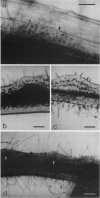
Abstract
The formation of first nodules inhibits subsequent nodulation in younger regions of alfalfa (Medicago sativa L.) roots by a feedback regulatory mechanism that controls nodule number systemically (G Caetano-Anollés, WD Bauer [1988] Planta 175: 546-557). Following inoculation with wild-type Rhizobium meliloti, almost all infections associated with cortical cell division developed into mature nodules. While the distribution of Rhizobium- induced cell divisions closely paralleled the distribution of first emergent nodules, only 9 to 15% of total cell division foci failed to become functional nodules. Nodule formation was restricted to the primary root when plants were inoculated before lateral root emergence. Excision of these primary root nodules allowed nodules to reappear in lateral roots clustered around the location of the root tip at the time of nodule removal. Apparently, this region regained susceptibility to infection within the first hours after excision of primary nodules and suppression of nodulation was restored a day later probably due to the development of new infection foci. Our results suggest that alfalfa controls nodulation during the onset of cell division in the root cortex and not during infection development as in soybean.
Full text
PDF


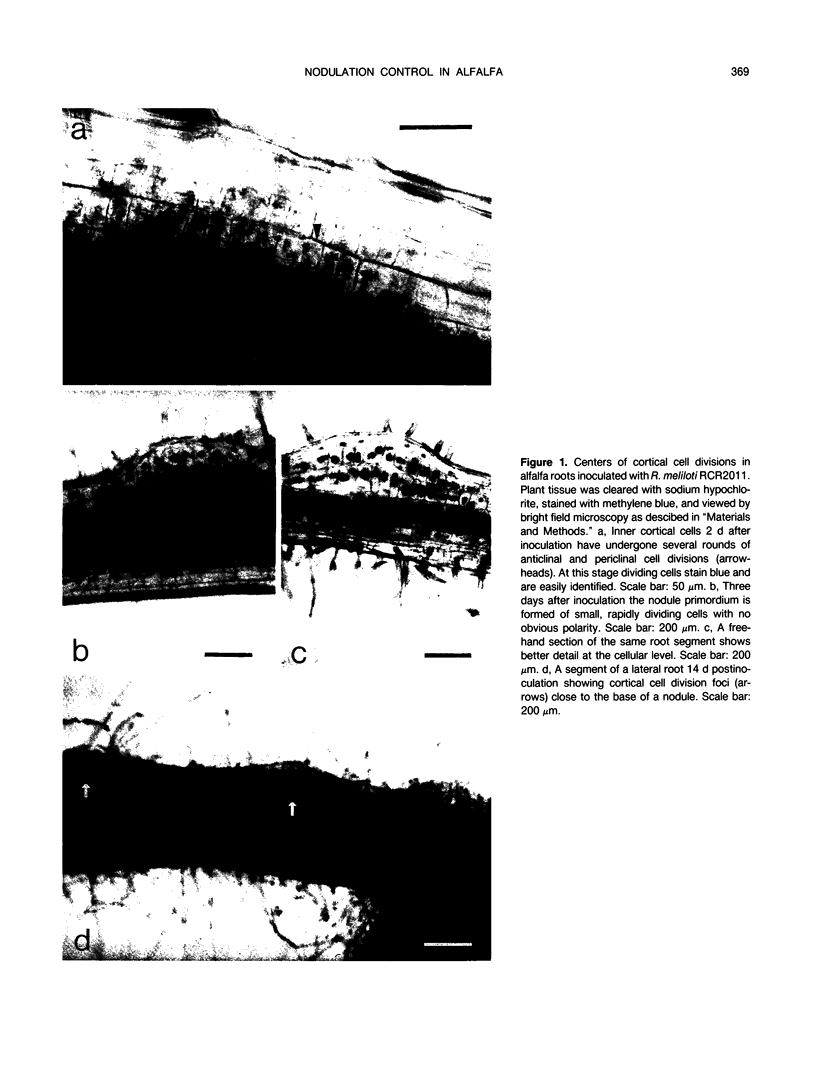
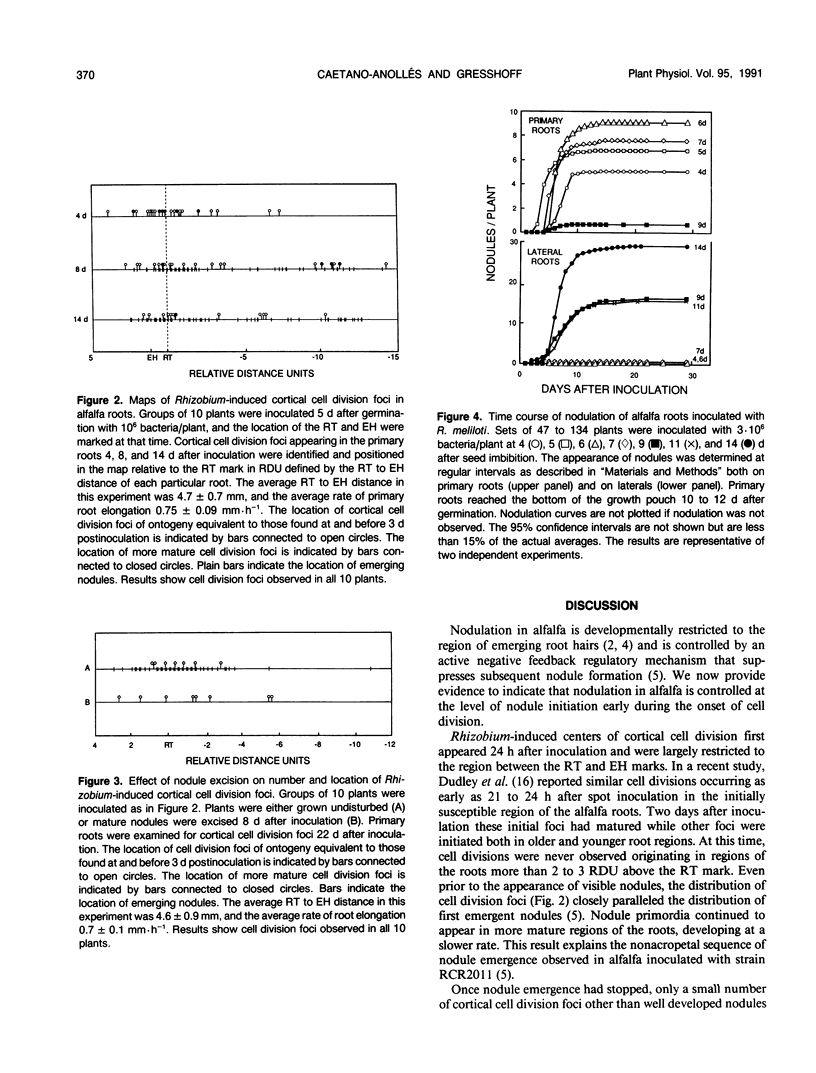
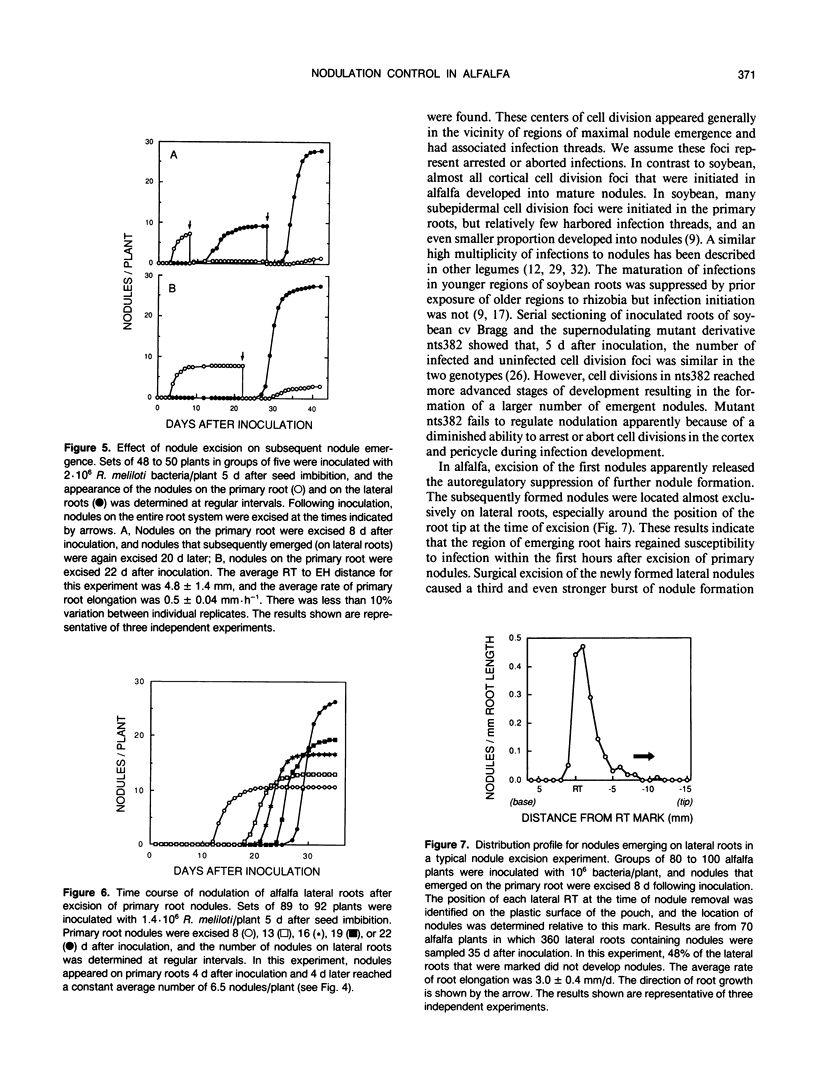


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Lagares A., Bauer W. D. Rhizobium meliloti exopolysaccharide Mutants Elicit Feedback Regulation of Nodule Formation in Alfalfa. Plant Physiol. 1990 Feb;92(2):368–374. doi: 10.1104/pp.92.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. J., McNeil D. L., Gresshoff P. M. A Supernodulation and Nitrate-Tolerant Symbiotic (nts) Soybean Mutant. Plant Physiol. 1985 May;78(1):34–40. doi: 10.1104/pp.78.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. J., McNeil D. L., Gresshoff P. M. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves A. C., Mathews A., Day D. A., Carter A. S., Carroll B. J., Gresshoff P. M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986 Oct;82(2):588–590. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M. E., Long S. R. A non-nodulating alfalfa mutant displays neither root hair curling nor early cell division in response to Rhizobium meliloti. Plant Cell. 1989 Jan;1(1):65–72. doi: 10.1105/tpc.1.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremaud M. F., Harper J. E. Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol. 1989 Jan;89(1):169–173. doi: 10.1104/pp.89.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Bhuvaneswari T. V., Torrey J. G., Bisseling T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Long S. R., Bang M., Haskins N., Ausubel F. M. Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J Bacteriol. 1982 Jul;151(1):411–419. doi: 10.1128/jb.151.1.411-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984 May;75(1):125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N. S., Bauer W. D. When does the self-regulatory response elicited in soybean root after inoculation occur? Plant Physiol. 1988 Nov;88(3):537–539. doi: 10.1104/pp.88.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J. E., Nakao P., Bohlool B. B., Gresshoff P. M. Lack of Systemic Suppression of Nodulation in Split Root Systems of Supernodulating Soybean (Glycine max [L.] Merr.) Mutants. Plant Physiol. 1989 Aug;90(4):1347–1352. doi: 10.1104/pp.90.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Wingender R., John M., Wieneke U., Schell J. Rhizobium meliloti nodA and nodB genes are involved in generating compounds that stimulate mitosis of plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8578–8582. doi: 10.1073/pnas.85.22.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]