Abstract
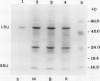
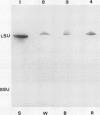
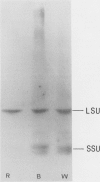
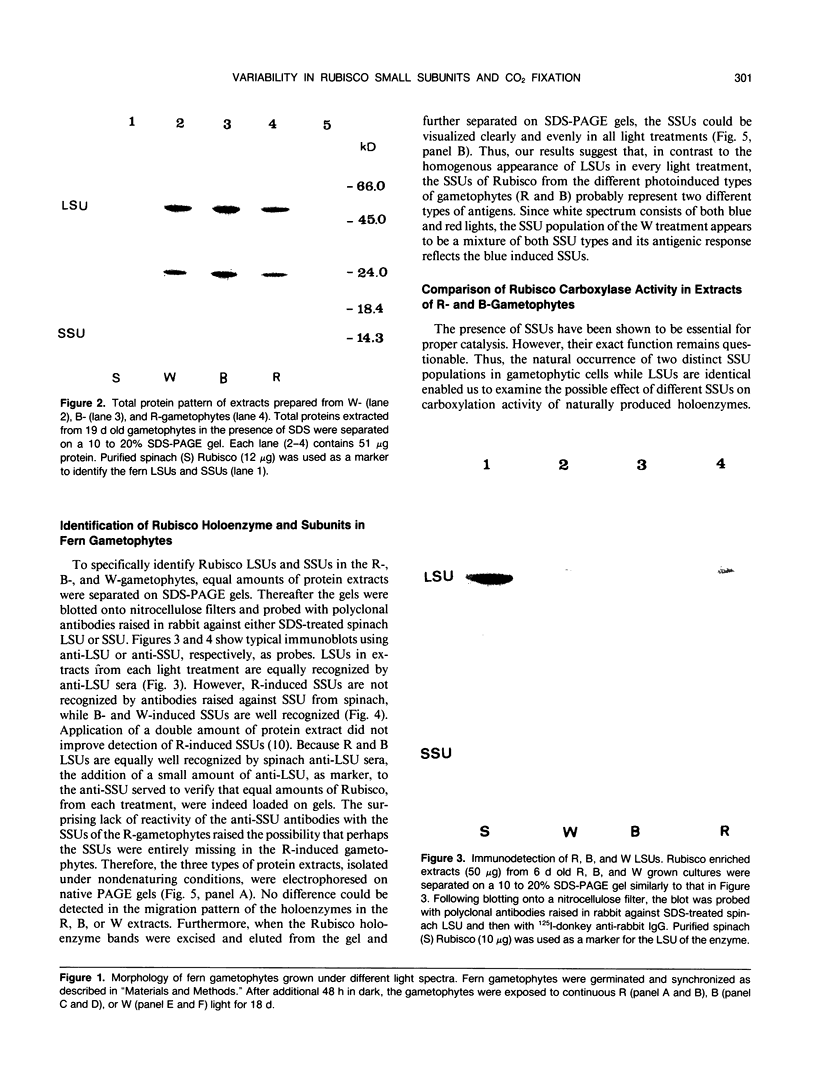
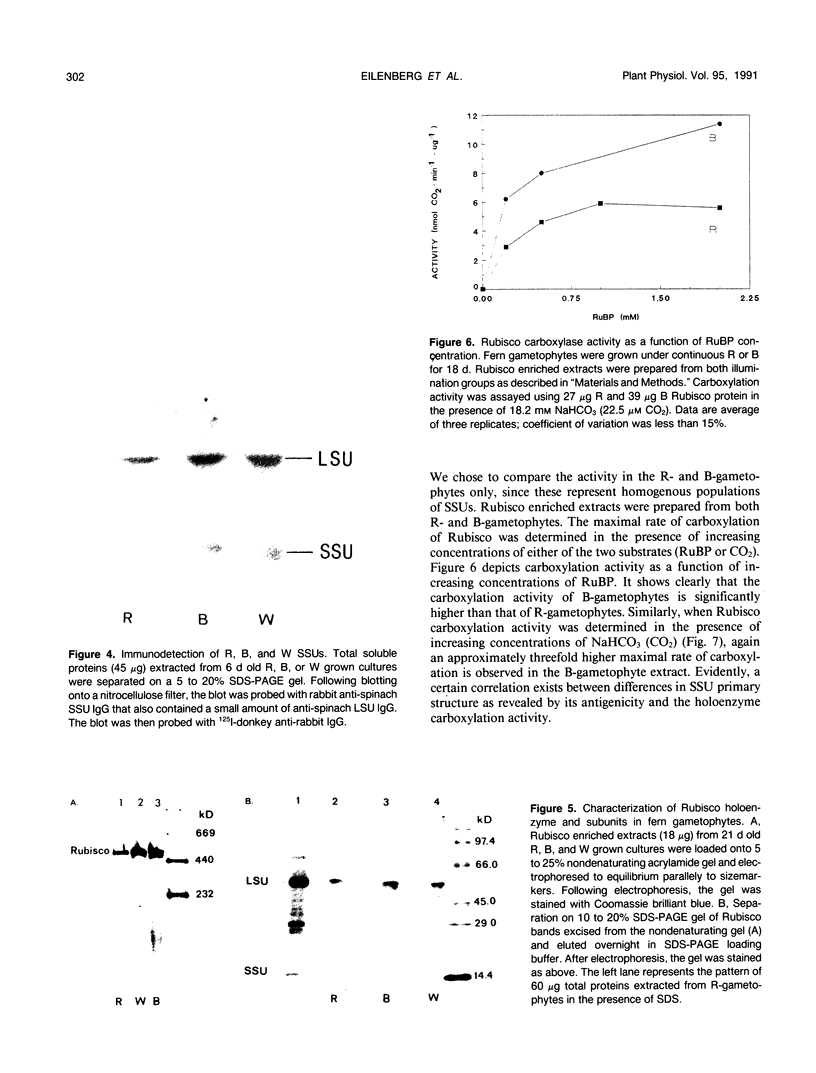
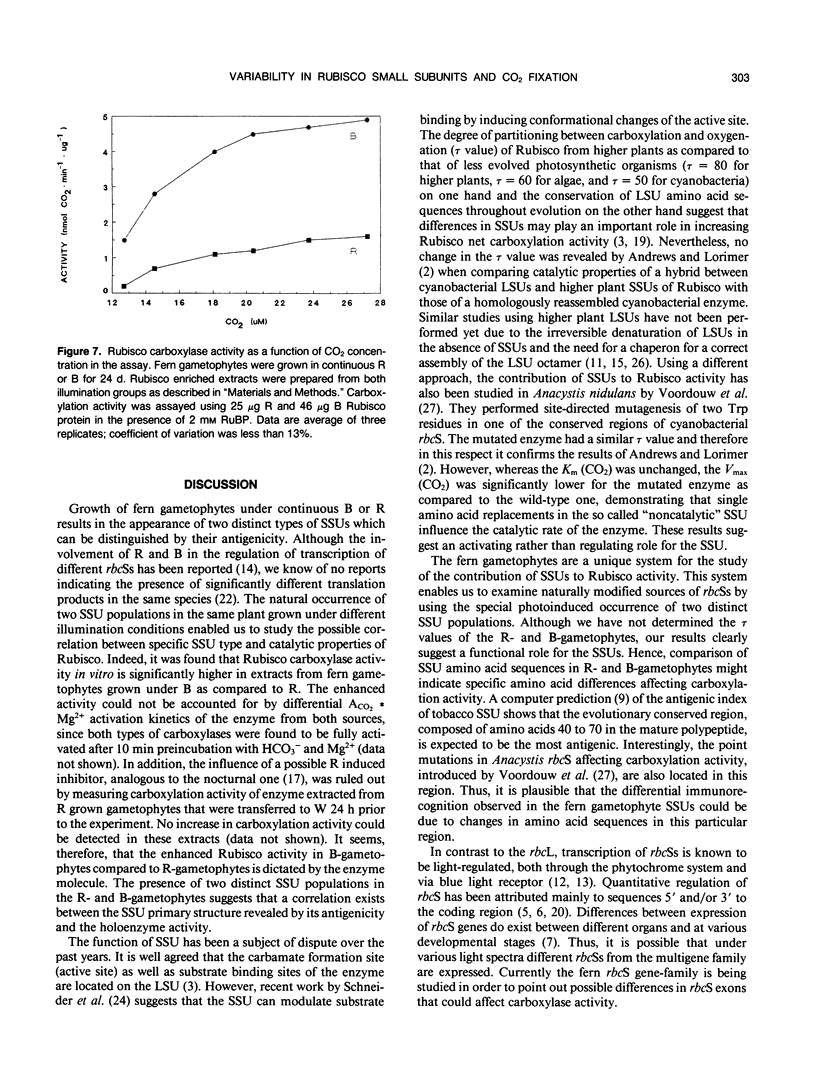
Two distinct ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) small subunit (SSU) populations were observed in Pteris vittata gametophytes grown under different illumination conditions. Exposure of the fern gametophytes to continuous red light (R) resulted in Rubisco SSUs that were not recognized by polyclonal antibodies raised against SSUs from spinach. Unlike the R-induced SSUs, blue light (B) induced SSUs were well recognized. This difference in SSU composition also reflected in Rubisco activity. In vitro, B-induced Rubisco exhibits a significantly higher carboxylation activity as compared to the R-induced Rubisco. Approximately a two- to threefold increase in the Vmax value of the B-induced carboxylase as compared to the R-induced one was measured. It thus seems very likely that certain domains in the SSU molecule affect enzyme activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Ballment B. The function of the small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1983 Jun 25;258(12):7514–7518. [PubMed] [Google Scholar]
- Andrews T. J., Lorimer G. H. Catalytic properties of a hybrid between cyanobacterial large subunits and higher plant small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1985 Apr 25;260(8):4632–4636. [PubMed] [Google Scholar]
- Avni A., Edelman M., Rachailovich I., Aviv D., Fluhr R. A point mutation in the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase affects holoenzyme assembly in Nicotiana tabacum. EMBO J. 1989 Jul;8(7):1915–1918. doi: 10.1002/j.1460-2075.1989.tb03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Favreau M., Bedbrook J., Dunsmuir P. Sequences 5' to translation start regulate expression of petunia rbcS genes. Plant Cell. 1989 Feb;1(2):209–215. doi: 10.1105/tpc.1.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Favreau M., Bond-Nutter D., Bedbrook J., Dunsmuir P. Sequences downstream of translation start regulate quantitative expression of two petunia rbcS genes. Plant Cell. 1989 Feb;1(2):201–208. doi: 10.1105/tpc.1.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. W., Gruissem W. Control of plastid gene expression during development: the limited role of transcriptional regulation. Cell. 1987 May 8;49(3):379–387. doi: 10.1016/0092-8674(87)90290-x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gruissem W. Chloroplast gene expression: how plants turn their plastids on. Cell. 1989 Jan 27;56(2):161–170. doi: 10.1016/0092-8674(89)90889-1. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C., Fluhr R., Green P. J., Chua N. H. Sequences in the pea rbcS-3A gene have homology to constitutive mammalian enhancers but function as negative regulatory elements. Genes Dev. 1987 May;1(3):247–255. doi: 10.1101/gad.1.3.247. [DOI] [PubMed] [Google Scholar]
- Schneider G., Knight S., Andersson I., Brändén C. I., Lindqvist Y., Lundqvist T. Comparison of the crystal structures of L2 and L8S8 Rubisco suggests a functional role for the small subunit. EMBO J. 1990 Jul;9(7):2045–2050. doi: 10.1002/j.1460-2075.1990.tb07371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw G., De Vries P. A., Van den Berg W. A., De Clerck E. P. Site-directed mutagenesis of the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from Anacystis nidulans. Eur J Biochem. 1987 Mar 16;163(3):591–598. doi: 10.1111/j.1432-1033.1987.tb10908.x. [DOI] [PubMed] [Google Scholar]
- Voordouw G., van der Vies S. M., Bouwmeister P. P. Dissociation of ribulose-1,5-bisphosphate carboxylase/oxygenase from spinach by urea. Eur J Biochem. 1984 Jun 1;141(2):313–318. doi: 10.1111/j.1432-1033.1984.tb08193.x. [DOI] [PubMed] [Google Scholar]
- Wolter F. P., Fritz C. C., Willmitzer L., Schell J., Schreier P. H. rbcS genes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci U S A. 1988 Feb;85(3):846–850. doi: 10.1073/pnas.85.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel E., Gepstein S. Immunological evidence for the presence of ribulose bisphosphate carboxylase in guard cell chloroplasts. Plant Physiol. 1985 Jul;78(3):586–590. doi: 10.1104/pp.78.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]