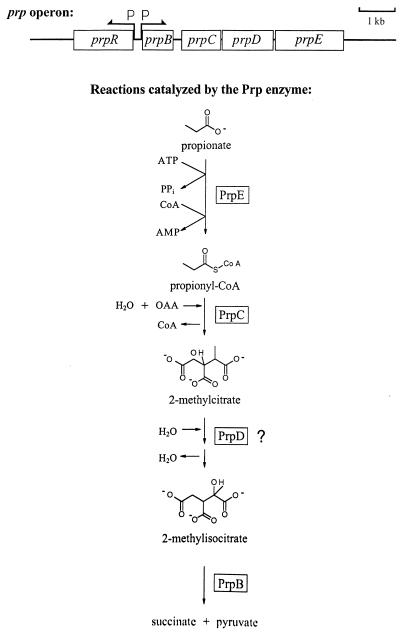
In the recent past, the catabolism of propionic acid in Salmonella typhimurium LT2 (11, 13, 34) and Escherichia coli (9, 32) has been investigated. The cluster of genes required for the catabolism of propionate in these bacteria was first identified in and characterized for S. typhimurium LT2 (11, 13), with the closely related gene cluster in E. coli being reported as part of the genome project of this bacterium (3). These genes are referred to as the prp genes and are located in the 8.5-centisome region of the chromosome. These genes constitute a locus comprised of five genes organized in two transcriptional units (Fig. 1). One of these units contains the prpR gene, which encodes a putative member of the sigma-54 (RpoN) family of transcriptional activators (27). PrpR activity is required for the catabolism of propionate in S. typhimurium (20). The second transcriptional unit contains the prpBCDE gene cluster, which is organized as an operon that encodes propionate-degrading enzymes (13, 14). Work with E. coli has identified PrpC as the 2-methylcitrate synthase (9, 32). The 2-methylcitric acid cycle was first discovered in Yallowia lipolytica (2, 29, 30), and in this cycle propionyl-coenzyme A (CoA) is α-oxidized to pyruvate (Fig. 1).
FIG. 1.
Organization of the prpRBCDE gene cluster and reactions proposed to be catalyzed by the prp gene products in the 2-methylcitric acid cycle. PrpE, propionyl-CoA synthetase (14); PrpC, 2-methylcitrate synthase (15); PrpD, putative 2-methylisocitrate synthase; PrpB, putative 2-methylisocitrate lyase. P, promoter.
Our laboratory discovered that in addition to the prpRBCDE genes, catabolism of propionate in S. typhimurium requires the activity encoded by the cobB gene (34). The CobB gene product was first identified as an activity required for the expression of a phosphoribosyltransferase enzyme that restored synthesis of adenosylcobalamin in strains defective in the late steps of the biosynthesis of this coenzyme (33).
We have also reported that the inability of cobB mutants to catabolize propionate was corrected by the induction of the pdu genes required for the catabolism of 1,2-propanediol (1,2-PDL) (34). This observation was of interest to us because propionate, or an activated form of it (not clear at this point), is the end product of the pathway responsible for the catabolism of 1,2-PDL (4, 16, 37). We noted that induction of the pdu operon, without catabolism of 1,2-PDL, was sufficient to correct the Prp− phenotype of cobB mutants. This finding was interpreted to mean that 1,2-PDL-dependent correction of this phenotype was due to the synthesis of a Pdu protein that compensated for the lack of CobB when propionate was the sole source of carbon and energy (34).
To better understand the implications of these results, it was essential to learn more about the roles of the Pdu and CobB proteins in propionate catabolism. These proteins may be enzymes that catalyze the same reaction, they may be regulatory proteins, or they may be both. Of particular interest to us was the possibility that CobB plays a regulatory role in the catabolism of propionate, since compensating for its absence through the induction of the pdu genes would provide evidence for pathway networking.
The regulation of expression of the prpBCDE operon is complex. PrpR is required, and at this point we assume that it has a coregulator, although it has not yet been identified. Propionate per se is not the coregulator, since it fails to induce transcription of the operon. This finding was shown by using transcriptional fusions of MudI1734 (lacZ+) elements (5) under the control of the prpBCDE promoter (PprpBCDE) (11). Expression of the fusion was observed only in merodiploid strains that carry a wild-type copy of prpBCDE and a second copy of the operon, into which the MudI1734 element was inserted. These results were interpreted to mean that a catabolite of propionate was the signal for the presence of propionate in the environment.
In this paper, we show that CobB, PrpC, and PrpE are needed for transcription of the prpBCDE operon. We also show that the pdu function that allows growth of cobB mutants on propionate does so by fully restoring transcription of the prpBCDE operon. We suggest that 2-methylcitrate, the proposed product of the reaction catalyzed by PrpC (9, 32) or a derivative of it, is the coregulator needed for PrpR to activate transcription of the operon. Consistent with the requirement for PrpC activity, reduction of the intracellular levels of propionyl-CoA in a prpC+ strain mimicked the effect of a prpC mutation on the expression of the prpBCDE operon. The roles of CobB and of the uncharacterized Pdu protein are discussed within the framework of metabolic pathway integration and physiological strategies used by the cell to ensure expression of target genes in response to multiple environmental stimuli.
MATERIALS AND METHODS
Bacterial strains, medium, and growth conditions.
All strains used in this work were derivatives of S. typhimurium LT2, and their genotypes and all plasmids used are listed in Table 1. Cells were grown as detailed in Table 1 or the figure legends. The no-carbon E (NCE) medium was used as minimal medium. Nutrients and their concentrations in the medium were as follows: propionate, 30 mM; 1,2-PDL, 12 mM; glycerol, 22 mM; methionine, 0.5 mM; d-(+)-arabinose, 500 μM; and MgSO4, 1 mM. Antibiotics were used at concentrations previously reported (8).
TABLE 1.
Strain and plasmid list
| Straina or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| LT2 | Wild type | Lab collection |
| Derivatives of LT2 | ||
| SMS209 | pta209::Tn10 | R. LaRossa |
| SMS408 | ack408::Tn10 | R. LaRossa |
| JE4265 | DUP1033[proC-pyrC] prpC114::MudI1734b DEL1184cobB | |
| JE4334 | DUP1033[proC-pyrC] prpC114::MudJ | |
| TR6583 (formerly SA2979) | metE205 ara-9 | K. Sanderson via J. Roth |
| Derivatives of TR6583 | ||
| JE4044 | pPRP25 | |
| JE4388 | prpB210/pPRP25 | |
| JE4389 | prpB195/pPRP25 | |
| JE4390 | prpC173/pPRP25 | |
| JE4391 | prpC167/pPRP25 | |
| JE4392 | prpD174/pPRP25 | |
| JE4393 | prpD169/pPRP25 | |
| JE4394 | prpE213::kan/pPRP25 | |
| JE4395 | prpE213::kan DEL1231 acs/pPRP25 | |
| JE4396 | prpE213::kan DEL1231 acs ack408::Tn10/pPRP25 | |
| JE4397 | prpE213::kan DEL1231 acs pta209::Tn10/pPRP25 | |
| JE4199 | prpC114::MudJ | |
| JE4359 | DUP1033[proC-pyrC] prpC114::MudJ pdu-8::MudA DEL1184cobB | |
| JE4401 | prpC114::MudI1734/pBAD30 | |
| JE4402 | JE4265/pCOBB5 cobB+ cat+ (vector pSU19) | |
| JE4521 | prpE213::kan ack408::Tn10 | |
| JE4524 | DEL1231 acs ack408::Tn10 | |
| JE4334 | DUP1033[proA-pyrC] prpC114::MudJ | |
| JE4265 | DEL1184cobB DUP1033[proA-pyrC] prpC114::MudJ | |
| Plasmids | ||
| pRS551 | Cloning vector with a promoterless lacZ+ gene, kan+ | 28 |
| pSU19 | Cloning vector, cat+ (Cmr) | 17 |
| pBAD30 | Expression vector, ParaBAD bla+ (Apr) | 10 |
| pPRP21 | prpB+ in pBAD30, bla+ (Apr) | 13 |
| pPRP25 | PprpBCDE-lacZ kan+ | |
| pPRP35 | prpC+ in pBAD30, bla+ (Apr) | 13 |
| pPRP36 | prpD+ in pBAD30, bla+ (Apr) | 13 |
| pPRP54 | prpE+ in pBAD30, bla+ (Apr) | 14 |
| pCOBB5 | cobB+ cloned into pSU19 cat+ (Cmr) | 35 |
All strains used were derivatives of S. typhimurium LT2, and unless otherwise stated, they were constructed during the course of this study.
Hereafter and throughout the text, MudI1734 is also referred to as MudJ.
Genetic techniques. (i) Transductions.
All genetic crosses were performed with the high-level-transduction mutant phage P22 HT105 int-201 (25, 26) as described elsewhere (7). Transductants were freed of phage by streaking on indicator plates (6).
(ii) Complementation analysis of prp point mutants.
The prpB, prpC, prpD, and prpE mutants used in experiments aimed at determining the involvement of prp functions in the expression of the prpBCDE operon were isolated after localized chemical mutagenesis with hydroxylamine (7, 12) as previously described (13). It was assumed that a single mutation was responsible for the inactivation of a gene product; however, it was not determined how many mutations were present in each affected gene. It should be noted that each one of these mutants was complemented by plasmids carrying the wild-type allele of the appropriate gene under the control of the arabinose-inducible promoter ParaBAD (13). The construction of these plasmids has been reported previously (13, 14). Plasmids were introduced into recipient strains by transformation (31). Inheritance of the plasmid was ensured by selecting for the antibiotic resistance carried by the cloning vector. Transformants were replica printed to NCE minimal medium supplemented with magnesium, propionate, and methionine.
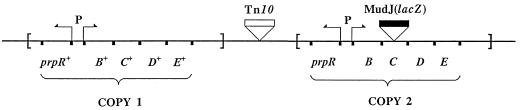
(iii) Construction of a cobB mutant carrying a duplication of the prpRBCDE genes.
A Tn10-held duplication of the S. typhimurium LT2 spanning the 8- to 26-centisome region of the chromosome (DUP1033[proA-pyrC]) was moved by transduction into a strain carrying a deletion of cobB by selecting for tetracycline resistance. The insertion prpC114::MudJ was placed by transduction into one of the copies of the prpBCDE operon present within the duplicated region (Fig. 2). As reported elsewhere, the resulting cobB mutant was unable to grow on propionate (34). cobB mutant strains containing this duplication displayed a Prp− phenotype and were routinely grown in the presence of tetracycline to avoid segregation of the duplicated material (1).
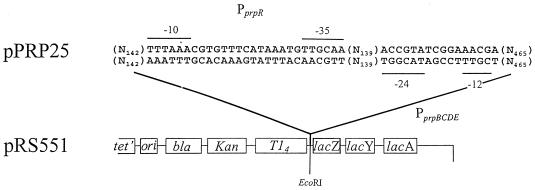
FIG. 2.
Structure of reporter plasmid pPRP25. Details of the cloning are described in Materials and Methods. The orientation of the cloned fragment was verified by sequencing.
Recombinant DNA techniques. (i) DNA sequencing.
Plasmid DNA was isolated with a QIAprep Spin Plasmid Miniprep kit of Qiagen Inc. (Chatsworth, Calif.) by following the manufacturer’s instructions without modifications. PCR sequencing reaction mixtures were prepared with an ABI PRISM Dye Terminator Cycle Sequencing kit (Perkin-Elmer, Norwalk, Conn.) according to the manufacturer’s instructions. Reaction mixtures were purified in AutoSeq G-50 columns (Pharmacia Biotech, Piscataway, N.J.), dried in a SpeedVac concentrator (Savant Instruments, Farmingdale, N.Y.), and sequenced at the Biotechnology Center of the University of Wisconsin—Madison.
(ii) Plasmid constructions.
A ca.-790-bp EcoRI fragment from plasmid pPRP8 (13) containing PprpBCDE was cloned into the EcoRI site of plasmid pRS551 (28) to place the promoterless lacZ+ gene in pRS551 under the control of PprpBCDE. The resulting plasmid, pPRP25 (Fig. 3), was used as a reporter of promoter activity. The orientation of this fragment was confirmed by DNA sequencing. Plasmid pPRP35 carries a wild-type allele of prpC under the arabinose-inducible promoter ParaBAD. The construction of this plasmid has been reported previously (13).
FIG. 3.
Tn10-held chromosomal tandem duplication of the region containing the prpRBCDE loci. The MudJ insertion places the lacZ gene under the control of PprpBCDE. P, promoter.
(iii) Electroporation.
Plasmids were introduced into recipient cells by electroporation under conditions described elsewhere (19). Resistance to the antibiotic encoded by the plasmid was used to assess inheritance. Plasmid DNA was isolated as described above.
β-Galactosidase enzyme activity assay.
β-Galactosidase activity assays were performed as described elsewhere (8). A unit of activity was defined as the amount of enzyme required to catalyze the hydrolysis of 1 nmol of o-nitrophenyl-α-d-galactopyranoside (ONPG) per min. Specific activity was reported as units per unit of absorbance at 650 nm (A650).
RESULTS
In a previous report, we showed that expression of a prp-lacZ transcriptional fusion occurs only in merodiploid strains that carry a wild-type copy of the prpBCDE operon. This result suggested that the activity of one or more of the Prp proteins was needed to generate the catabolite of propionate required for activation of transcription of the operon (11).
Identification of prp functions encoded by the prpBCDE operon needed for expression of prpBCDE.
To determine which functions encoded by the prpBCDE operon were required for its transcription, we investigated the effect that missense mutations in prpB, prpC, or prpD had on the expression of a plasmid-encoded lacZ reporter gene placed under the control of PprpBCDE (plasmid pPRP25). Allele prpE213, a kanamycin resistance cassette, was inserted into prpE by recombinant means (14). This insertion element ensured inactivation of prpE function, and since prpE is the most downstream gene in the operon, polarity of this insertion was not a concern.
Strains carrying mutations in prpB, prpC, or prpD displayed a severe Prp− phenotype that, in all cases, was corrected by introducing into the strains a plasmid carrying a wild-type allele of the gene affected by the mutation. prpE mutants with an otherwise wild-type genetic background did not display a Prp− phenotype due to redundant functions in the background (13, 14). When these functions were inactivated, however, the Prp− phenotype of prpE mutants was complemented by a wild-type allele of prpE.
Complementation of the Prp− phenotype of prpB, prpC, prpD, and prpE mutant strains by a single-gene plasmid showed that the mutations tested did not affect the synthesis of other gene products in the operon. Except for prpE, two independently isolated alleles of each gene were used in these studies to help validate the conclusions drawn. All strains tested carried a wild-type allele of prpR, the putative activator of prpBCDE transcription (13).
Loss of prpC function resulted in a severe reduction (ca. 95%) in the level of expression of the operon relative to the level of expression in the wild-type strain (Table 2). Lack of any of the remaining prp functions did not have as severe an effect. Noteworthy was the effect observed in strains lacking PrpD. In these mutants the level of prpBCDE expression was reduced ca. 66% (Table 2). Lack of PrpE reduced the level of expression by 30% (Table 2), and lack of PrpB resulted in a ca. 10% reduction in the level of expression of the operon relative to that in the wild type (Table 2). The roles of PrpD and PrpE are discussed further below.
TABLE 2.
Effect of prp mutations on the expression of the prpBCDE operon
| Strain | Relevant genotypea | Mean β-galactosidase activity ± SD (U/A650 unit)b | % Expressionc |
|---|---|---|---|
| JE4043 | prpRBCDE+/pRS551 (vector control) | 27 ± 17 | NA |
| JE4044 | prpRBCDE+/pPRP25 (PprpBCDE-lacZ+) | 20,696 ± 850 | 100 |
| JE4390 | prpC173/pPRP25 (PprpBCDE-lacZ+) | 1,320 ± 130 | 6 |
| JE4391 | prpC167/pPRP25 (PprpBCDE-lacZ+) | 974 ± 160 | 5 |
| JE4393 | prpD169/pPRP25 (PprpBCDE-lacZ+) | 6,987 ± 687 | 34 |
| JE4392 | prpD174/pPRP25 (PprpBCDE-lacZ+) | 7,568 ± 655 | 36 |
| JE4394 | prpE213/pPRP25 (PprpBCDE-lacZ+) | 14,039 ± 1,578 | 68 |
| JE4389 | prpB195/pPRP25 (PprpBCDE-lacZ+) | 18,767 ± 1,102 | 91 |
| JE4388 | prpB210/pPRP25 (PprpBCDE-lacZ+) | 18,267 ± 395 | 88 |
All strains were derivatives of strain TR6583 (metE205 ara-9).
Assays were performed with mid-log-phase cultures grown in NCE medium supplemented with propionate, glycerol, and methionine. Assays conditions have been described elsewhere (7). A unit of activity was defined as the amount of enzyme that catalyzed the hydrolysis of 1 nmol of ONPG per min.
Obtained by dividing the level of expression of the lacZ gene in the mutant by the level of expression of the lacZ gene in the wild-type strain and multiplying by 100. Numbers are rounded up to the closest integer. NA, not applicable.
These results indicated that PrpC activity was necessary for expression of the prpBCDE operon. We hypothesize that PrpC is probably involved in synthesizing the coregulator needed by PrpR to activate transcription of the prpBCDE operon.
As shown below, regulation of expression of the prpBCDE operon requires additional activities; that is, although PrpC activity was necessary, it was not sufficient to ensure expression of the operon.
PrpC provided in trans restores activity of the prpBCDE promoter in a haploid strain.
Data presented in Table 3 demonstrate that the lack of PrpC activity was the reason why the prpC114::MudJ transcriptional fusion present in the chromosome was not expressed if the strain did not carry an additional, wild-type copy of the prpBCDE operon (11). When prpC+ was provided in trans, a 28-fold increase in expression of the prpC114::MudJ fusion was measured.
TABLE 3.
prpC function provided in trans restores prpBCDE operon expression in a haploid strain
| Strain | Relevant genotypea | Mean β-galactosidase activity ± SD (U/A650 unit)b
|
|
|---|---|---|---|
| − Arabinose | + Arabinose | ||
| JE4401 | prpC114::MudJ/pBAD30 (vector control) | 56 ± 7 | 61 ± 2 |
| JE4199 | prpC114::MudJ/pPRP35 ParaBAD prpC+ | 1,390 ± 136 | 1,564 ± 59 |
All strains were derivatives of strain TR6583 (metE205 ara-9).
Assays were performed with mid-log-phase cultures grown in NCE medium supplemented with propionate (30 mM), glycerol (22 mM), methionine (0.5 mM), and arabinose (0.5 mM) whenever indicated. Assay conditions have been described elsewhere (8). A unit of activity was defined as the amount of enzyme that catalyzed the hydrolysis of 1 nmol of ONPG per min.
We note that similar levels of expression were measured when arabinose was not included in the medium, indicating that residual expression of prpC in the absence of the inducer resulted in levels of PrpC protein that were sufficient to restore full expression of the fusion. In contrast, the presence of arabinose was required to correct the Prp− phenotype of a prpC missense mutant; that is, complementation of function was not observed in the absence of arabinose (data not shown). These results demonstrated the ability of arabinose to substantially increase the level of PrpC in the cell. As expected, the growth phenotype of strain JE4199 (prpC114::MudJ/pPRP35 ParaBAD prpC+) was not corrected in the presence or absence of arabinose (data not shown but see reference 13). These results were consistent with the need for prpD and prpE gene products to catabolize propionate. Neither PrpD nor PrpE is synthesized in strain JE4199 due to the polar effect of the MudJ element on prpD and prpE.
The dramatic increase in expression of the fusion in the absence of prpD and prpE gene products strongly suggested that the propionate catabolite needed for expression of the prpBCDE operon was either 2-methylcitrate (Fig. 1) or a derivative of it.
The pathway shown in Fig. 1 predicted that expression of the prpBCDE operon in a strain deficient in the synthesis of propionyl-CoA would not be restored by providing prpC+ in trans. This prediction was tested and proven to be correct. The results of the experiments addressing this point are discussed below.
Propionyl-CoA is a precursor of the catabolite that signals the presence of propionate in the environment.
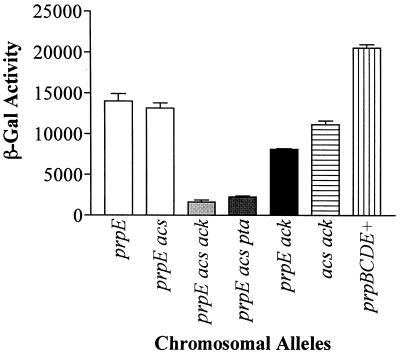
Figure 4 shows the effect of the lack of propionyl-CoA on the expression of the prpBCDE operon. Work from our laboratory recently demonstrated that the prpE gene encodes a specific propionyl-CoA synthetase (14). It was also demonstrated that prpE mutants compensate for the lack of this enzyme through the activity of the acetyl-CoA synthetase encoded by the acs gene.
FIG. 4.
Propionyl-CoA is a required precursor for the signal that triggers prpBCDE expression. β-Galactosidase specific activity is expressed in units per A650 unit. The unit of activity for this enzyme is defined in Materials and Methods. The genes listed are ack (encodes acetate kinase), pta (encodes phosphotransacetylase), prpE (encodes propionyl-CoA synthetase), and acs (encodes acetyl-CoA synthetase). All mutations used were insertion alleles which are described in Table 1.
As discussed above, expression of the lacZ gene in plasmid pPRP25 in prpE mutant strain JE4394 was only slightly reduced relative to that in the prpE+ strain JE4044 (Table 2). Data in Fig. 4 show that expression of the reporter in the prpE acs double-mutation strain JE4395 was not significantly reduced relative to the level measured in the prpE acs+ strain JE4394 (36 and 32% reductions, respectively). A very drastic reduction in the expression of the reporter, however, was observed in triple-mutation strains carrying null alleles of prpE acs ack (strain JE4396) or prpE acs pta (strain JE4397). The Ack (acetate kinase) and Pta (phosphotransacetylase) enzymes are known to contribute to the synthesis of acetyl-CoA in the cell (18, 36). In strain JE4396 the level of β-galactosidase activity (mean ± standard deviation, 1,661 ± 407 U/A650 unit) was reduced 92% relative to that in the wild-type strain JE4044 (20,696 ± 850 U/A650 unit). In strain JE4397 the level of activity was reduced 89% relative to the level in the control strain JE4044 (2,286 ± 206 versus 20,696 ± 850 U/A650 unit). Thus, the acetyl-CoA-synthesizing enzymes in S. typhimurium can activate propionate into propionyl-CoA. In addition, these results mimic those obtained with strains carrying mutations in prpC, indicating that PrpE (propionyl-CoA synthetase) activity is also required for expression of the prpBCDE operon.
It should be pointed out that a strain deficient in the synthesis of PrpE and Acs, but wild type for ack and pta, did not grow on propionate (14). In such a strain, expression of the prpBCDE operon was reduced only by a third relative to the level expressed by the wild-type strain (Fig. 4), and yet this strain failed to grow on propionate. To help explain this result, we argue that in the context of growth, Ack and Pta are poor substitutes for Acs or PrpE and that these enzymes cannot keep up with the demand of the pathway for propionyl-CoA to sustain cell growth. On the other hand, Ack and Pta can clearly generate enough propionyl-CoA to activate transcription of the prpBCDE operon. These results warn us about the risks of concluding that a high level of transcription of any gene necessarily means that the function of the corresponding gene product is sufficient for the cell to grow.
Although transcription of the prpBCDE operon in a prpE ack double mutant (strain JE4521) was only 39% of the level measured in the wild-type strain JE4044 (8,148 ± 171 versus 20,696 ± 850 U/A650 unit), strain JE4521 grew well on propionate, indicating that, unlike Ack and Pta, Acs can satisfy the demand of the pathway for propionyl-CoA by itself and that the cell can grow on propionate. In the acs ack double mutant (strain JE4524), transcription of prpBCDE was high (11,196 ± 785 or 50% of wild-type expression) and the strain grew well on propionate. These results were not surprising since strain JE4524 was wild type for prpE.
cobB function is required for transcription of the prpBCDE operon.
As alluded to in the introduction, one possible explanation for the inability of cobB mutants to grow on propionate was that CobB activity is required for the expression of the prpBCDE operon. To test this idea we measured the expression of the prpC114::MudJ transcriptional fusion in a merodiploid strain as a function of CobB. Data in Table 4 clearly show that cobB mutants fail to express the prpBCDE operon (Table 4). In the absence of CobB, expression of the operon was reduced 24-fold. This drastic reduction was due exclusively to the lack of CobB, since when a wild-type allele of cobB was provided in trans, transcription of the operon was restored to the levels observed in the cobB+ strain (Table 4). Plasmid pCOBB5 carries only a wild-type allele of cobB (35).
TABLE 4.
CobB or an uncharacterized Pdu protein is required for prpBCDE transcription in response to propionate or 1,2-PDL
| Strainb | Relevant genotype | Mean β-galactosidase activity ± SD (U/A650 unit) in cells grown in minimal mediuma supplemented with:
|
Growth on Prpc | Growth on Prp, 1,2-PDLd | |
|---|---|---|---|---|---|
| No additions | 1,2-PDL | ||||
| JE4334 | cobB+ DUP1033[proA-pyrC] prpC114::MudJ | 663 ± 51 | 770 ± 20 | Yes | Yes |
| JE4265 | DEL1184cobB DUP1033[proA-pyrC] prpC114::MudJ | 28 ± 7 | 750 ± 37 | No | Yes |
| JE4359 | DEL1184cobB DUP1033[proA-pyrC] prpC114::MudJ pdu-8::MudA | 21 ± 3 | 17 ± 2 | No | No |
| JE4402 | JE4265/pCOBB5 cobB+ | 692 ± 50 | NDe | Yes | Yes |
NCE medium (7) containing glycerol (22 mM) and propionate (30 mM).
All strains were derivatives of strain TR6583 (metE205 ara-9).
NCE medium with 30 mM propionate (Prp) was the sole carbon and energy source.
NCE medium with 30 mM propionate (Prp) and 12 mM 1,2-PDL. The medium did not contain cobalamin to prevent 1,2-PDL degradation.
ND, not determined.
The ability of the strains used in these experiments to grow on propionate was consistent with the observed pattern of prpBCDE expression (Table 4).
Induction of the propanediol utilization (pdu) operon restores expression of prpBCDE and growth on propionate in cobB mutants.
As mentioned above, we previously reported that induction of the propanediol utilization (pdu) genes by 1,2-PDL was sufficient to restore the ability of cobB mutants to grow on propionate as the carbon and energy source (34). Data in Table 4 show that induction of the pdu operon in a cobB mutant resulted in expression of the prpBCDE operon to levels observed in strains carrying a wild-type allele of cobB (Table 4). Consistent with previous phenotypic observations (34), introduction of the insertion element pdu-8::MudA into cobB mutant strain JE4265 completely eliminated the ability of the resulting strain (JE4359) to express the prpBCDE operon; hence, strain JE4359 failed to grow on propionate as the sole carbon and energy source in spite of the presence of 1,2-PDL in the medium (Table 4). These results further define our understanding of the role of CobB in propionate catabolism and provide a good example of pathway networking in the cell.
DISCUSSION
The work reported herein makes two important contributions to our understanding of the physiology of S. typhimurium: (i) it provides insights into the complex regulation of transcription of the prpBCDE operon, which encodes functions required for the catabolism of propionate in this bacterium, and (ii) it provides strong evidence for metabolic pathway integration.
Propionyl-CoA synthetase (PrpE), 2-methylcitrate synthase (PrpC), and CobB activities are needed to activate prpBCDE operon transcription.
Data reported in this paper show that three proteins are required for expression of this operon, namely, PrpE, PrpC, and CobB or its alternative, an uncharacterized Pdu protein. All these proteins are needed in addition to PrpR, the sigma-54 activator encoded by prpR (13, 20).
Two of these proteins have documented biochemical activities associated with them: PrpE has propionyl-CoA synthetase activity (14) and PrpC has 2-methylcitrate synthase activity (9, 15, 32). The role of PrpE and PrpC in prpBCDE transcription can be rationalized on the basis of the proposed pathway for propionate catabolism in this bacterium (Fig. 1). On the basis of our data, we hypothesize that the catabolite of propionate that signals the presence of this fatty acid in the environment is either 2-methylcitrate or a derivative of it. This metabolite may be the coregulator needed by PrpR to activate transcription of prpBCDE.
Since PrpC catalyzes the synthesis of 2-methylcitrate from propionyl-CoA and oxaloacetate, it is clear that PrpE activity is central to the generation of one of the substrates for PrpC. That is why the effect of blocking propionyl-CoA synthesis in prpC+ strains mimics the effect that mutations in prpC have on the expression of the prpBCDE operon.
Role of the PrpD and PrpB proteins in prpBCDE expression.
Our data on the involvement of PrpB in the regulation of the prpBCDE operon suggest that this putative 2-methylisocitrate lyase enzyme activity most likely does not play a role in the generation of the catabolites that signals the presence of propionate in the environment.
Although the data for PrpD are less clear, we also conclude that PrpD activity is not needed for the generation of the signal. The data show a significant reduction in prpBCDE expression in prpD mutants that suggests that PrpD activity may be required to generate the signal needed to activate prpBCDE transcription. However, this reduction should be interpreted with caution. The same effect would be observed if the absence of PrpD had the net effect of reducing the amount of 2-methylcitrate made by PrpC, in which case, the observed reduction of prpBCDE operon expression in prpD mutants would be the result of suboptimal concentrations of the signal needed by PrpR to activate transcription of the operon. An additional argument supporting the idea that PrpD activity may not be needed to generate the signal comes from the experiments that analyzed the expression of the prpC114::MudJ fusion in a haploid strain with or without prpC+ in trans (Table 3). In these experiments, PrpC was sufficient to restore full expression of the fusion in a strain where expression of prpD was presumably eliminated by the MudJ element upstream of this gene. At this point, we cannot rule out the possibility that a small but sufficient amount of PrpD was synthesized in this strain. Such a small amount of PrpD would have to be sufficient to allow regulation at the wild-type level to occur.
One important difference between these experiments and the ones performed with strains carrying duplications of the prpBCDE operon should be kept in mind. In the experiments with the haploid strain, prpC+ was carried by a high-copy-number plasmid; thus, the concentration of PrpC protein was greater than the concentration afforded by a single chromosomal copy of prpC in the strain with the duplication. This high concentration of PrpC, we argue, is likely to generate sufficient 2-methylcitrate to make PrpR fully functional in the absence of PrpD.
Roles of the CobB and Pdu proteins in prpBCDE operon expression.
The roles that the CobB protein and its alternative, Pdu protein, play in the transcription of prpBCDE are not obvious. The CobB and Pdu proteins may have one or more enzymatic activities needed for the generation of a metabolite required for prpBCDE transcription activation. Alternatively, they may be proteins without any enzymatic activity that play some role in either transcription activation or attenuation, or in a more complex scenario these proteins may have one or more enzymatic activities in addition to playing a more direct role in transcription activation and/or posttranscriptional regulation of the prpBCDE operon expression. How these proteins affect expression of the prpBCDE operon remains under investigation.
Integration of the 1,2-PDL and propionate catabolism pathways.
Regardless of how the CobB and Pdu proteins affect transcription of the prpBCDE operon, we believe that the existence of these redundant functions reflects physiological strategies to ensure that synthesis of the propionate degrading enzymes occurs under different environmental conditions. Since 1,2-PDL catabolism is likely to proceed via propionyl-CoA, it is clear that expression of the prpBCDE operon is needed for the cell to be able to use 1,2-PDL as a carbon and energy source.
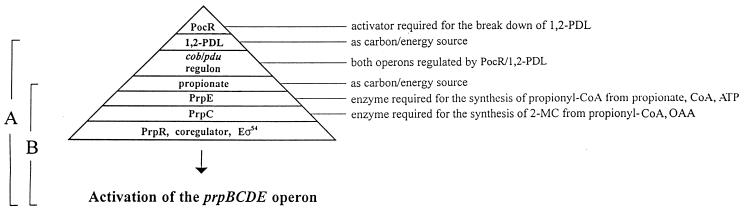
It appears that the Pdu alternative for CobB function is a mechanism that the cell has evolved to ensure the timely synthesis of the PrpB, -C, -D, and -E enzymes to further degrade propionyl-CoA into central metabolites. Figure 5 illustrates how we think the expression of the prpBCDE operon is ensured when either 1,2-PDL or propionate is present in the environment. When 1,2-PDL is the sole carbon and energy source, it binds to the PocR protein (21, 22) and the PocR–1,2-PDL complex activates transcription of the cob or pdu regulon (23, 24). Since this Pdu function is available only upon induction of the operon by 1,2-PDL, S. typhimurium would be unable to use propionate as a carbon and energy source in an environment devoid of 1,2-PDL. One way to solve this problem would be to have a redundant function encoded outside of the pdu operon, with the expression of such a gene being independent of the presence of 1,2-PDL. In this case, the redundant function is encoded by cobB. Learning more about how the CobB and Pdu proteins affect prpBCDE operon expression will shed light on one mechanism used by this bacterium to integrate its metabolism.
FIG. 5.
Elements controlling prpBCDE operon expression in the presence or absence of propionate in the environment. Under all conditions, propionyl-CoA synthetase (PrpE) and 2-methylcitrate synthase (PrpC) activities are needed to synthesize the coregulator (2-methylcitrate or a derivative of it) required by PrpR to activate transcription of the operon. Under all conditions, core (E) RNA polymerase with sigma-54 (RpoN) is needed for expression. (A) Under conditions where 1,2-PDL is the sole source of carbon and energy, expression of the prpBCDE operon CobB activity is dispensable due to the synthesis of an uncharacterized protein encoded by the pdu operon. This Pdu protein compensates for the lack of CobB in cobB mutants. (B) Under conditions where propionate is the sole source of carbon and energy, CobB activity is required. 2-MC, 2-methylcitrate.
ACKNOWLEDGMENTS
This work was supported by NSF grant MCB9724924 and USDA Hatch grant WIS3765 to J.C.E.-S.
We acknowledge the participation of Michael Smits in the initial stages of this work. Michael Smits was a participant of the 1997 REU Summer Research Program of the Department of Bacteriology at the University of Wisconsin—Madison, which is sponsored by the NSF, the Graduate School, and the College of Agricultural and Life Sciences of the University of Wisconsin—Madison. We thank R. LaRossa for strains.
REFERENCES
- 1.Anderson R P, Roth J R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Uchiyama H, Umetsu H, Tabuchi T. Isolation of 2-methylcitrate dehydratase, a new enzyme serving in the methylcitric acid cycle for propionate metabolism, from Yallowia lipolytica. Biosci Biotechnol Biochem. 1995;59:1825–1828. [Google Scholar]
- 3.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bobik T A, Xu Y, Jeter R M, Otto K E, Roth J R. Propanediol utilization genes (pdu) of Salmonella typhimurium: three genes for the propanediol dehydratase. J Bacteriol. 1997;179:6633–6639. doi: 10.1128/jb.179.21.6633-6639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castilho B A, Olfson P, Casadaban M J. Plasmid insertion mutagenesis and lac gene fusions with mini-Mu bacteriophage transposons. J Bacteriol. 1984;158:488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan R K, Botstein D, Watanabe T, Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high transducing lysate. Virology. 1972;50:883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- 7.Davis R W, Botstein D, Roth J R. A manual for genetic engineering: advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 8.Escalante-Semerena J C, Roth J R. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987;169:2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerike U, Hough D W, Russell N J, Dyall-Smith M L, Danson M J. Citrate synthase and 2-methylcitrate synthase: structural, functional and evolutionary relationships. Microbiology. 1998;144:929–935. doi: 10.1099/00221287-144-4-929. [DOI] [PubMed] [Google Scholar]
- 10.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammelman T A, O’Toole G A, Trzebiatowski J R, Tsang A W, Rank D, Escalante-Semerena J C. Identification of a new prp locus required for propionate catabolism in Salmonella typhimurium LT2. FEMS Microbiol Lett. 1996;137:233–239. doi: 10.1111/j.1574-6968.1996.tb08111.x. [DOI] [PubMed] [Google Scholar]
- 12.Hong J-S, Ames B N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci USA. 1971;68:3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horswill A R, Escalante-Semerena J C. Propionate catabolism in Salmonella typhimurium LT2: two divergently transcribed units comprise the prp locus at 8.5 centisomes, prpR encodes a member of the sigma-54 family of activators, and the prpBCDE genes constitute an operon. J Bacteriol. 1997;179:928–940. doi: 10.1128/jb.179.3.928-940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horswill, A. R., and J. C. Escalante-Semerena. The prpE gene of Salmonella typhimurium LT2 encodes propionyl-CoA synthetase. Submitted for publication. [DOI] [PubMed]
- 15.Horswill, A. R., T. Grimek, and J. C. Escalante-Semerena. 1998. Unpublished results.
- 16.Jeter R M. Cobalamin-dependent 1,2-propanediol utilization by Salmonella typhimurium. J Gen Microbiol. 1990;136:887–896. doi: 10.1099/00221287-136-5-887. [DOI] [PubMed] [Google Scholar]
- 17.Martínez E, Bartolomé B, de la Cruz F. pACY184-derived cloning vectors containing the multiple cloning site and lacZα reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68:159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- 18.McCleary W R, Stock J B, Ninfa A J. Is acetyl phosphate a global signal in Escherichia coli? J Bacteriol. 1993;175:2793–2798. doi: 10.1128/jb.175.10.2793-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Toole G A, Rondon M R, Escalante-Semerena J C. Analysis of mutants of Salmonella typhimurium defective in the synthesis of the nucleotide loop of cobalamin. J Bacteriol. 1993;175:3317–3326. doi: 10.1128/jb.175.11.3317-3326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palacios, S., and J. C. Escalante-Semerena. 1998. Unpublished results.
- 21.Rondon M R, Escalante-Semerena J C. In vitro analysis of the interactions between the PocR regulatory protein and the promoter region of the cobalamin biosynthetic (cob) operon of Salmonella typhimurium LT2. J Bacteriol. 1996;178:2196–2203. doi: 10.1128/jb.178.8.2196-2203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rondon M R, Escalante-Semerena J C. The poc locus is required for 1,2-propanediol-dependent transcription of the cobalamin biosynthetic (cob) and propanediol utilization (pdu) genes of Salmonella typhimurium. J Bacteriol. 1992;174:2267–2272. doi: 10.1128/jb.174.7.2267-2272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rondon M R, Trzebiatowski J R, Escalante-Semerena J C. Biochemistry and molecular genetics of cobalamin biosynthesis. Prog Nucleic Acid Res Mol Biol. 1997;56:347–384. doi: 10.1016/s0079-6603(08)61010-7. [DOI] [PubMed] [Google Scholar]
- 24.Roth J R, Lawrence J G, Bobik T A. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 25.Schmieger H. A method for detection of phage mutants with altered transduction ability. Mol Gen Genet. 1971;100:378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- 26.Schmieger H, Bakhaus H. The origin of DNA in transducing particles of P22 mutants with increased transduction frequencies (HT-mutants) Mol Gen Genet. 1973;120:181–190. doi: 10.1007/BF00267246. [DOI] [PubMed] [Google Scholar]
- 27.Shingler V. Signal sensing by sigma 54-dependent regulators: derepression as a control mechanism. Mol Microbiol. 1996;19:409–416. doi: 10.1046/j.1365-2958.1996.388920.x. [DOI] [PubMed] [Google Scholar]
- 28.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 29.Tabuchi T, Serizawa N. A hypothetical cyclic pathway for the metabolism of odd-carbon n-alkanes or propionyl-CoA via seven-carbon tricarboxylic acids in yeasts. Agric Biol Chem. 1975;39:1055–1061. [Google Scholar]
- 30.Tabuchi T, Umetsu H, Aoki H, Uchiyama H. Characteristics of 2-methylisocitrate dehydratase, isolated from Yallowia lipolytica, in comparison with aconitase. Biosci Biotechnol Biochem. 1995;59:2013–2017. [Google Scholar]
- 31.Tang X, Nakata Y, Li H-O, Zhang M, Gao H, Fujita A, Sakatsume O, Ohta T, Yokoyama K. The optimization of preparations of competent cells for transformation of E. coli. Nucleic Acids Res. 1994;22:2857–2858. doi: 10.1093/nar/22.14.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Textor S, Wendisch V F, De Graaf A A, Müller U, Linder M I, Linder D, Buckel W. Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch Microbiol. 1997;168:428–436. doi: 10.1007/s002030050518. [DOI] [PubMed] [Google Scholar]
- 33.Trzebiatowski J R, O’Toole G A, Escalante-Semerena J C. The cobT gene of Salmonella typhimurium encodes the NaMN:5,6-dimethylbenzimidazole phosphoribosyl transferase responsible for the synthesis of N1-(5-phospho-α-d-ribosyl)-5,6-dimethylbenzimidazole, an intermediate in the synthesis of the nucleotide loop of cobalamin. J Bacteriol. 1994;176:3568–3575. doi: 10.1128/jb.176.12.3568-3575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang A W, Escalante-Semerena J C. cobB function is required for the catabolism of propionate in Salmonella typhimurium LT2. Evidence for the existence of a substitute function for CobB within the 1,2-propanediol utilization (pdu) operon. J Bacteriol. 1996;178:7016–7019. doi: 10.1128/jb.178.23.7016-7019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang A W, Escalante-Semerena J C. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase activity in cobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J Biol Chem. 1998;273:31788–31794. doi: 10.1074/jbc.273.48.31788. [DOI] [PubMed] [Google Scholar]
- 36.Van Dyk T K, LaRossa R A. Involvement of ack-pta operon products in α-ketobutyrate metabolism in Salmonella typhimurium. Mol Gen Genet. 1987;207:435–440. doi: 10.1007/BF00331612. [DOI] [PubMed] [Google Scholar]
- 37.Walter D, Ailion M, Roth J. Genetic characterization of the pdu operon: use of 1,2-propanediol in Salmonella typhimurium. J Bacteriol. 1997;179:1013–1022. doi: 10.1128/jb.179.4.1013-1022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]