Abstract
Background
Nosocomial infections caused by Serratia marcescens mostly occurred in pediatrics and it was very rarely reported after adult surgery. Here, an intracranial abscess caused by Serratia marcescens was reported.
Case summary
We report a rare case of a postoperative intracranial abscess caused by Serratia marcescens in a 63-year-old male patient with a left parietal mass. The patient underwent resection of the mass on June 1, 2022, and the postoperative pathology revealed an angiomatous meningioma, WHO I. He then experienced recurrent worsening of right limb movements, and repeated cranial CT scans showed oozing blood and obvious low-density shadows around the operation area. Delayed wound healing was considered. Subsequently, a large amount of pus was extracted from the wound. The etiological test showed that Serratia marcescens infection occurred before the removal of the artificial titanium mesh. Antibiotics were initiated based on the results of drug susceptibility tests. At present, the patient is recovering well and is still closely monitored during follow-up.
Conclusion
It is rare for Serratia marcescens to cause brain abscesses without any obvious signs of infection. This report provided in detail our experience of a warning postoperative asymptomatic brain abscess caused by an uncommon pathogen.
Keywords: Intracranial abscess, Serratia marcescens, Titanium mesh, Hair removal, Antibiotics
Introduction
Bacterial infections of the central nervous system (CNS) remain to be an important cause of morbidity and mortality after craniotomies. The incidence rate is 1–3%, with Staphylococcus aureus (S. aureus) being the most common pathogen. Serratia marcescens is a gram-negative enteric bacillus that rarely colonizes human hosts. It is widely distributed in hospital environments and is regarded as an opportunistic pathogen that occasionally causes various infections, such as wound infections and pneumonia [1–4]. Most brain abscesses caused by Serratia marcescens occurred in settings such as intensive care units (ICUs), especially neonatal units (NICUs) [5–9]. Serratia marcescens, a member of the Enterobacteriaceae, is a well-known pathogen of the respiratory tract, urinary tract, and a common etiology of wound infections, which has raised concerns as a causative pathogen of nosocomial infections [10, 11]. However, Serratia marcescens is a rare pathogen of adult CNS infections [12]. Here, we present a case of brain abscess caused by S. marcescens, with accompanying review of literatures.
Case presentation
A 63-year-old man presented with complaints of right limb weakness over the past 4 months and was brought to the emergency department after a seizure episode. The patient had a 10-year history of unstable angina and underwent skin grafting 5 years prior. There had no recent history of fever, excessive sleepiness, or nuchal rigidity. Neurological and systemic examinations revealed grade 4 + on the right limb on manual muscle testing. Computed tomography (CT) of the brain showed a lesion occupying the left parietal lobe with a mild mass effect and a midline shift. Convex meningioma was presumptively diagnosed based on magnetic resonance imaging (MRI) results (Fig. 1).
Fig. 1.
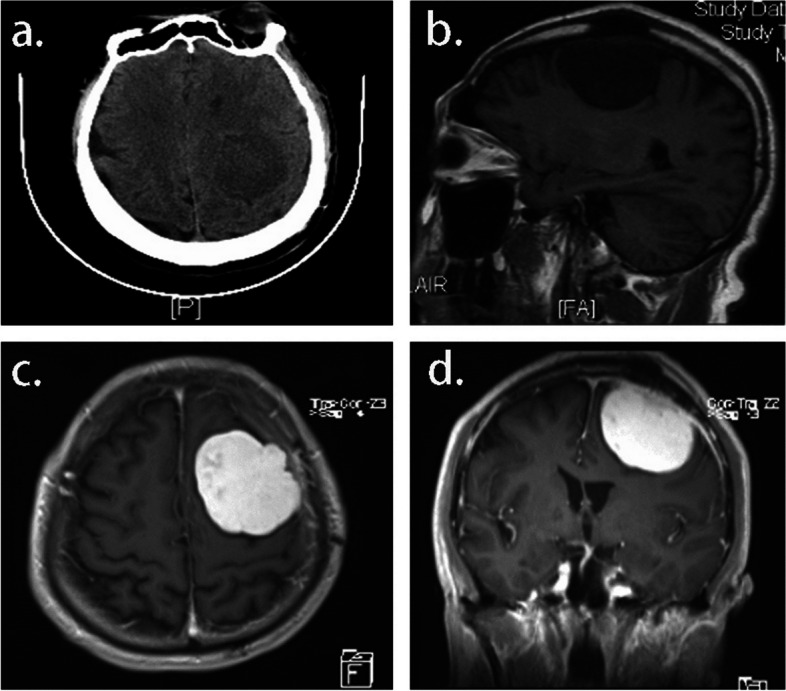
A Immediate preoperative CT performed at initial encounter demonstrated a minimally mass effect in the left parietal region. B Large well-defined mass lesion in the left posterior region with adjacent skull erosion was shown in sagittal T1. On axial (C) and coronal (D) enhanced T1, strong post-contrast enhancement was observed with dural tail sign, exerting mass effect on the left lateral ventricle with resultant shift of midline structures to the right
On June 1, 2022, the patient underwent left parietal craniotomy with total resection of the tumor. Consistent with Fig. 1B, the lesion exhibited bony erosion of the entire skull during craniotomy (Fig. 2). A histopathological examination of an intra-operative fast frozen section biopsy (Identification number D22-27096; Qingdao, China) demonstrated a meningeal epithelial tumor with mild-moderate cellular atypia and rich vessel supply, predisposed to atypical meningiomas (WHO II-III). It was recommended to wait for a large number of paraffin samples and immunohistochemistry (IHC) results to exclude the possibility of higher-grade lesions. The tumor had invaded the dura mater and skull. We performed an excision of the involved dura mater and skull, reaching a Simon Grade 5 level of resection. Consequently, in the process of cranial closure, we used an artificial dura mater for precise suturing of the dura mater and a titanium mesh to reconstruct the affected areas of the skull. This approach also potentially contributed to the risk of persistent and spreading infection in the patient. We sterilized the titanium mesh rigorously before it was implanted.
Fig. 2.
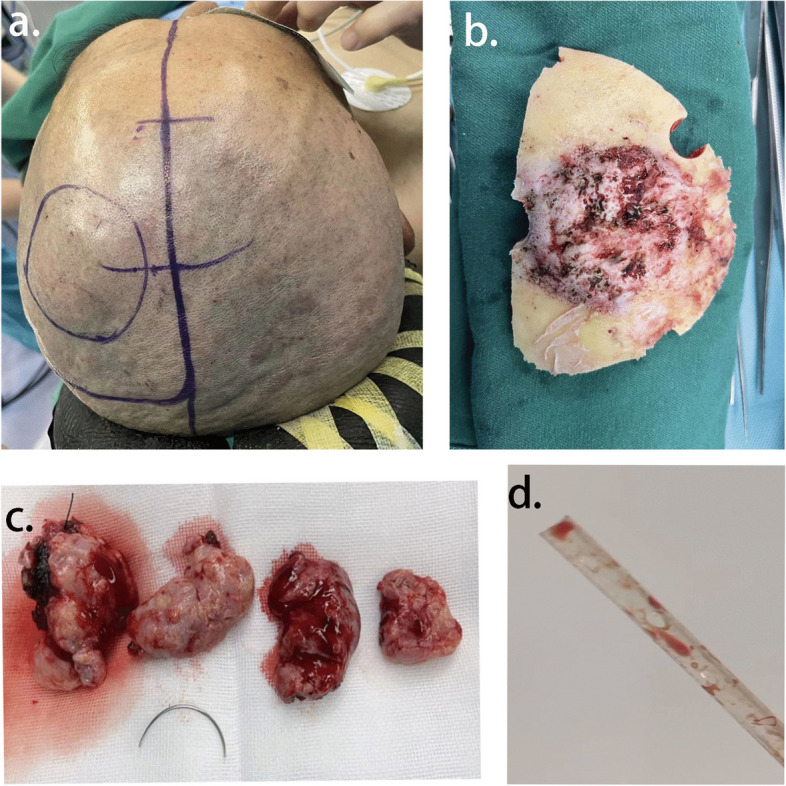
A Operative position, the hair was totally removed without obvious scalp injury. B In accordance with preoperative imaging data, skull erosion was found during craniotomy. C The operative specimen was removed in a piece meal fashion. D Prophylactic use of a subcutaneous closed suction drain was necessary for prevention of postoperative surgical site hematoma in craniotomy. Then, the drain was removed within 48 h after insertion without any apparent secretions
However, 8 days after the craniotomy, when all sutures were removed, the wound healed poorly. We changed dressings more frequently and, at the same time, applied vancomycin based on weight and creatinine clearance. Notably, the patient showed no signs of local and systemic infection. Thirteen days after craniotomy, the final histopathological diagnosis (Identification number D22-26985; Qingdao, China) confirmed the diagnosis of angiomatous meningioma, which was defined by hypervascularity, with tumoral blood vessels exceeding 50% of the total volume (WHO I) [13]. The IHC provided the definitive information –Vimentin ( +), SSTR2 ( +), PR ( +), CD34 ( +), STAT6 ( +), GFAP (-), S-100 (-), Olig-2 (-), Ki-67 (+ ,2%) (Fig. 3).
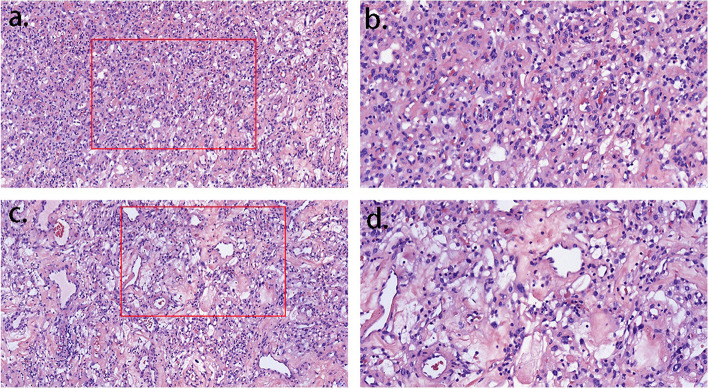
Fig. 3.
The final histological examination of the case confirmed marked hypervascularity with extensive hyalinization of both large and small vessels, along with the characteristics of angiomatous meningiomas
However, when we planned to remove the secondary sutures around the poorly healing wound on June 27, 2022, a copious amount of a thin yellow liquid was squeezed out of the wound. It was then contemplated that titanium implants should be removed immediately after performing another MRI examination. The imaging findings showed an evident light-yellow empyema around the titanium plate (Fig. 4).
Fig. 4.
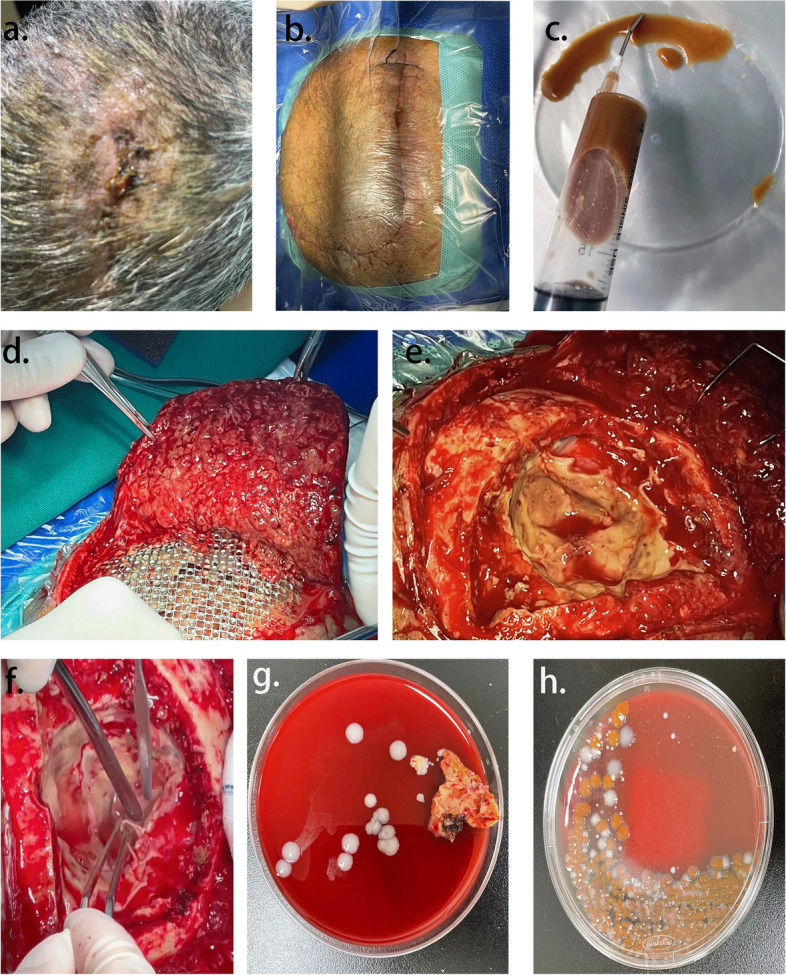
A Delayed wound healing was demonstrated. B In the same operative position, again the hair was totally removed. C Pus was removed from wound with sterile injection syringe. D-F Debridement of brain: First, artificial materials (sutures, titanium mesh and neuro-patch) were removed; Second, rinsed with large amount of gentamycin saline, betadine saline and hydrogen peroxide; Third, the surgical instruments and surgical films were replaced, and then sutured by layers. G, H Representative photograph of isolation of S. marcescens from brain pus of the case
We reviewed the postoperative cranial MR on June 28, 2022. Cranial MR suggests postoperative changes in the left frontoparietal tumor, a high likelihood of a left frontal lobe hematoma, and a high likelihood of bilateral parietal subcutaneous hematomas or effusions (Fig. 5).
Fig. 5.
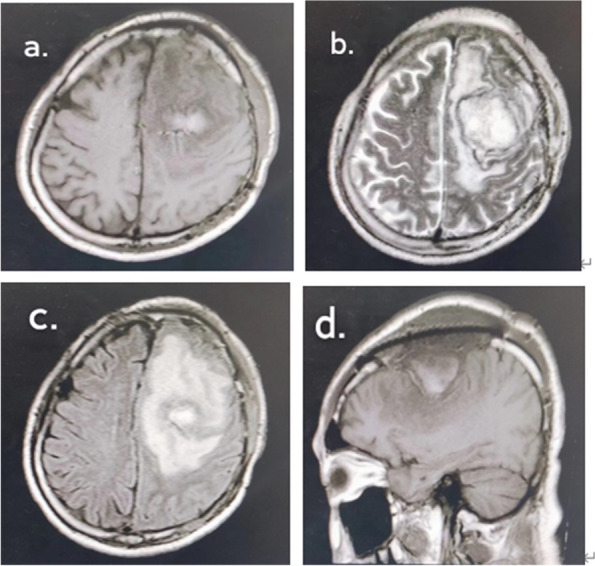
a-d Irregular equal-length mixed T1 inhomogeneous long T2 signal was seen in the left frontal lobe, and a short T2 signal ring was seen at the edge, with a cross-sectional size of about 46 mm × 46 mm × 40 mm, and a large cerebral edema was seen in the surrounding area
Surgical repair and debridement were performed by removing the titanium plate and cleaning up the suppurative secretions. Before discharge, five laboratory tests were conducted to identify possible pathogenic microorganisms. The first bacterial culture was obtained from wound discharge and the other three organisms were isolated from soft tissues obtained during the surgical procedure. Only Serratia marcescens was isolated from all four cultures (Fig. 4G), which was identified to be not antibiotic-resistant. On the 10th day after brain debridement, the sutures were removed and the wound healed well (Fig. 6). After the patient was treated with sensitive antibiotics based on the drug susceptibility test results, the condition gradually improved, and he was transferred to a rehabilitation facility.
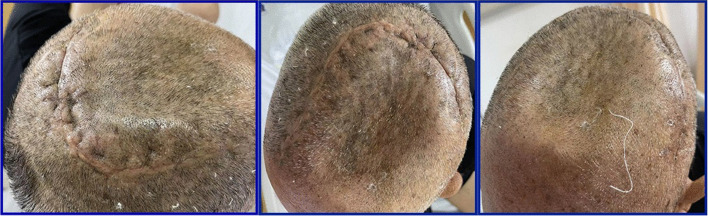
Fig. 6.
Patient's scalp fully healed at discharge
Literature review and discussion
Herein, we present a unique case of an uncommon occurrence in which a 63-year-old male patient with a mass in the left parietal region developed an intracranial abscess caused by Serratia marcescens following surgery. Through this case, we would like to discuss several problems as follows. When and how to make hair preparation before surgery? How to quickly identify postoperative intracranial infection? We should be wary of Serratia marcescens in neurosurgery clinical work. How to deal with the challenges posed by infection after titanium mesh implantation.
In this case, the patient's preoperative preparation was to shave his head the day before the operation instead of cutting hair. Whether the presence of hair at surgical sites increases the risk of neurosurgical infections remains controversial. Preoperative head shaving improves the visibility of the incision line and reduces postoperative surgical site infections. However, many recent studies have reported that shaving the head can change the normal flora around the surgical area and usually results in minor trauma to the scalp, and both of which increase the risk of neurosurgical infection [14–25].
NICE guidelines identified that surgeons should opt to use clippers rather than shaving for hair removal [26]. Razors cause microtrauma, facilitating microbial entry and proliferation in incisions. Studies indicate increased SSIs with shaving versus clipping [27, 28]. Interestingly, few studies have evaluated the risk of SSIs between hair clipping and no hair removal. Forensic evidence shows individual-specific bacterial flora in hair, highlighting endogenous flora as a primary SSI source [29–31]. The necessity and efficacy of hair removal in preventing SSIs are debatable, as are the effectiveness of disinfection methods for unshaved hair and strategies to mitigate clipping-associated risks, such as optimal timing and location of hair removal.
Serratia marcescens is a gram-negative bacterium that has garnered attention due to its association with healthcare-associated infections [10]. Serratia marcescens, commonly found in hospital environments, medical equipment, and on healthcare workers' hands, can infect various clinical samples like sputum, urine, and blood. Although most skin flora are gram-positive bacteria, Serratia marcescens infections have been reported in both healthy and immunocompromised individuals. Serratia marcescens has exhibited increasing resistance to multiple antibiotics, including β-lactams, aminoglycosides, and fluoroquinolones [32–34]. Its resistance mechanisms mainly include efflux pumps, downregulation or alteration of outer membrane pore proteins, chromosomally encoded antibiotic-modifying enzymes, plasmid-encoded ribosomal methyltransferases, and aminoglycoside-modifying enzymes [32].
Recent studies have focused on understanding the mechanisms of resistance and exploring alternative treatment options [32]. Combination therapies, such as the use of β-lactamase inhibitors in combination with β-lactam antibiotics, have shown efficacy in combating resistant strains [35, 36]. Additionally, research is underway to investigate the potential of novel antimicrobial agents and therapeutic approaches like phage therapy [37, 38]. Genomic analysis of Serratia marcescens provides a comprehensive understanding of its genetic composition, including virulence factors, antibiotic resistance genes, and regulatory networks [39–41]. It facilitates the identification of potential targets for therapeutic interventions, the development of diagnostic tools, and the implementation of effective strategies for controlling infections caused by this bacterium.
Antibiotics, the behind-the-scenes heroes of modern medicine, are used to treat various infectious diseases. However, several bacteria have become resistant to most currently available antibiotics. Staphylococci and drug-resistant gram-negative bacilli are the most likely causes of post-neurosurgical intracranial infections [42, 43]. The mortality rate of intracranial infections caused by gram-negative bacteria is significantly higher than that caused by other pathogenic bacteria [44]. Drug susceptibility tests indicated that only a few drugs, such as polymyxins and aminoglycosides, were effective against gram-negative bacteria. However, conventional drug delivery routes have low brain penetration rates, making it difficult to achieve effective therapeutic results. Thus, it is suggested to try some new routes of drug delivery such as intrathecal and intracerebroventricular administration [45]. In addition, the optimal antibiotic treatment for S. marcescens CNS infections remains controversial.
Currently, no clinical or preclinical studies demonstrate a direct relationship between the staging of meningiomas and infection. The pathology report after the patient's surgery showed that he had an angiomatous meningioma, which is a type of brain tumor classified as WHO I. Serratia marcescens infection occurred after surgery, possibly due to intraoperative contamination or suboptimal preoperative shaving, and was not significantly associated with the meningioma stage. We don't detect this latent infection very quickly. Therefore, exploring some methods for rapid identification of postoperative intracranial infection is necessary.
Some cytokines can be used as markers to diagnose bacterial infections. Significantly elevated levels of cerebrospinal fluid IL-6, IL-8, and INF-α occur in bacterial intracranial infections. Cerebrospinal fluid (CSF) analysis is currently considered the gold standard for diagnosing intracranial infections, and biomarkers such as CSF lactate, heparin-binding protein, C-reactive protein, and procalcitonin play ancillary roles [46–49]. With the development of molecular biology, laboratory diagnostic tools have evolved from the cellular to the molecular DNA levels [50, 51]. Pathogenic high-throughput genetic testing enables the rapid detection of disease-causing microorganisms. In addition, diffusion-weighted MR imaging (DWI) and apparent diffusion coefficient (ADC) help distinguish abscesses from other pathologies.
Infection of titanium mesh implants is a common postcranioplasty complication. We analyzed the various causes of titanium implant-associated infections after cranioplasty, such as poor underlying conditions of patients, failure to achieve strict aseptic operation or contamination of the titanium mesh during the procedure, poor local blood supply due to pressure on the scalp caused by a poorly shaped titanium mesh, and local tissue necrosis due to excessive intraoperative hemostasis [52, 53]. Second, cranioplasty requires breakthroughs in materials science, and finding new alternative biomaterials is an urgent clinical requirement. Recent studies revealed that PEEK has the lowest risk of cranioplasty revision and new hydrogel biomaterials show promising antibacterial properties and bone regeneration effects [54–57]. In this case, the autogenous bone was discarded after the meningioma was found to erode the skull, and a titanium mesh was selected for repair during the craniotomy procedure. However, it is questionable to rashly use allogeneic metal materials for repair when the grade of meningioma is not clear enough.
Conclusion
Serratia marcescens is a rare but significant cause of postoperative brain abscesses, often leading to high mortality despite advanced antibiotics and radiology. This pathogen, not previously reported in adult neurosurgery, poses a significant risk for nosocomial infections. The case and literature review aims to share insights for diagnosing and treating such infections.
Preventive measures include proper hair removal before surgery, monitoring postoperative inflammatory markers, and early diagnostic imaging for delayed wound healing. Effective treatment hinges on selecting appropriate antibiotics based on drug sensitivity and understanding their mechanisms. New therapies, including phage therapy and antibiotic combinations, are promising against this pathogen. Additionally, correct titanium mesh implantation and stringent sterilization are crucial to prevent related infections, with research on infection-resistant materials ongoing. Ultimately, timely removal of artificial materials and abscess resection are key to managing these infections.
Authors’ contributions
Xiaolin Song did the literature search; Wenzheng Liu, M.D.,Phd. and Ridong Feng, M.D.,Phd. wrote the paper. Hai Zhao conceived the subject, and revised the manuscript.
Funding
This work was funded by the Shandong Province Natural Science Foundation grants ZR2022QH372.
Availability of data and materials
If someone wants to request the data and materials from this study, they should contact Wenzheng Liu/lwzdoct@163.com.
Declarations
Ethics approval and consent to participate
We confirm that the informed consent of all subjects was obtained during the study.
Consent for publication
We confirm that informed consent has been obtained from the patient for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Russo TA, Johnson JR. Diseases Caused by Gram-Negative Enteric Bacilli. Harrison’s Principles of Internal Medicine, 19e. 2015.
- 2.Passaro DJ, Waring L, Armstrong R, Bolding F, Bouvier B, Rosenberg J, et al. Postoperative Serratia marcescens wound infections traced to an out- of-hospital source. J Infect Dis. 1997 doi: 10.1086/514008. [DOI] [PubMed] [Google Scholar]
- 3.Vigeant P, Loo VG, Bertrand C, Dixon C, Hollis R, Pfaller MA, et al. An outbreak of Serratia marcescens infections related to contaminated chlorhexidine. Infect Control Hosp Epidemiol. 1998 doi: 10.2307/30141429. [DOI] [PubMed] [Google Scholar]
- 4.Okuda T, Endo N, Osada Y, Zen-Yoji H. Outbreak of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol. 1984 doi: 10.1128/jcm.20.4.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patra S, Bhat YR, Lewis LE, Purakayastha J, Sivaramaraju VV. Cerebral abscess due to Serratia marcescens. Indian J Pediatr. 2015;82:199–200. doi: 10.1007/s12098-014-1528-y. [DOI] [PubMed] [Google Scholar]
- 6.Faisal W, Burnton G, Imlay-Gillespie L, Robilliard J. Cerebral abscesses and septic pulmonary emboli due to serratia marcescens infection arising from a portacath. J Microbiol Immunol Infect. 2010;43:538–541. doi: 10.1016/S1684-1182(10)60083-7. [DOI] [PubMed] [Google Scholar]
- 7.Wu YM, Hsu PC, Yang CC, Chang HJ, Ye JJ, Huang CT, et al. Serratia marcescens meningitis: epidemiology, prognostic factors and treatment outcomes. J Microbiol Immunol Infect. 2013;46:259–265. doi: 10.1016/j.jmii.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Luttmann K, Starnes VR, Haddad M, Duggan J. Serratia marcescens, a rare and devastating cause of endocarditis: a case report and review of the literature. Cureus. 2022;14:1–9. doi: 10.7759/cureus.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristina ML, Sartini M, Spagnolo AM. Serratia marcescens infections in neonatal intensive care units (NICUs) Int J Environ Res Pub Health. 2019;16:610. doi: 10.3390/ijerph16040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 11.Jadhav SB, Shah N, Rathi A, Rathi V, Rathi A. Serratiopeptidase: Insights into the therapeutic applications. Biotechnol Rep. 2020;28:e00544. doi: 10.1016/j.btre.2020.e00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS, et al. Acute bacterial meningitis in adults – a review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 13.Hasselblatt M, Nolte KW, Paulus W. Angiomatous Meningioma. Am J Surg Pathol. 2004;28:390–393. doi: 10.1097/00000478-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Simona V, Sara P. Cranial neurosurgery without hair removal and shampoo care: retrospective analysis of 450 cases. Pediatrics Ther. 2016 doi: 10.4172/2161-0665.1000282. [DOI] [Google Scholar]
- 15.Richards A, Zaben M, Patel C, Leach P. The need for hair removal in paediatric brain tumour surgery? Br J Neurosurg. 2021 doi: 10.1080/02688697.2021.1872777. [DOI] [PubMed] [Google Scholar]
- 16.Pinotti S, Vergna S. 31 Cranial neurosurgery without hair removal and shampoo care: retrospective analysis of 450 cases. Arch Dis Child. 2012 doi: 10.1136/archdischild-2012-302724.0031. [DOI] [Google Scholar]
- 17.Seeman K. Clinical Issues—February 2022. AORN Journal. 2022. [DOI] [PubMed]
- 18.Luther E, Berry K, McCarthy D, Sandhu J, Mayrand R, Guerrero C, et al. Hair-sparing technique using absorbable intradermal barbed suture versus traditional closure methods in supratentorial craniotomies for tumor. Acta Neurochir. 2020 doi: 10.1007/s00701-020-04239-3. [DOI] [PubMed] [Google Scholar]
- 19.Sheinberg MA, Ross DA. Cranial procedures without hair removal. Neurosurgery. 1999 doi: 10.1097/00006123-199906000-00054. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda S, Ikawa F, Ohba H, Yoshiyama M, Hidaka T, Kurisu K, et al. Questionnaire survey regarding prevention of surgical site infection after neurosurgery in Japan: Focus on perioperative management and administration of surgical antibiotic prophylaxis. Neurol Med Chir. 2019 doi: 10.2176/nmc.oa.2018-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piatt JH, Oregon P, Steinbok P. Hair and Neurosurgery Neurosurgery. 1994 doi: 10.1097/00006123-199404000-00042. [DOI] [PubMed] [Google Scholar]
- 22.Broekman MLD, Van Beijnum J, Peul WC, Regli L. Neurosurgery and shaving: What’s the evidence? A review. J Neurosurgery. 2011;115(4):670–8. doi: 10.3171/2011.5.JNS102003. [DOI] [PubMed] [Google Scholar]
- 23.Miyagi Y, Shima F, Ishido K. Implantation of deep brain stimulation electrodes in unshaved patients: technical note. J Neurosurg. 2002 doi: 10.3171/jns.2002.97.6.1476. [DOI] [PubMed] [Google Scholar]
- 24.Tang K, Yeh JS, Sgouros S. The influence of hair shave on the infection rate in neurosurgery: a prospective study. Pediatr Neurosurg. 2001 doi: 10.1159/000050379. [DOI] [PubMed] [Google Scholar]
- 25.Liu W-J, Duan Y-C, Chen M-J, Tu L, Yu A-P, Jiang X-L. Effectiveness of preoperative shaving and postoperative shampooing on the infection rate in neurosurgery patients: a meta-analysis. Int J Nurs Stud. 2022;131:104240. doi: 10.1016/j.ijnurstu.2022.104240. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment NICE guideline (NG125). https://www.nice.org.uk/terms. 2019. [PubMed]
- 27.Alexander JW, Fischer JE, Boyajian M, Palmquist J, Morris MJ. The influence of hair-removal methods on wound infections. Arch Surg. 1983 doi: 10.1001/archsurg.1983.01390030079013. [DOI] [PubMed] [Google Scholar]
- 28.Balthazar ER, Colt JD, Nichols RL. Preoperative hair removal: a random prospective: study of shaving versus clipping. South Med J. 1982 doi: 10.1097/00007611-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Tridico SR, Murray DC, Addison J, Kirkbride KP, Bunce M. Metagenomic analyses of bacteria on human hairs: a qualitative assessment for applications in forensic science. Investigative Genet. 2014 doi: 10.1186/s13323-014-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishi E, Watanabe K, Tashiro Y, Sakai K. Terminal restriction fragment length polymorphism profiling of bacterial flora derived from single human hair shafts can discriminate individuals. Leg Med. 2017 doi: 10.1016/j.legalmed.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Tridico SR, Murray DC, Bunce M, Kirkbride KP. DNA profiling of bacteria from human hair: Potential and pitfalls. In: Forensic Microbiology. 2017.
- 32.Tavares-Carreon F, de Anda-Mora K, Rojas-Barrera IC, Andrade A. Serratia marcescens antibiotic resistance mechanisms of an opportunistic pathogen: a literature review. PeerJ. 2023 doi: 10.7717/peerj.14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sleigh JD. Antibiotic resistance in Serratia marcescens. British Med J. 1983;287(6406):1651. doi: 10.1136/bmj.287.6406.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray C, Shenoy AT, Orihuela CJ, González-Juarbe N. Killing of Serratia marcescens biofilms with chloramphenicol. Ann Clin Microbiol Antimicrob. 2017 doi: 10.1186/s12941-017-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vázquez-Ucha JC, Alonso-Garcia I, Guijarro-Sánchez P, Lasarte-Monterrubio C, Álvarez-Fraga L, Cendón-Esteve A, et al. Activity of aztreonam in combination with novel β-lactamase inhibitors against metallo-β-lactamase-producing Enterobacterales from Spain. Int J Antimicrob Agents. 2023;61:106738. doi: 10.1016/j.ijantimicag.2023.106738. [DOI] [PubMed] [Google Scholar]
- 36.Krishnamoorthy R, Athinarayanan J, Periyasamy VS, Alshuniaber MA, Alshammari G, Hakeem MJ, et al. Antibacterial mechanisms of zinc oxide nanoparticle against bacterial food pathogens resistant to beta-lactam antibiotics. Molecules. 2022 doi: 10.3390/molecules27082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udomessien CK, Okon NE, Ubah CB, Oti VB, Ioannou M, Ufot EA. Enterobacteriaceae therapy using bacteriophages: a review. J Pharmaceutical Res Int. 2022 doi: 10.9734/jpri/2022/v34i43a36306. [DOI] [Google Scholar]
- 38.Tikhe C V., Dimopoulos G. Phage Therapy for Mosquito Larval Control: a Proof-of-Principle Study. mBio. 2022. 10.1128/mbio.03017-22. [DOI] [PMC free article] [PubMed]
- 39.Hines DA, Saurugger PN, Ihler GM, Benedik MJ. Genetic analysis of extracellular proteins of Serratia marcescens. J Bacteriol. 1988 doi: 10.1128/jb.170.9.4141-4146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva CR, Miller RM, Pereira BC, Aveleda L, Marin VA. Genomic analysis and plant growth-promoting potential of a Serratia marcescens isolated from food. Research, Society and Development. 2022. 10.33448/rsd-v11i1.24799.
- 41.Li Y, Han P, Pu M, Li F, Li M, An X, et al. Genomic analysis of the Serratia marcescens bacteriophage BUCT660. Microbiology Resource Announcements. 2022 doi: 10.1128/mra.00406-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis*. Clinical Infectious Diseases. 2017. 10.1093/cid/ciw861. [DOI] [PMC free article] [PubMed]
- 43.Tsimogianni A, Alexandropoulos P, Chantziara V, Vassi A, Micha G, Lagiou F, et al. Intrathecal or intraventricular administration of colistin, vancomycin and amikacin for central nervous system infections in neurosurgical patients in an intensive care unit. Int J Antimicrobial Agents. 2017;3(49):389–90. doi: 10.1016/j.ijantimicag.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Pomar V, Benito N, López-Contreras J, Coll P, Gurguí M, Domingo P. Spontaneous gram-negative bacillary meningitis in adult patients: Characteristics and outcome. BMC Infect Dis. 2013 doi: 10.1186/1471-2334-13-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imberti R, Iotti GA, Regazzi M. Intraventricular or intrathecal colistin for the treatment of central nervous system infections caused by multidrug-resistant Gram-negative bacteria. Expert Rev Anti-Infective Ther. 2014;12(4):471–8. doi: 10.1586/14787210.2014.896740. [DOI] [PubMed] [Google Scholar]
- 46.Kong Y, Ye Y, Ma J, Shi G. Accuracy of heparin-binding protein for the diagnosis of nosocomial meningitis and ventriculitis. Crit Care. 2022 doi: 10.1186/s13054-022-03929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mintegi S, García S, Martín MJ, Durán I, Arana-Arri E, Fernandez CL, et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 doi: 10.1542/PEDS.2020-1126. [DOI] [PubMed] [Google Scholar]
- 48.Zatta M, Di Bella S, Bottazzi B, Rossi F, D’Agaro P, Segat L, et al. Determination of pentraxin 3 levels in cerebrospinal fluid during central nervous system infections. Eur J Clin Microbiol Infect Dis. 2020 doi: 10.1007/s10096-019-03767-w. [DOI] [PubMed] [Google Scholar]
- 49.Sakushima K, Hayashino Y, Kawaguchi T, Jackson JL, Fukuhara S. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. J Infect. 2011;62(4):255–62. doi: 10.1016/j.jinf.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Stebner A, Ensser A, Geißdörfer W, Bozhkov Y, Lang R. Molecular diagnosis of polymicrobial brain abscesses with 16S-rDNA-based next-generation sequencing. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 51.Jang Y, Kim S, Kim N, Son H, Ha EJ, Koh EJ, et al. Nanopore 16S sequencing enhances the detection of bacterial meningitis after neurosurgery. Ann Clin Translatl Neurol. 2022 doi: 10.1002/acn3.51517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conen A, Fux CA, Vajkoczy P, Trampuz A. Management of infections associated with neurosurgical implanted devices. Expert Rev Anti-Infective Ther. 2017;15(3):241–55. doi: 10.1080/14787210.2017.1267563. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Hu Y, Yuan Q, Yu J, Wu X, Du Z, et al. Association between metal hypersensitivity and implant failure in patients who underwent titanium cranioplasty. J Neurosurg. 2019 doi: 10.3171/2018.1.JNS171804. [DOI] [PubMed] [Google Scholar]
- 54.Yang X, Huang J, Chen C, Zhou L, Ren H, Sun D. Biomimetic design of double-sided functionalized silver nanoparticle/bacterial cellulose/hydroxyapatite hydrogel mesh for temporary cranioplasty. ACS Appl Mater Interfaces. 2023 doi: 10.1021/acsami.2c22771. [DOI] [PubMed] [Google Scholar]
- 55.Wu M, Wu P, Xiao L, Zhao Y, Yan F, Liu X, et al. Biomimetic mineralization of novel hydroxyethyl cellulose/soy protein isolate scaffolds promote bone regeneration in vitro and in vivo. Int J Biol Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Zhang G, Wei G, Su Z. One-pot, in-situ synthesis of 8-armed poly(ethylene glycol)-coated ag nanoclusters as a fluorescent sensor for selective detection of Cu2+ Biosensors. 2020 doi: 10.3390/BIOS10100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Li MD, Shi JY, Su HF, Liu JW, Feng L, et al. In Situ Capture of a Ternary Supramolecular Cluster in a 58-Nuclei Silver Supertetrahedron. CCS Chemistry. 2022. 10.31635/ccschem.021.202100880.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If someone wants to request the data and materials from this study, they should contact Wenzheng Liu/lwzdoct@163.com.