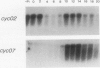
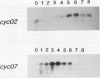
Abstract
A cDNA library was screened for genes that may be involved in the progression of the cell cycle of cells of higher plants. The Catharanthus roseus L. (G) Don. cells were synchronized by the double phosphate starvation method, and a λgt11 cDNA library was prepared using poly(A)+ RNA from cells in the S phase of the cell cycle. Two independent sequences, cyc02 and cyc07, were identified by differential screening. The levels of cyc02 and cyc07 mRNAs increased dramatically, but transiently, at the G1/S boundary of the cell cycle. High levels of cyc02 mRNA, but not of cyc07 mRNA, were also present in cells arrested at the G1 phase by phosphate starvation. In an asynchronous batch culture, cyc02 and cyc07 mRNAs accumulated transiently at different stages of the growth cycle, cyc02 mRNA early in the stationary phase, and cyc07 mRNA in the midlogarithmic phase. When the proliferation of cells was arrested by nutrient starvation, i.e. by sucrose or nitrogen starvation, the relative amounts of the cyc02 and cyc07 mRNAs decreased. These results indicate that cyc02 and cyc07 contain nucleotide sequences from growth-related genes. The analysis of nucleotide sequence of cyc02 shows that the predicted product of this gene is basic and is composed of 101 amino acids. No significant homology to other known proteins was detected.
Full text
PDF

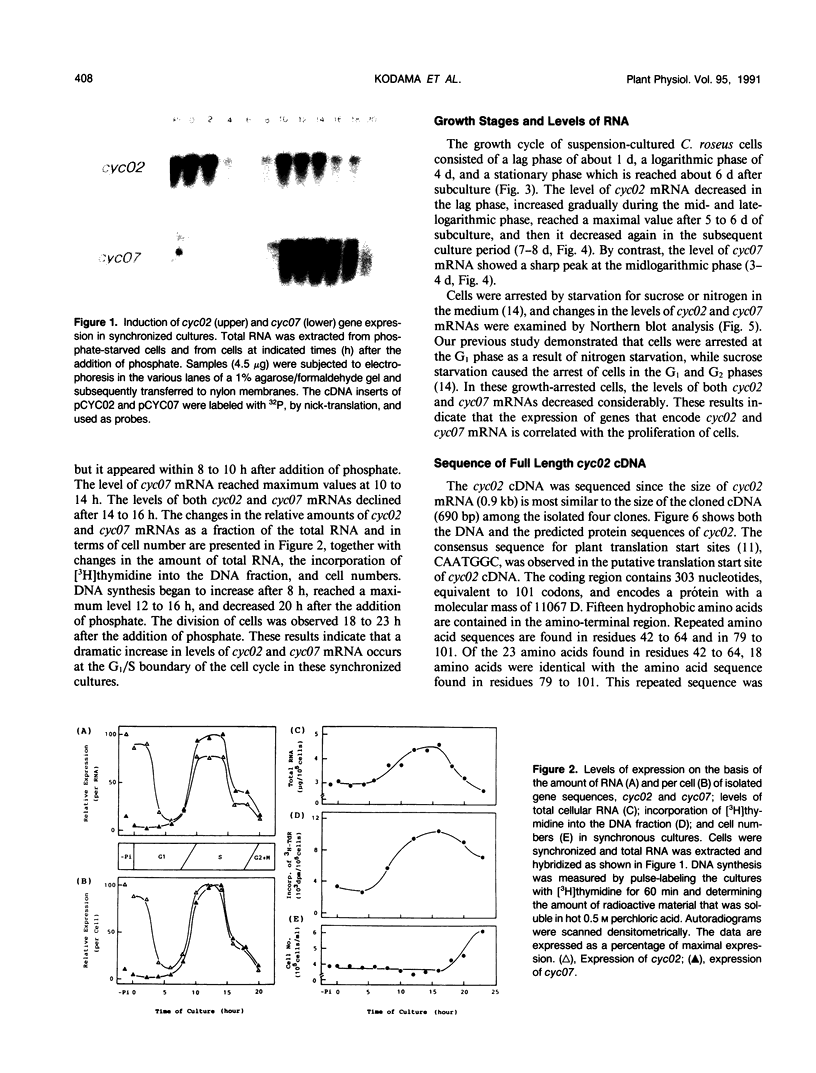
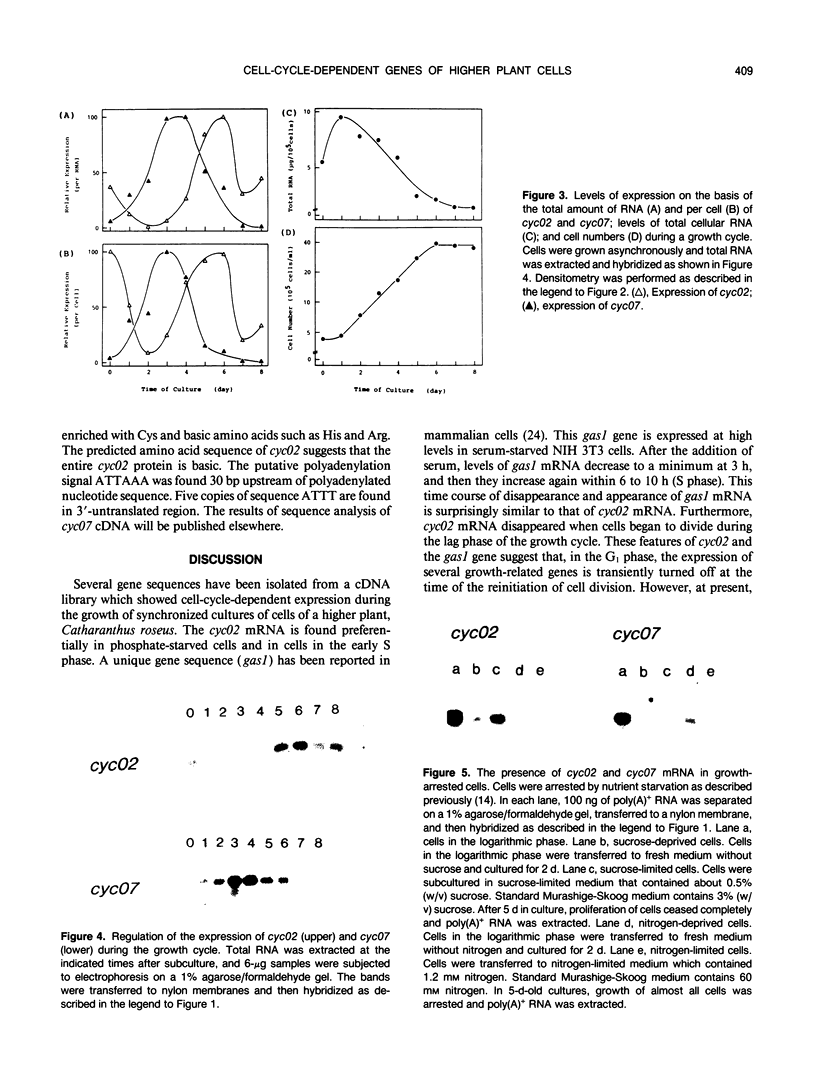


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Kaneda S., Takeishi K., Seno T. Cell-cycle-directed regulation of thymidylate synthase messenger RNA in human diploid fibroblasts stimulated to proliferate. J Mol Biol. 1986 Aug 20;190(4):559–567. doi: 10.1016/0022-2836(86)90241-x. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Ittmann M., Greco A., Basilico C. Isolation of the human gene that complements a temperature-sensitive cell cycle mutation in BHK cells. Mol Cell Biol. 1987 Oct;7(10):3386–3393. doi: 10.1128/mcb.7.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H., Kawakami N., Watanabe A., Komamine A. Phase-Specific Polypeptides and Poly(A) RNAs during the Cell Cycle in Synchronous Cultures of Catharanthus roseus Cells. Plant Physiol. 1989 Mar;89(3):910–917. doi: 10.1104/pp.89.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Suzuka I., Daidoji H., Matsuoka M., Kadowaki K., Takasaki Y., Nakane P. K., Moriuchi T. Gene for proliferating-cell nuclear antigen (DNA polymerase delta auxiliary protein) is present in both mammalian and higher plant genomes. Proc Natl Acad Sci U S A. 1989 May;86(9):3189–3193. doi: 10.1073/pnas.86.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kuroda H., Tanaka T., Machida Y., Takebe I., Nagata T. Isolation of an auxin-regulated gene cDNA expressed during the transition from G0 to S phase in tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9279–9283. doi: 10.1073/pnas.86.23.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]