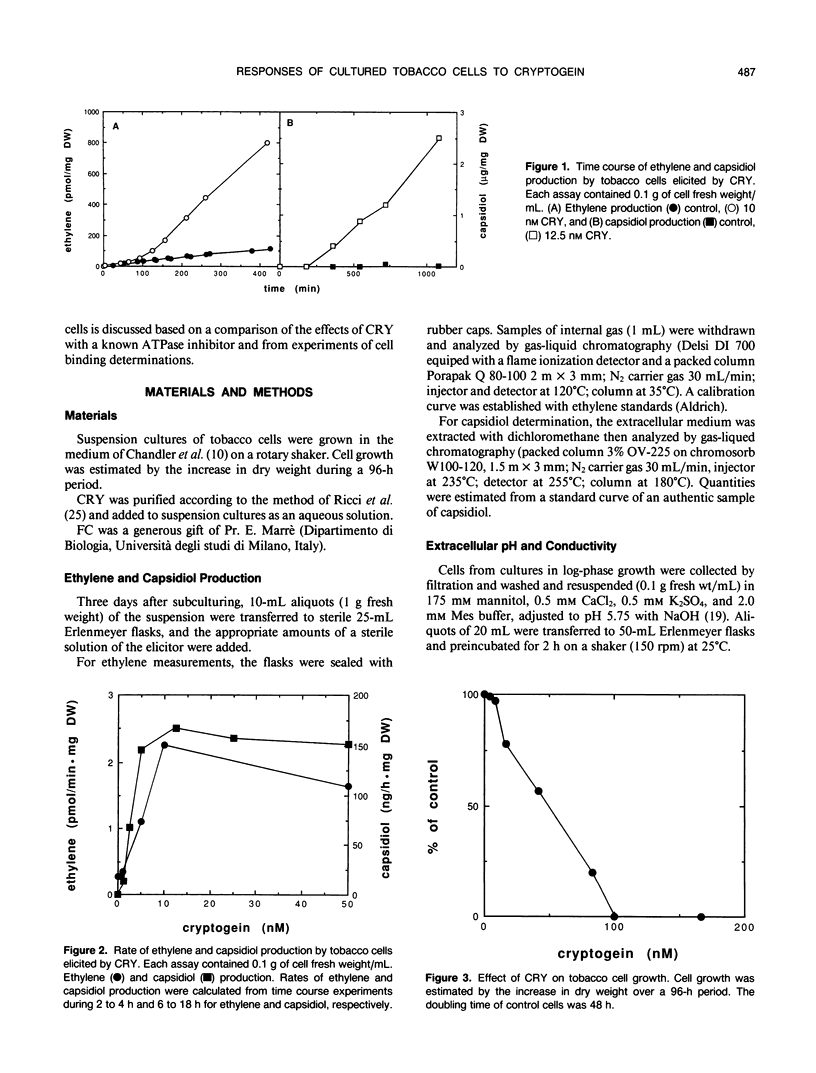
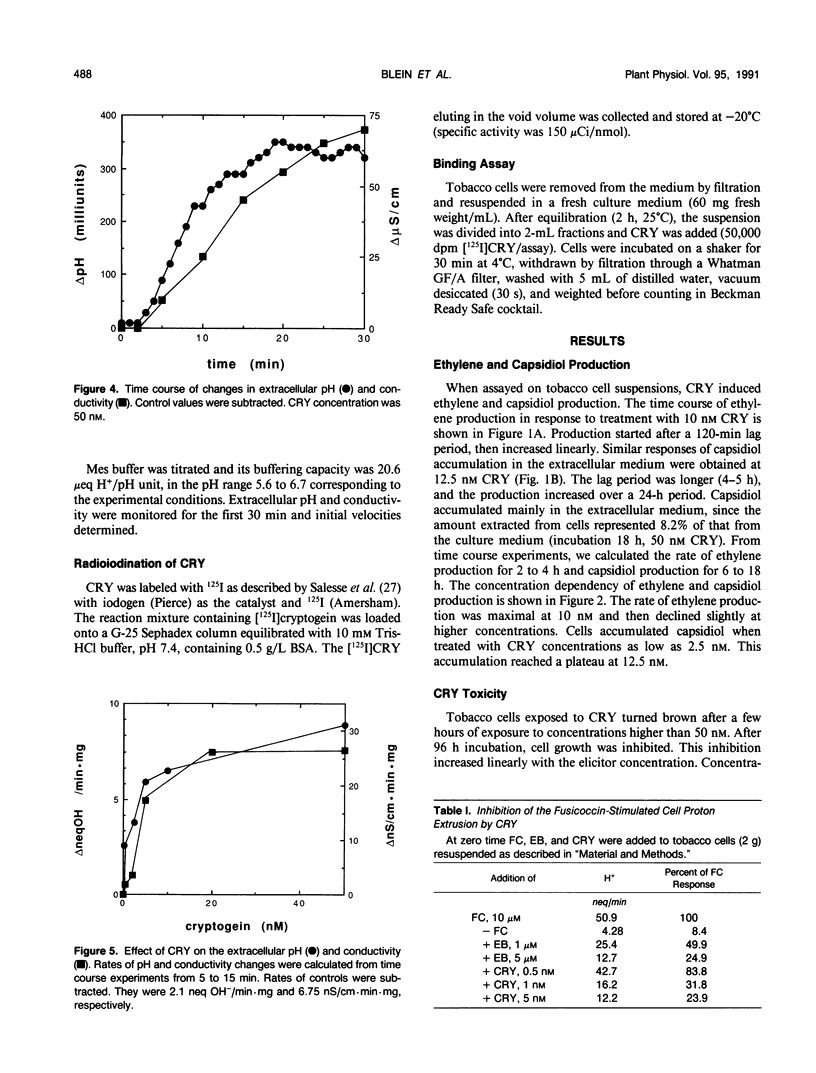
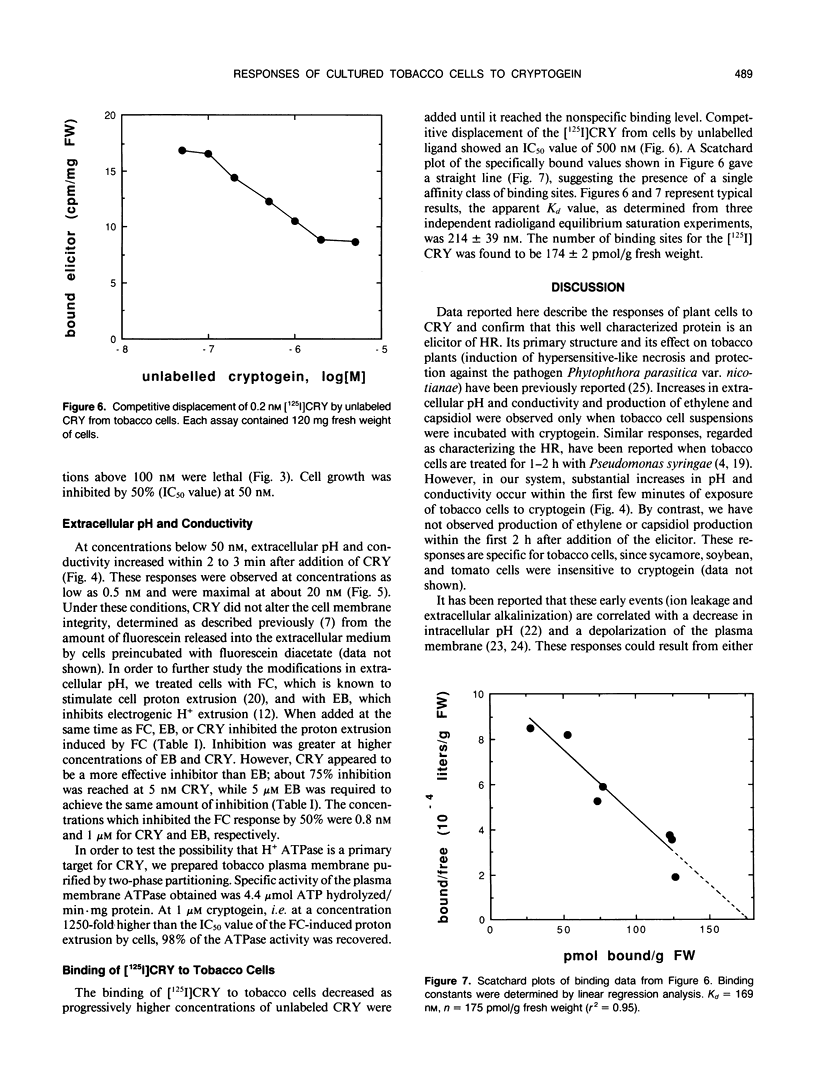
Abstract
In culture, the phytopathogenic fungus Phytophthora cryptogea secretes a protein which elicits hypersensitive-like necroses and protects tobacco plants against invasion by the pathogen Phytophthora parasitica var. nicotianae. This protein, named cryptogein, has been purified and its amino acid sequence determined. In this work, we studied the effect of cryptogein on tobacco cell suspension cultures. Cryptogein was lethal at about 0.10 micromolar. When added at sublethal doses, it elicited the production of ethylene and phytoalexins. It also induced a rapid increase in pH and conductivity of the extracellular medium without affecting the integrity of the plasma membrane. Cryptogein reduced the fusicoccin-induced acidification of the extracellular medium. The concentration which inhibited the fusicoccin response by 50% was 0.8 nanomolar, while 1 micromolar erythrosine B, an ATPase inhibitor, was needed to produce the same inhibition. However, cryptogein did not inhibit the activity of a purified plasma membrane ATPase. Results of binding studies with whole cells suggested the presence of elicitor-binding sites with a high affinity for cryptogein. The involvement of the plasma membrane during the initial interaction between elicitor and cells is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aducci P., Ballio A., Blein J. P., Fullone M. R., Rossignol M., Scalla R. Functional reconstitution of a proton-translocating system responsive to fusicoccin. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7849–7851. doi: 10.1073/pnas.85.21.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Baker C. J., Collmer A. Transient Activation of Plasmalemma K Efflux and H Influx in Tobacco by a Pectate Lyase Isozyme from Erwinia chrysanthemi. Plant Physiol. 1986 Sep;82(1):142–146. doi: 10.1104/pp.82.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Baker C. J. Role of the Plasmalemma H-ATPase in Pseudomonas syringae-Induced K/H Exchange in Suspension-Cultured Tobacco Cells. Plant Physiol. 1989 Sep;91(1):298–303. doi: 10.1104/pp.91.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Huang J. S., Knopp J. A. The Hypersensitive Reaction of Tobacco to Pseudomonas syringae pv. pisi: Activation of a Plasmalemma K/H Exchange Mechanism. Plant Physiol. 1985 Nov;79(3):843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio E. G., Pöpperl H., Schmidt W. E., Ebel J. High-affinity binding of fungal beta-glucan fragments to soybean (Glycine max L.) microsomal fractions and protoplasts. Eur J Biochem. 1988 Aug 1;175(2):309–315. doi: 10.1111/j.1432-1033.1988.tb14198.x. [DOI] [PubMed] [Google Scholar]
- Ojalvo I., Rokem J. S., Navon G., Goldberg I. P NMR Study of Elicitor Treated Phaseolus vulgaris Cell Suspension Cultures. Plant Physiol. 1987 Nov;85(3):716–719. doi: 10.1104/pp.85.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P., Bonnet P., Huet J. C., Sallantin M., Beauvais-Cante F., Bruneteau M., Billard V., Michel G., Pernollet J. C. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989 Aug 15;183(3):555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salesse R., Genty N., Garnier J. Lutropin is processed much more rapidly than human choriogonadotropin by porcine Leydig cells in primary culture. Biol Cell. 1983;49(2):187–190. doi: 10.1111/j.1768-322x.1984.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Keen N. T., Wang M. C. A receptor on soybean membranes for a fungal elicitor of phytoalexin accumulation. Plant Physiol. 1983 Oct;73(2):497–506. doi: 10.1104/pp.73.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


