Abstract
Myxococcus xanthus is a social bacterium that lives in the soil and undergoes spectacular development to form multicellular fruiting bodies. It contains a large family of eukaryote-like serine/threonine protein kinases. We found that a number of inhibitors for eukaryotic protein serine, threonine, and tyrosine kinases could inhibit the development and sporulation of M. xanthus to various degrees. These results suggest that serine/threonine and tyrosine phosphorylation may be involved in development of M. xanthus. None of the inhibitors tested had any effect on vegetative growth of M. xanthus. Most of them seemed to act during the early stages of development. However, the expression of a very early development-specific gene, Ω4521, was not significantly affected by the inhibitors. The patterns of protein phosphorylation during development were also not significantly altered by the inhibitors, suggesting that the targets of the inhibitors are minor or unstable phosphoproteins but play key roles in fruiting-body formation in M. xanthus.
Myxococcus xanthus is a gram-negative soil bacterium which undergoes spectacular multicellular development and cellular differentiation. Upon starvation at high cell density (2 × 109 cells/ml) on a semisolid surface, cells move to aggregation centers, where they form mounds called fruiting bodies. Within the fruiting bodies, many cells lyse while others differentiate to become sonication- and heat-resistant spores (for a recent review, see reference 3). Its social behavior and morphogenesis resemble those of eukaryotic slime molds like Dictyostelium discoideum, in which a signal transduction system consisting of a receptor, a G-protein, an effector, and protein serine/ threonine kinases is involved in sensing starvation and regulating gene expression for development. Due to the close resemblance of the life cycles of Dictyostelium and M. xanthus, it has been proposed that a similar signal transduction system might exist in this developmental bacterium. In particular, the recent discovery of a large family of eukaryote-like protein serine/threonine kinases in M. xanthus (3, 5, 29, 31, 32) provided an intriguing opportunity to examine the role of these kinases in prokaryote development.
The first eukaryote-like protein serine/threonine kinase found in bacteria was discovered in M. xanthus (20) and found to be required for normal development. Subsequently, M. xanthus was found to contain a family of at least 13 eukaryote-like protein serine/threonine kinases (31). The cloning and sequencing of these 13 protein serine/threonine kinases have revealed that all of them retain the conserved structural features of eukaryotic protein kinases (11). Many of these protein kinases are transmembrane proteins (5, 28, 32). It seems very likely that these transmembrane protein kinases sense certain environmental signals and are involved in various signal transduction pathways leading to regulation of growth and development. Because of the sequence similarity between eukaryotic and M. xanthus protein serine/threonine kinases, it is possible that known inhibitors for eukaryotic kinases affect the activity of protein kinases of M. xanthus. In this study, we have examined the effect of some of these protein kinase inhibitors on fruiting-body formation as well as spore formation by M. xanthus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The M. xanthus strain used was DZF1. The cells were grown vegetatively in CYE medium (1% Casitone, 0.5% yeast extract, 0.1% MgSO4), and development was studied on CF agar (10 mM Tris HCl [pH 7.6], 8 mM MgSO4, 0.02% Casitone, 0.2% NH4SO4, 1 mM potassium phosphate buffer [pH 7.6], 0.2% sodium citrate, 0.1% sodium pyruvate, 1.5% agar), supplemented with protein kinase inhibitors in 48-well microtiter plates (Falcon Inc). For quantitation of β-galactosidase activity, strain DK6620 carrying Ω4521 (kindly provided by H. Kaplan, University of Texas Medical School, Houston, Tex.) was used (12).
Protein kinase inhibitors.
Staurosporine and genistein were purchased from Sigma (St. Louis, Mo.); K252c was purchased from Calbiochem (San Diego, Calif.); and chelerythrin, KN-62, bisindolylmaleimide, daidzein and tyrphostin B52 were from Alexis Co. (Woburn, Mass.).
Inhibition of development of M. xanthus by various inhibitors.
To study the development of M. xanthus under starvation conditions, cells were grown in CYE medium until they reached a turbidity of 100 Klett units, at which time they were harvested, washed once with TM buffer (10 mM Tris HCl [pH 7.6], 8 mM MgSO4), and resuspended in TM buffer at 4,000 Klett units. Cell suspension (2 μl) was spotted on each well of a 48-well microtiter plate containing 300 μl of CF agar and the individual protein kinase inhibitors at 5 μM. The inhibitors were dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in CF agar wells was 0.5%. The control plates contained 0.5% DMSO only. The plates were incubated at 30°C, and development of M. xanthus was monitored every 8 h under a dissecting microscope. To study the effect of inhibitors during vegetative growth, cells were grown to a turbidity of 100 Klett units in CYE medium, 2 μl of growing culture was spotted onto each well of a 48-well microtiter plate containing 300 μl of CYE agar and the individual protein kinase inhibitors at 5 μM, and the plates were incubated at 30°C. The effect on growth and motility of cells was assessed by the ability of cells to grow and move away from the growing spot. To study the effect of addition of protein kinase inhibitor 5 h after the onset of development, the cells were allowed to develop on CF agar in a 24-well microtiter plate. After 5 h, the agar was gently lifted from one end and protein kinase inhibitor was added at the bottom of the agar to a final concentration of 5 μM.
Effect of inhibitors on sporulation.
To count the number of spores produced in the presence of protein kinase inhibitors on CF medium, M. xanthus cells were allowed to develop in 48-well microtiter plates in the presence of the individual inhibitors at 5 μM, as described above. After 5 days, all the cells (containing well-developed fruiting bodies in some cases) were scraped off the surface of the agar, washed once with 200 μl of TM buffer, resuspended in 200 μl of TM buffer, and sonicated to disrupt the fruiting bodies. Sonication-resistant refractile spores were counted under the microscope. The numbers listed in Table 1 reflect the yield of spores from 2 μl of cell suspension that was spotted on the surface of CF agar.
TABLE 1.
Effect of protein kinase inhibitors on the development of M. xanthus
| Inhibitor | Development phenotype | Spore yield | Remarks |
|---|---|---|---|
| None | 1.25 × 107 | ||
| Staurosporine | Inhibited | 0.87 × 107 | General and potent inhibitor of Ser/Thr kinases; inhibits PKC (IC50 = 2.7 nM) and PKA (IC50 = 8.2 nM) |
| K-252c | Inhibited | 0 | Inhibits PCK (IC50 = 2.45 μM) and PKA (IC50 = 25.7 μM) |
| Chelerythrin | Inhibited | 0 | Selective inhibitor of PKC (IC50 = 660 nM) |
| KN-62 | Delayed | 1.0 × 107 | Inhibits Ca2+/CaM kinase (Ki = 900 nM); can also inhibit PKA, PKC, and PKG |
| Bisindolylmaleimide | Delayed | 0.75 × 107 | Selective inhibitor of PKC (Ki = 10 nM) |
| Genistein | No effect | 1.23 × 107 | Inhibits Tyr kinase by competing with ATP |
| Daidzein | Delayed | 0.75 × 107 | Inhibits Tyr kinase by competing with ATP; can also arrest the cell cycle at G1 |
| Tyrphostin B52 | Inhibited | 0 | Structurally resembles Tyr and so competitively inhibits Tyr kinase |
Effect of inhibitors on activity of protein kinases in vitro.
To study the inhibition of protein kinases of M. xanthus in vitro by protein kinase inhibitors, the inhibitors were added to 0.1 μg of purified protein at a final concentration of 5 μM, in a total volume of 20 μl. The reaction was carried out in kinase buffer (50 mM Tris HCl [pH 7.6], 50 mM KCl, 10 mM MgCl2, 5 mM NaF, 0.1 mM ATP, 1 mM β-mercaptoethanol) in the presence of 10 μCi of [γ-32P]ATP at room temperature for 15 min. For Pkn2, MgCl2 was replaced by MnCl2, and for Pkn11, 5 mM CaCl2 was included in the reaction mixture. The reaction was terminated by addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and heating at 90°C for 10 min. The autophosphorylated protein was subjected to SDS-PAGE and visualized by autoradiography.
Assay of β-galactosidase activity.
To study the expression of Ω4521, at the indicated times 40 μl of cells was scraped off the surface of CF agar containing different protein kinase inhibitors and washed once with TM buffer, and the cell pellet was stored at −20°C. β-Galactosidase activity was assayed by the method of Kroos et al. (15).
In vitro phosphorylation during development.
To study the pattern of phosphorylated proteins during development, at the indicated times 40 μl of cells was scraped off the surface of agar containing different protein kinase inhibitors and sonicated to disrupt the cells (the sonication buffer contained 10 mM Tris HCl [pH 7.6], 1 mM EDTA, and 5 mM phenylmethylsulfonyl fluoride). Unbroken cells were removed by centrifugation, and total-cell extract was used in an in vitro phosphorylation reaction as described above. After the proteins were separated by SDS-PAGE, they were transferred to a polyvinylidene difluoride membrane. The membrane was washed with 10% trichloroacetic acid (TCA) at 55°C for 2 h to remove acid-sensitive phosphorylation and autoradiographed.
RESULTS AND DISCUSSION
When M. xanthus cells are spotted on CF agar at a high cell density (2 × 109 cells/ml), they aggregate to form fruiting bodies. This process is completed within 48 h. To elucidate the roles of protein serine/threonine kinases during vegetative growth and the developmental cycle of M. xanthus, a series of single and multiple deletions of protein kinases have been constructed by positive-negative KG cassettes (11). These deletions include a quadruple deletion mutant with pkn6, pkn7, pkn11, and pkn13. Examination of fruiting-body formation and sporulation by this mutant strain revealed that fruiting-body formation was initiated earlier in the mutant than in the wild-type strain but the fruiting bodies showed normal morphology and could produce normal numbers of spores. These results indicated a redundancy in the functions of protein kinases of M. xanthus. Therefore, to investigate the overall function of protein kinases during the development and sporulation of M. xanthus, we tested the effects of several different protein kinase inhibitors on development of M. xanthus. We found that in the presence of protein kinase inhibitors, which included both serine/threonine and tyrosine protein kinase inhibitors, the formation of fruiting bodies was either delayed or completely inhibited (Table 1).
Effect of staurosporine and other protein serine/threonine kinase inhibitors.
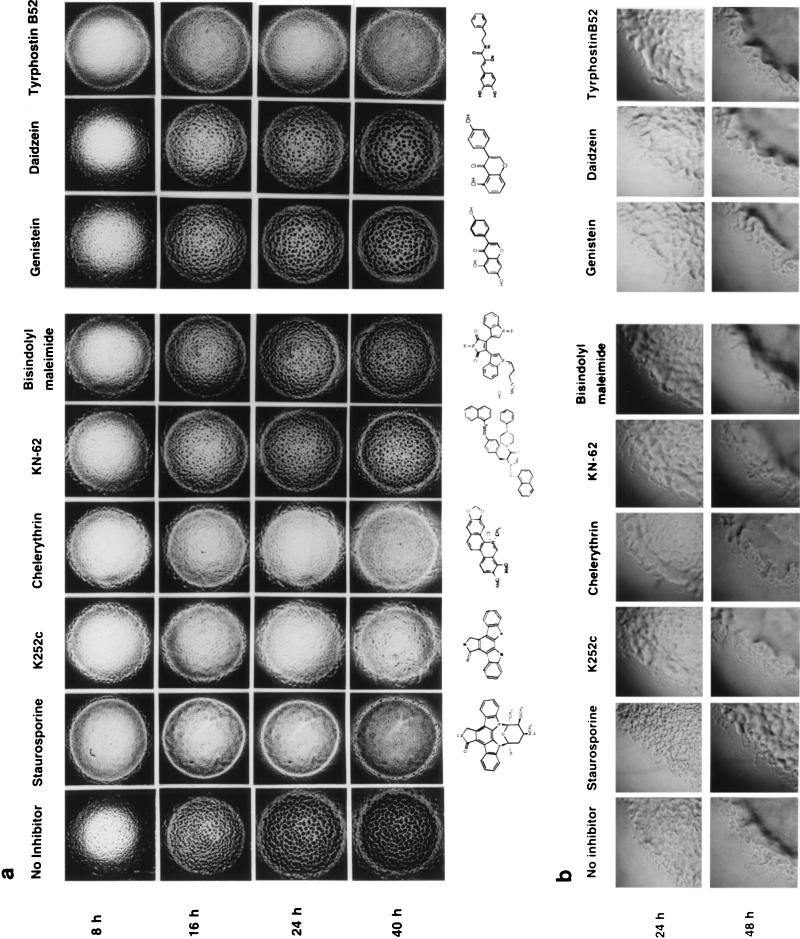
Staurosporine is the most potent inhibitor of kinases in vitro that has been found to date (26). It has been found to inhibit a variety of serine/threonine and tyrosine kinases, e.g., protein kinase C (PKC) (50% inhibitory concentration [IC50] = 2.7 nM), PKA (IC50 = 8.2 nM), tyrosine kinase p60v-src (IC50 = 6.4 nM) (21), and tyrosine kinase epidermal growth factor receptor (IC50 = 630 nM) (16). It has been found to interact with the catalytic domain of protein kinases and prevent the binding of ATP to protein kinases. In in vitro experiments, staurosporine inhibits the autophosphorylation activity of Pkn2, a serine/threonine protein kinase of M. xanthus (28). At 5 μM, staurosporine completely blocked the formation of fruiting bodies in M. xanthus (Fig. 1a); however, at 1 μM, fruiting-body formation, although delayed by 24 h, could occur. Interestingly, even though staurosporine could completely inhibit the formation of fruiting bodies at 5 μM, it did not inhibit the formation of spores (Table 1). It has been known for a long time that the pathways leading to fruiting-body formation and differentiation into spores are not always coupled. Many nonfruiting mutants of M. xanthus are able to form normal numbers of spores (18). Staurosporine and other inhibitors tested did not inhibit vegetative growth and motility of M. xanthus, as indicated by the presence of a normal “flare” on CYE plates (Fig. 1b).
FIG. 1.
Effect of protein kinase inhibitors on the development (a) and vegetative growth (b) of M. xanthus.
K252c is another indole carbazole protein kinase inhibitor isolated from microorganisms (13). Like staurosporine, K252c has a broad specificity for inhibiting protein kinases, although it is less potent than staurosporine. It inhibits Ca2+/calmodulin-dependent protein kinase (CaM kinase) (Ki = 18 nM), myosin light-chain kinase (Ki = 17 nM), PKA (Ki = 25 nM), PKC (Ki = 25 nM), and PKG (Ki = 20 nM). K252c at 5 μM also could inhibit fruiting-body formation and sporulation of M. xanthus. It is interesting that although staurosporine and K252c have very similar structures, as shown in Fig. 1a, K252c could inhibit both sporulation (Table 1) and fruiting-body formation (Fig. 1a) whereas staurosporine could not inhibit sporulation.
Chelerythrin chloride inhibits PKC (IC50 = 660 nM) but does not inhibit PKA or CaM kinase (7). It is not competitive with ATP, suggesting that it binds to a different region of the catalytic domain than staurosporine and its analogs. As shown in Fig. 1a and Table 1, 5 μM chelerythrin chloride could inhibit fruiting-body formation and sporulation.
KN-62 selectively inhibits CaM kinase II (Ki = 900 nM) by binding directly to the calmodulin-binding site of the enzyme (27). KN-62 at 5 μM could delay the formation of fruiting bodies in M. xanthus by 16 h (Fig. 1a). The fruiting bodies formed in the presence of KN-62 contained nearly normal numbers of spores (Table 1).
Bisindolylmaleimide is known to be a selective inhibitor of PKC (Ki = 10 nM) (19, 27). It is structurally similar to staurosporine and is a competitive inhibitor with respect to ATP. However, 5 μM bisindolylmaleimide showed only a weak inhibition of M. xanthus development (Fig. 1a).
The spores produced in the presence of various inhibitors were checked for their ability to germinate on CYE medium, and all of them were found to be viable (data not shown).
It is also interesting that compounds like calphostin C, sphingosine, and hypericin, which are known to specifically inhibit PKC by competitively inhibiting the binding of diacylglycerol to the regulatory domain of PKC (6, 14, 25), failed to show any effect on development of M. xanthus (data not shown). Sphingosine has also been thought to inhibit phospholipase A2 and phospholipase D by acting as the biosynthetic precursor of sphingolipids. This could mean that a signal transduction pathway involving phospholipases, diacylglycerol, and inositol triphosphate (22), similar to that found in eukaryotes, probably does not operate during development of M. xanthus. However, although Benaissa et al. (2) have shown that synthesis and degradation of inositol phospholipids occur during clumping in Stigmatella aurantiaca and suggested that a phosphoinositol cycle might operate, the relation between protein kinases and a phosphatidylinositol cycle in M. xanthus remains to be investigated, although the role of Ca2+ in clumping of M. xanthus is well known (24, 30). In this respect, it is interesting that several serine/threonine protein kinases identified in M. xanthus require Ca2+ for their activity (32). It is possible that Ca2+ influxes, along with starvation, activate certain protein kinases and trigger a signal transduction pathway to initiate development.
The effect of the H-7 series of compounds (which are isoquinolinesulfonamide derivatives) (8, 9) on fruiting-body formation and sporulation by M. xanthus was also tested (data not shown). These compounds inhibit PKA (Ki = 1.2 to 3.0 μM), PKC (Ki = 6.0 to 40 μM), PKG (Ki = 480 nM to 5.8 μM), and myosin light-chain kinase (Ki = 3.6 to 150 μM). The compounds tested are HA-100, HA-1004, H-7, Iso H-7, H-8, and H-9. None of these compounds could inhibit fruiting-body formation and sporulation in M. xanthus, probably because their Ki values are too high (their inhibitory activity is 103-fold lower than that of K252c) and so at 5 μM they failed to show any effect.
Olomoucine is a specific inhibitor of the cdc/cdk family of cyclin-dependent protein kinases, which are involved in regulation of the cell cycle. Olomoucine did not have any effect on vegetative growth or development of M. xanthus (data not shown).
Effect of tyrosine kinase inhibitors.
In general, the tyrosine kinase inhibitors can be divided into two groups: genistein (and its analogs) and tyrphostins.
Genistein competitively inhibits the binding of ATP and thus inhibits protein tyrosine kinase (1). However, since genistein itself bears no structural resemblance to ATP, it has been suggested that the inhibition might not be due to true competition but the binding sites of genistein and ATP might overlap. Genistein is almost inactive against protein serine/threonine kinases and so is used to distinguish between the two groups of kinases (1). In the present experiments, no effect of genistein on the development of M. xanthus was observed; however, a genistein analog, daidzein, could delay fruiting-body formation by 24 h in M. xanthus, suggesting that all the features of eukaryotic tyrosine kinases might not be conserved in protein kinases of M. xanthus. The number of spores produced in the presence of daidzein were close to normal, and these spores were viable.
Interestingly, genistein has been shown to inhibit histidine kinases (10, 23), with an IC50 of 110 μM, which is much higher than the concentration of genistein used in the present experiments (see Materials and Methods). Moreover, all the kinase inhibitors used in the present experiments were tested for inhibition of autophosphorylation of purified EnvZ, a histidine kinase of Escherichia coli; however, at 5 μM none could inhibit the autophosphorylation activity of purified EnvZ (data not shown). These results indicate that inhibition of the development of M. xanthus by the kinase inhibitors used in the present experiments is not due to inhibition of histidine kinases.
Tyrphostins are synthetic compounds which structurally resemble tyrosine and thus act as specific inhibitors of tyrosine kinases. Many of them have broad specificities, while some are selective (16). We found that 5 μM tyrphostin B52 was able to inhibit fruiting-body formation and sporulation of M. xanthus on CF plates. This indicates the existence of tyrosine phosphorylation, which may play important roles during the development of M. xanthus. However, a protein tyrosine kinase has not been identified in M. xanthus, although the presence of phosphotyrosine has been reported (4), and the patterns of tyrosine-phosphorylated proteins were shown to vary during development. There is no evidence at present to show that phosphorylation of tyrosine occurs by a tyrosine-specific kinase, although its presence in M. xanthus has been speculated (4). It is also possible that some of the M. xanthus serine/threonine kinases possess dual specificity and phosphorylate on tyrosine residues in addition to serine/threonine and that these kinases are inhibited by tyrphostins as well as genistein analogs.
When M. xanthus cells were spotted on CF agar plates containing 1 μM staurosporine or tyrphostin, their development was delayed by 24 h (data not shown). However, when other inhibitors were tested at 1 μM, the cells could develop normally.
It is also important to note that the inhibitory activities of different protein kinase inhibitors also depend on the permeability of the cell membrane and their stability.
Effect of addition of protein kinase inhibitors after the onset of development.
The process of development is accompanied by the expression of several developmentally regulated genes. The products of these genes are probably required for development. By analyzing 36 different developmentally expressed genes, Kroos et al. (15) have divided them into three groups: group I, the initiation/aggregation group, which is expressed from h 0 to 5; group II, the postaggregation group, which is expressed from h 9 to 15; and group III, the sporulation group, which is expressed from h 18 to 24.
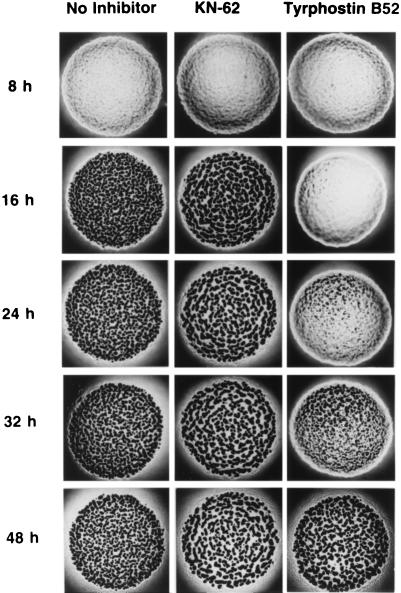
We tried to determine if protein kinases of M. xanthus affect the early or late stages of development. If these inhibitors affect late stages of development, their addition after the onset of starvation should be able to inhibit development. However, if they inhibit early stages of development, their addition after the onset of starvation might fail to show any effects. Thus, protein kinase inhibitors were added to developing cells 5 h after the onset of development, to allow the expression of group I genes, as described in Materials and Methods. The results are shown in Fig. 2. We found that most of the protein kinase inhibitors failed to inhibit the development of M. xanthus if they were added 5 h after the onset of development, except for tyrphostin B52, which was able to delay the development. KN-62 also failed to inhibit development; however, the fruiting bodies produced were larger than those produced in the absence of any inhibitor. These results show that most of the protein kinase inhibitors inhibit steps occurring during the early stages of development.
FIG. 2.
Effect of addition of protein kinase inhibitors after the onset of development.
Effect of kinase inhibitors on gene expression during early development.
One of the earliest genes to be expressed during development has been identified as Ω4521 (15), a commonly used marker for development of M. xanthus. Ω4521 was originally identified by Tn5 lac insertion in this gene (15). The Tn5 lac transposon carries a promoterless lacZ gene, which can be expressed under the transcriptional control of any transcriptionally active gene into which the transposon is inserted in the right orientation. Strain DK6620 (12) carries such an insertion, called Ω4521, and was used for this experiment. The expression of Ω4521 requires both an A-signal, which is a mixture of amino acids and their peptides, and nutritional starvation during development (17). The expression of Ω4521 is detectable as early as 1.5 h after the onset of starvation and reaches a peak at 21 h of development (15). Since most inhibitors seem to act during the early stages of development, we attempted to examine whether any of the kinases could block the signal transduction pathways leading to induction of Ω4521. The results are shown in Table 2 (the numbers shown are the averages of the results of two independent experiments). The β-galactosidase activity values obtained in the absence of inhibitors are in good agreement with that reported by Kroos et al. (15).
TABLE 2.
Effect of protein kinase inhibitors on the expression of Ω4521
| Inhibitor | Sp act of β-galactosidase
|
|
|---|---|---|
| 5 h after starvation | 20 h after starvation | |
| None | 49.2 | 117.2 |
| Staurosporine | 28.7 | 110.3 |
| K252c | 27.0 | 55.0 |
| Chelerythrin | 65.4 | 200.9 |
| KN-62 | 38.6 | 107.3 |
| Bisindolylmaleimide | 49.6 | 103.3 |
| Genistein | 20.3 | 88.0 |
| Daidzein | 35.6 | 130.7 |
| Tyrphostin B52 | 27.1 | 77.9 |
It is interesting that genistein, which had no effect on the development of M. xanthus, was a potent inhibitor of Ω4521 expression during the early stages of development, indicating the involvement of tyrosine phosphorylation in the signal transduction pathway leading to expression of Ω4521. Other kinase inhibitors also showed inhibition of Ω4521 to various degrees; e.g., K252c and tyrphostin B52, which inhibited fruiting-body formation and sporulation (Fig. 1), showed inhibition of Ω4521 expression to the same degree as genistein did. Interestingly, chelerythrin, although completely inhibiting development, had no effect on expression of Ω4521 but, rather, seemed to enhance the expression of Ω4521. This supports the previous observation by Kroos et al. (15) that development of M. xanthus does not completely depend on expression of Ω4521, so that a strain carrying a Tn5 lac insertion in this gene can develop normally. In other words, the process of development does not absolutely require the gene product of Ω4521 and the expression of Ω4521 does not guarantee that development can occur. That is why, even though expression of Ω4521 is found to be normal in some cases, development is inhibited, probably by inhibition of pathways other than the one leading to expression of Ω4521. Another possibility is that the kinases inhibited by these inhibitors, e.g., staurosporine and chelerythrin, are situated downstream of Ω4521 in the developmental pathway.
Effect of protein kinase inhibitors on autophosphorylation of protein kinases of M. xanthus.
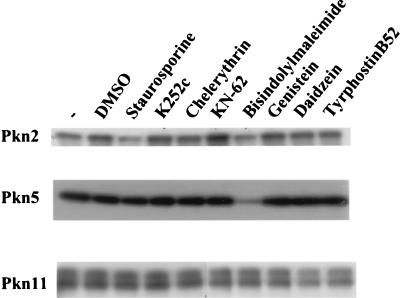
The autophosphorylation of purified Pkn2, Pkn5, and Pkn11 was examined in the presence of different protein kinase inhibitors, as described in Materials and Methods. It was found that Pkn5 was inhibited only by bisindolylmaleimide (Fig. 3) whereas Pkn2 was inhibited by both staurosporine and bisindolylmaleimide (Fig. 3). The IC50 for inhibition of Pkn2 by staurosporine is 440 nM (29). Pkn11 was not inhibited by any of the protein kinase inhibitors that we have tried so far (Fig. 3).
FIG. 3.
Effect of protein kinase inhibitors on autophosphorylation of serine/threonine protein kinases of M. xanthus.
Effect of protein kinase inhibitors on the pattern of phosphorylated proteins during development.
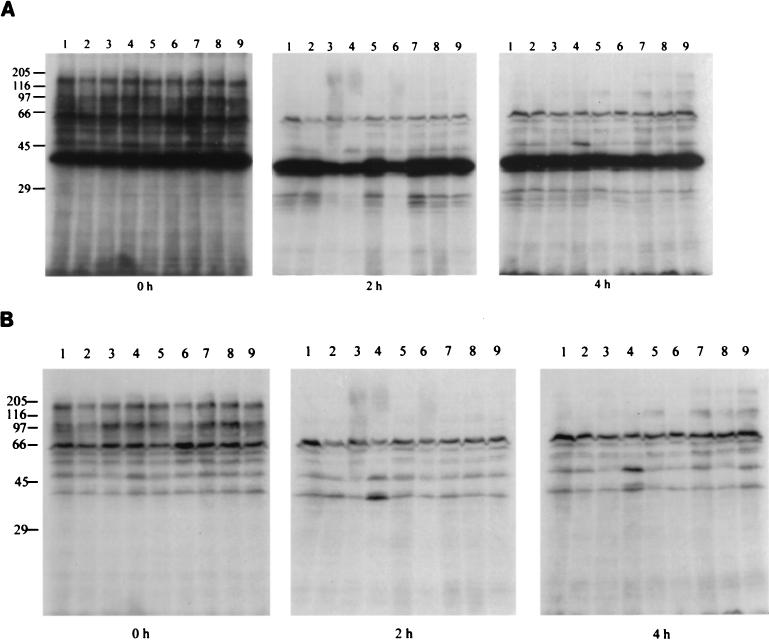
The pattern of phosphorylated proteins was examined as described in Materials and Methods. As shown in Fig. 4, no significant difference in the phosphorylation pattern was detected in the cells which were developing on CF agar plates containing different protein kinase inhibitors. However, at the onset of development, several phosphorylated bands disappeared, indicating downregulation of some kinases or activation of some specific phosphatases. The lack of any obvious difference in the pattern of phosphorylation of proteins in cells developing on different inhibitors could be due to the targets of phosphorylation being minor proteins which could not be detected in the experimental system we used.
FIG. 4.
Effect of protein kinase inhibitors on the pattern of in vitro-phosphorylated proteins of M. xanthus during development. (A) Before the filter is washed with TCA. (B) After the filter is washed with TCA. Lanes: 1, no inhibitor; 2, staurosporine; 3, K252c; 4, chelerythrin; 5, KN-62; 6, bisindolylmaleimide; 7, genistein; 8, daidzein; 9, tyrphostin B52. The differences between lane 1 and the other lanes seen at 2 and 4 h of development were not consistently observed.
In conclusion, we have shown that general eukaryotic protein kinase inhibitors can inhibit the development of M. xanthus. A wide variety of protein kinase inhibitors can inhibit the development of M. xanthus to various degrees, suggesting that they have different specific targets in the cell. The fact that protein kinases of M. xanthus can be directly inhibited by inhibitors of eukaryotic protein kinases indicates that serine/threonine protein kinases of M. xanthus have catalytic domains similar to those of PKC and PKA and also that these kinases are involved in developmental pathways. Interestingly, inhibitors with different known specificities could inhibit the development of M. xanthus, suggesting the involvement of more than one kinase in the regulation of development. Most of the protein kinase inhibitors showed only a partial inhibition of Ω4521 expression, indicating that the kinases might act in a different pathway leading to development. Nutritional starvation and an A-signal could activate many different pathways, including elevation of intracellular levels of guanosine tetraphosphate and cyclic AMP, all of which contribute to the process of fruiting-body formation and sporulation; the expression of Ω4521 is just one of the effects of these pathways. Each of the protein kinase inhibitors could affect one or more of these steps. For example, it is possible that genistein inhibits one or more steps leading to the synthesis of Ω4521, while allowing the other steps to go on. This might result in inhibition of expression of Ω4521 although development can proceed. On the other hand, some other inhibitors, such as staurosporine, might inhibit the pathways leading to development while allowing the expression of Ω4521. Moreover, inhibitors such as K252c and tyrphostin B52 might inhibit many different pathways, which leads to inhibition not only of development and sporulation but also of expression of Ω4521.
It is important to note that these inhibitors did not inhibit vegetative growth (data not shown), indicating that they are not toxic to the cell and supporting the previous hypothesis that kinase activity is required for the development of M. xanthus.
ACKNOWLEDGMENTS
We thank Heidi Kaplan, University of Texas Medical School, Houston, Tex., for providing strain DK6620 and William Hanlon, Merck Co., Rahway, N.J., for helpful discussions. We also thank Masayori Inouye, UMDNJ, Piscataway, N.J., for critical reading of the manuscript.
This work was supported by Public Health Service grant GM26843 from the National Institutes of Health.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawara S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Benaissa M, Vieyres-Lubochinsky J, Odeide R, Lubochinsky B. Stimulation of inositide degradation in Stigmatella aurantiaca. J Bacteriol. 1994;176:1390–1393. doi: 10.1128/jb.176.5.1390-1393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dworkin M. Recent advances in the social and developmental biology of myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frasch S, Dworkin M. Tyrosine phosphorylation in Myxococcus xanthus, a multicellular prokaryote. J Bacteriol. 1996;178:4084–4088. doi: 10.1128/jb.178.14.4084-4088.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanlon W A, Inouye M, Inouye S. Pkn9, a Ser/Thr protein kinase involved in the development of Myxococcus xanthus. Mol Microbiol. 1996;23:459–471. doi: 10.1046/j.1365-2958.1997.d01-1871.x. [DOI] [PubMed] [Google Scholar]
- 6.Hannun Y A, Loomis C R, Merrill A H, Jr, Bell R M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261:12604–12609. [PubMed] [Google Scholar]
- 7.Herbert J M, Augereau J M, Gleye J, Maffrand J P. Chelerythrin is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 8.Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- 9.Hidaka H, Watanabe M, Kobayashi R. Properties and use of H-series compounds as protein kinase inhibitors. Methods Enzymol. 1991;201:328–339. doi: 10.1016/0076-6879(91)01029-2. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Nasr M, Kim Y, Matthews H R. Genistein inhibits protein histidine kinase. J Biol Chem. 1992;267:15511–15515. [PubMed] [Google Scholar]
- 11.Inouye, S., et al. Unpublished data.
- 12.Kaplan H B, Kuspa A, Kaiser D. Suppressors that permit A-signal independent developmental gene expression in Myxococcus xanthus. J Bacteriol. 1991;173:1460–1470. doi: 10.1128/jb.173.4.1460-1470.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028c), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 15.Kroos L, Kuspa A, Kaiser D. A global analysis of developmentally regulated genes in M. xanthus. Dev Biol. 1986;117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 16.Levitzki A. Tyrphostins—potential antiproliferative agents and novel molecular tools. Biochem Pharmacol. 1990;40:913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell S, Kaiser D. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 1995;9:1633–1644. doi: 10.1101/gad.9.13.1633. [DOI] [PubMed] [Google Scholar]
- 18.Morrison C E, Zusman D R. Myxococcus xanthus mutants with temperature-sensitive, stage-specific defects: evidence for independent pathways in development. J Bacteriol. 1979;140:1036–1042. doi: 10.1128/jb.140.3.1036-1042.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muid R E, Dale M, Davis P D, Elliot L H, Hill C H, Kumar H, Lawton G, Twomey B M, Wadsworth J, Wilkinson S E, Nixon J S. A novel conformationally restricted protein kinase C inhibitor, R0-31-8425, inhibits human neutrophil superoxide generation by soluble, particulate and post-receptor stimuli. FEBS Lett. 1991;293:169–172. doi: 10.1016/0014-5793(91)81178-b. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Dorado J, Inouye S, Inouye M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell. 1991;67:995–1006. doi: 10.1016/0092-8674(91)90372-6. [DOI] [PubMed] [Google Scholar]
- 21.Nakano H, Kobayashi E, Takahashi I, Tamaoki T, Kuzuu Y, Iba H. Staurosporine inhibits tyrosine-specific kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot. 1987;40:706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- 22.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 23.Ogawara H, Akiyama T, Ishida J, Watanabe S, Suzuki K. A specific inhibitor for tyrosine protein kinase from Pseudomonas. J Antibiot. 1986;39:606–609. doi: 10.7164/antibiotics.39.606. [DOI] [PubMed] [Google Scholar]
- 24.Shimkets L J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986;166:842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi I, Nakanishi S, Kobayashi E, Nakano H, Suzuki K, Tamaoki T. Hypericin and pseudohypericin specifically inhibit protein kinase C: possible relation to their antiretroviral activity. Biochem Biophys Res Commun. 1989;165:1207–1212. doi: 10.1016/0006-291x(89)92730-7. [DOI] [PubMed] [Google Scholar]
- 26.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 27.Toullec D, Pianetti P, Coste H, Belleverque P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203x is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 28.Udo H, Munoz-Dorado J, Inouye M, Inouye S. Myxococcus xanthus, a gram-negative bacterium, contains a transmembrane protein serine/threonine kinase that blocks the secretion of β-lactamase by phosphorylation. Genes Dev. 1994;9:972–983. doi: 10.1101/gad.9.8.972. [DOI] [PubMed] [Google Scholar]
- 29.Udo H, Inouye M, Inouye S. Biochemical characterization of Pkn2, a Ser/Thr protein kinase from M. xanthus, a gram-negative developmental bacterium. FEBS Lett. 1997;400:188–192. doi: 10.1016/s0014-5793(96)01384-1. [DOI] [PubMed] [Google Scholar]
- 30.Womack B J, Gilmore D F, White D. Calcium requirement for gliding motility in myxobacteria. J Bacteriol. 1989;171:6093–6096. doi: 10.1128/jb.171.11.6093-6096.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Munoz-Dorado J, Inouye M, Inouye S. Identification of a putative eukaryotic-like protein kinase family in the developmental bacterium Myxococcus xanthus. J Bacteriol. 1992;174:5450–5453. doi: 10.1128/jb.174.16.5450-5453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Inouye M, Inouye S. Reciprocal regulation of the differentiation of M. xanthus by Pkn5 and Pkn6, eukaryotic like Ser/Thr protein kinases. Mol Microbiol. 1996;20:435–447. doi: 10.1111/j.1365-2958.1996.tb02630.x. [DOI] [PubMed] [Google Scholar]