Abstract
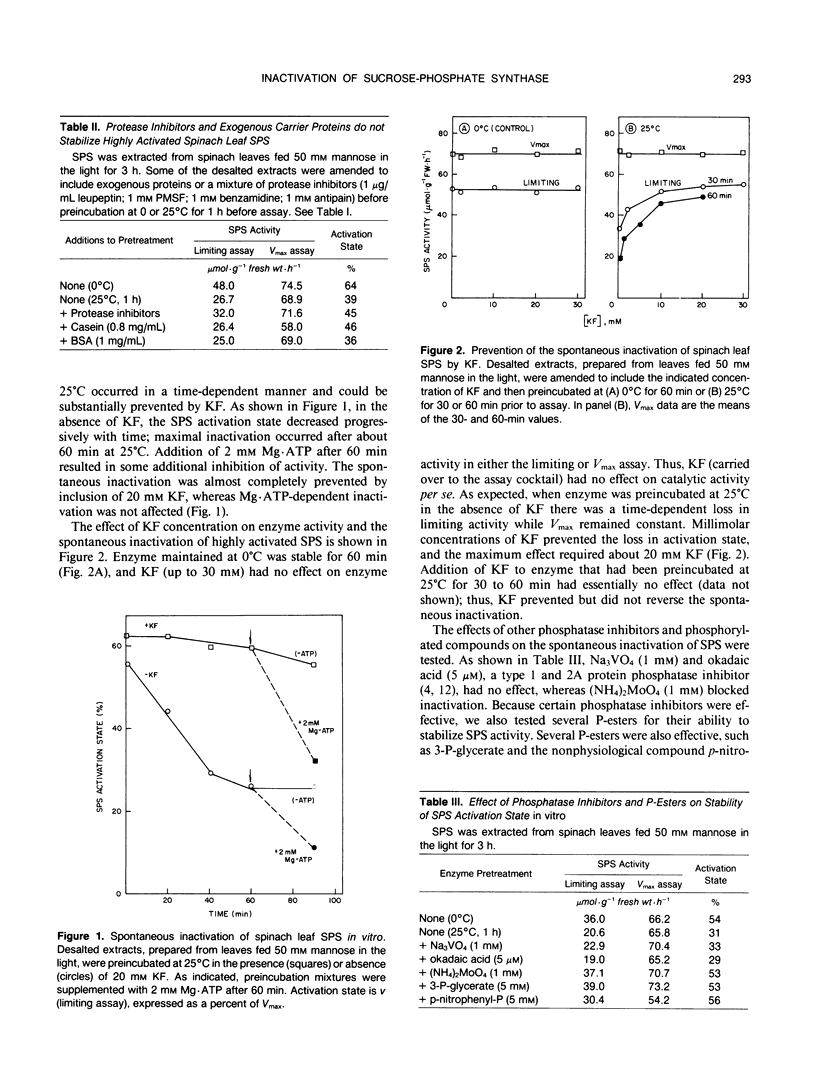
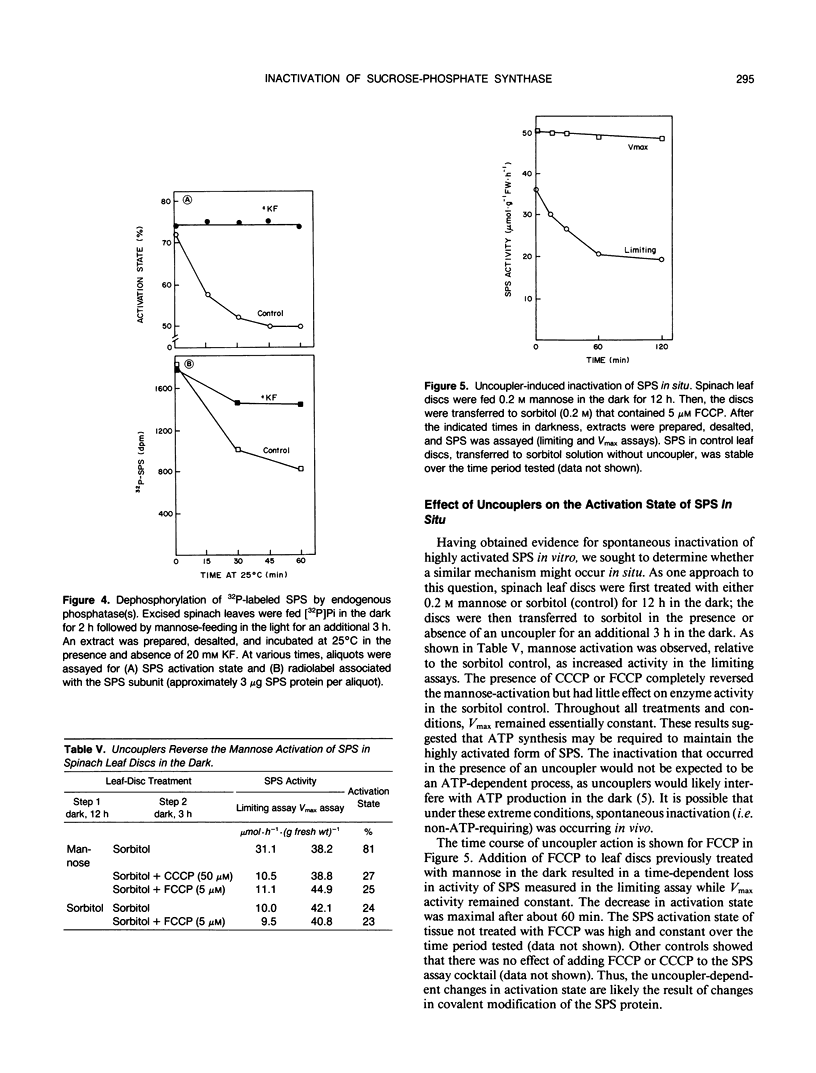
Spinach (Spinacia oleracea L.) leaf sucrose-phosphate synthase (SPS) can be phosphorylated and inactivated in vitro with [γ-32P]ATP (JLA Huber, SC Huber, TH Nielsen [1989] Arch Biochem Biophys 270: 681-690). Thus, it was surprising to find that SPS, extracted from leaves fed mannose in the light to highly activate the enzyme, could be inactivated in an ATP-independent manner when desalted crude extracts were preincubated at 25°C before assay. The “spontaneous” inactivation involved a loss in activity measured with limiting substrate concentrations in the presence of the inhibitor, Pi, without affecting maximum catalytic activity. The spontaneous inactivation was unaffected by exogenous carrier proteins and protease inhibitors, but was inhibited by inorganic phosphate, fluoride, and molybdate, suggesting that a phosphatase may be involved. Okadaic acid, a potent inhibitor of mammalian type 1 and 2A protein phosphatases, had no effect up to 5 micromolar. Inactivation was stimulated about twofold by exogenous Mg2+ and was relatively insensitive to Ca2+ and to pH over the range pH 6.5 to 8.5. Radioactive phosphate incorporated into SPS during labeling of excised leaves with [32P]Pi (initially in the dark and then in the light with mannose) was lost with time when desalted crude extracts were incubated at 25°C, and the loss in radiolabel was substantially reduced by fluoride. These results provide direct evidence for action of an endogenous phosphatase(s) using SPS as substrate. We postulate that highly activated SPS contains phosphorylated residue(s) that increase activation state, and that spontaneous inactivation occurs by removal of these phosphate group(s). Inactivation of SPS in vivo caused by feeding uncouplers to darkened leaf tissue that had previously been fed mannose in the dark, may occur by this mechanism. However, there is no evidence that this mechanism is involved in light-dark regulation of SPS in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budde R. J., Randall D. D. Regulation of steady state pyruvate dehydrogenase complex activity in plant mitochondria : reactivation constraints. Plant Physiol. 1988 Dec;88(4):1026–1030. doi: 10.1104/pp.88.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Huber J. L., Huber S. C., Nielsen T. H. Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch Biochem Biophys. 1989 May 1;270(2):681–690. doi: 10.1016/0003-9861(89)90551-1. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Huber J. L. Activation of sucrose-phosphate synthase from darkened spinach leaves by an endogenous protein phosphatase. Arch Biochem Biophys. 1990 Nov 1;282(2):421–426. doi: 10.1016/0003-9861(90)90138-o. [DOI] [PubMed] [Google Scholar]
- Kalt-Torres W., Kerr P. S., Usuda H., Huber S. C. Diurnal changes in maize leaf photosynthesis : I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987 Feb;83(2):283–288. doi: 10.1104/pp.83.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. P. Phosphoprotein Phosphatase of Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES . Plant Physiol. 1980 Sep;66(3):368–374. doi: 10.1104/pp.66.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Randall D. D. Some properties of pea mitochondrial phospho-pyruvate dehydrogenase-phosphatase. Plant Physiol. 1987 Feb;83(2):311–315. doi: 10.1104/pp.83.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Haritou M. Purification and characterization of two wheat-embryo protein phosphatases. Biochem J. 1988 Apr 15;251(2):357–363. doi: 10.1042/bj2510357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Rubin P. M. Plant Pyruvate Dehydrogenase Complex: II. ATP-Dependent Inactivation and Phosphorylation. Plant Physiol. 1977 Jan;59(1):1–3. doi: 10.1104/pp.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Williams M., Rapp B. J. Phosphorylation-dephosphorylation of pyruvate dehydrogenase complex from pea leaf mitochondria. Arch Biochem Biophys. 1981 Apr 1;207(2):437–444. doi: 10.1016/0003-9861(81)90051-5. [DOI] [PubMed] [Google Scholar]
- Rao K. P., Randall D. D. Plant pyruvate dehydrogenase complex: inactivation and reactivation by phosphorylation and dephosphorylation. Arch Biochem Biophys. 1980 Apr 1;200(2):461–466. doi: 10.1016/0003-9861(80)90377-x. [DOI] [PubMed] [Google Scholar]
- Sicher R. C., Kremer D. F. Possible control of maize leaf sucrose-phosphate synthase activity by light modulation. Plant Physiol. 1985 Nov;79(3):695–698. doi: 10.1104/pp.79.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Bailey D., Jones M. W., Markwell J. Chloroplast thylakoid protein phosphatase is a membrane surface-associated activity. Plant Physiol. 1989 Jan;89(1):238–243. doi: 10.1104/pp.89.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. L., Huber S. C. Purification and preliminary characterization of sucrose-phosphate synthase using monoclonal antibodies. Plant Physiol. 1989 Feb;89(2):518–524. doi: 10.1104/pp.89.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]


